Abstract
The frequent co-occurrence of Alzheimer disease (AD) pathology in patients with normal pressure hydrocephalus suggests a possible link between ventricular dilation and AD. If enlarging ventricles serve as a marker of faulty cerebrospinal fluid (CSF) clearance mechanisms, then a relationship may be demonstrable between increasing ventricular volume and decreasing levels of amyloid beta peptide (Aβ) in CSF in preclinical and early AD. CSF biomarker data (Aβ, tau, and phosphorylated tau) as well as direct measurements of whole brain and ventricular volumes were obtained from the Alzheimer's Disease Neuroimaging Initiative dataset. The ratio of ventricular volume to whole brain volume was derived as a secondary independent measure. Baseline data were used for the group analyses of 288 subjects classified as being either normal (n=87), having the syndrome of mild cognitive impairment (n=136), or mild AD (n=65). Linear regression models were derived for each biomarker as the dependent variable, using the MRI volume measures and age as independent variables. For controls, ventricular volume was negatively associated with CSF Aβ in APOE ε4 positive subjects. A different pattern was seen in AD subjects, in whom ventricular volume was negatively associated with tau, but not Aβ in ε4 positive subjects. Increased ventricular volume may be associated with decreased levels of CSF Aβ in preclinical AD. The basis for the apparent effect of APOE ε4 genotype on the relationship of ventricular volume to Aβ and tau levels is unknown, but could involve altered CSF-blood-brain barrier function during the course of disease.
Keywords: Alzheimer's, MRI, cerebrospinal fluid, A-beta
INTRODUCTION
It is well recognized that amyloid beta peptide (Aβ) levels are decreased in the cerebrospinal fluid (CSF), whereas levels of tau and phospho-tau are elevated in the CSF of Alzheimer’s disease (AD) compared to normal controls. Furthermore, these biomarkers are abnormal in the preclinical stage of mild cognitive impairment (MCI) [1]. These measurements are presently being explored in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study as potentially useful biomarkers of disease progression.
Tau probably arises from degeneration of neurofibrillary tangle-laden neurons and axons. It is elevated not only in AD, but also in other conditions including acute stroke, multiple sclerosis, AIDS dementia, head trauma, amyotrophic lateral sclerosis, frontotemporal dementias, corticobasal degeneration, and prion diseases.[2, 3]
Aβ is the major constituent of neuritic plaques in AD and appears to be deposited extracellularly in the brain very early in the pathological process of AD. There is evidence that this occurs in the preclinical stage of disease, many years before the onset of dementia symptoms. The so-called amyloid hypothesis is a prevailing theory of AD pathogenesis, which holds that Aβ deposition is a seminal event leading to a toxic cascade of neurodegenerative processes that culminate eventually in loss of synapses and neuronal death.[4, 5] The mechanism whereby CSF levels of Aβ decline in AD is not well understood, but may be related to increasing self-aggregation of Aβ in the brain.
Normal pressure hydrocephalus (NPH) is a clinical syndrome manifested by the triad of gait disturbance, bladder incontinence, and later dementia. Brain imaging studies reveal a pattern of ventricular dilation consistent with communicating type hydrocephalus, in which expansion of ventricles is out of proportion to the degree of cortical atrophy. Unlike cases of acute or subacute obstructive hydrocephalus related to infection, subarachnoid hemorrhage and trauma, response to shunting cases of NPH is unpredictable, and beneficial responses are often short-lived. Most cases of NPH occur in elderly patients without apparent cause, and many of these patients have evidence of degenerative brain disease postmortem, most commonly AD. One explanation for poor responders is that they have AD with hydrocephalus ex-vacuo rather than a chronic form of obstructive hydrocephalus. Given the frequent co-occurrence of AD pathology, ranging from 31–75% of patients with clinically diagnosed NPH who have been biopsied,[6–8] this association may be more than a matter of misdiagnosis, but rather a potentially pathogenic mechanism for some if not many cases of late-onset AD. Along similar lines of evidence, Silverberg et al. coined the term "NPH-AD" to describe a subset of patients with overlapping clinical features of AD and NPH.[9] More recently Chakravarty[10] and Wostyn[11] have reviewed the evidence suggesting a possible link between AD and NPH.
There is further evidence of a defect in homeostatic mechanisms involving CSF dynamics in the choroid plexus of AD that could reduce the sink effect on Aβ clearance from interstitial fluid into the vascular space.[12] In NPH, resistance to CSF absorption is the mechanism that leads to reduced CSF turnover, and by this mechanism one might expect an increased likelihood of interstitial Aβ accumulation.[13] Since amyloid accumulates in the arachnoid membranes of AD patients,[14, 15] reduced absorption as well as reduced production of CSF may be a particularly important mechanism leading to amyloid toxicity in late onset cases of AD in whom overproduction of Aβ may not be operative. If reduced absorption rather than reduced production is the primary mechanism of CSF stagnation in AD, then one would expect to see ventricular dilation out of proportion to cortical atrophy as the correlate of reduced Aβ levels in CSF.
We propose that ventricular dilation in AD serves as a marker of altered CSF dynamics that can be used as a biologic proxy for faulty CSF clearance mechanisms. If so, then a close relationship should exist between degree of ventricular dilation in AD and levels of Aβ in CSF. If such a relationship can be demonstrated, then therapies aimed at restoring normal CSF dynamics may prove to be palliative or even effective in slowing disease progression.
Of note, a recent controlled trial of shunting patients with AD failed to demonstrate efficacy, possibly due to insensitivity of the global end point (Global Deterioration Scale) over the nine months of the double-blind portion of the study.[16] Another reason for this failure may have been related to enrollment of too many moderate to severe patients. Like a number of current anti-amyloid experimental therapies that have failed in clinical trials, the intervention may also have been tried too late to restore or slow a well-established neurodegenerative cascade. Therefore, we examined the relationship of Aβ and ventricular volumes in persons with MCI and aged controls as well as those with well-established AD.
The goals of this study were to demonstrate that there is a significant relationship between ventricular dilation and CSF Aβ levels. We predicted that this relationship would be more significant for patients with AD than for normal controls, and that the relationship would be intermediate for those with MCI. Since tau is largely an intracellular protein, and CSF clearance mechanisms for tau are not well known, we predicted that CSF tau concentration may be more significantly related to brain volume than ventricular volume.
MATERIALS and METHODS
Subjects
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu\ADNI). The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials.[17, 18]
The Principal Investigator of this initiative is Michael W. Weiner, M.D., VA Medical Center and University of California – San Francisco. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the U.S. and Canada. The initial goal of ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research -- approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years, and 200 people with early AD to be followed for 2 years. For up-to-date information see www.adni-info.org.
All subjects undergo neuropsychological and behavioral evaluations every six months over three years, as well as periodic neuroimaging with MRI and PET, blood and urine samples. Over 50% are providing periodic lumbar CSF samples as well. Biological samples are banked at the University of Pennsylvania. The biomarkers being studied include apolipoprotein E (APOE) genotype, tau and phosphorylated tau181p (p-tau), Aβ 1–42, isoprostanes, and homocysteine.
Data from the ADNI study were downloaded from the website, including demographic, cognitive, CSF (total tau, hyperphosphorylated tau, and beta-amyloid peptide 1–42, ratios of tau and beta-amyloid), and MRI volumetric region of interest data (whole brain and ventricles). Diagnostic subgroups included subjects with AD, MCI, and healthy controls. All subjects underwent an extensive clinical diagnostic evaluation, including basic mental status tests, neuropsychological tests, physical and neurological examinations. Global measures of cognitive function included the Mini-mental State Examination (MMSE).[19] Dementia severity was graded by the Clinical Dementia Rating (CDR).[20] All AD patients satisfied NINCDS-ADRDA diagnostic criteria[21] for probable AD and had questionable to very mild dementia (CDR 0.5) or mild (CDR 1) dementia. MCI subjects score 24–30 on the MMSE, had a CDR of 0.5, and had memory complaints as well as objective evidence of memory impairment based on education-adjusted scores on the Wechsler Logical Memory II memory scale. By study entry criteria, “any significant neurologic disease, such as Parkinson’s disease, multi-infarct dementia, Huntington’s disease, normal pressure hydrocephalus, brain tumor, progressive supranuclear palsy, seizure disorder, subdural hematoma, multiple sclerosis, or history of significant head trauma followed by persistent neurologic defaults or known structural brain abnormalities.” In this manner, cases with normal pressure hydrocephalus were excluded by clinical criteria without a specific radiologic exclusion of any person with enlarged ventricles.
Biomarkers
CSF specimens for biomarkers were processed by the Biomarker Core of ADNI at the Translational Research Laboratory, Department of Pathology & Laboratory Medicine at the University of Pennsylvania Medical School, under the direction of Drs. Leslie M. Shaw and John Trojanowski. Methods for measuring CSF biomarkers have been described previously.[22] The Luminex multiplex immunoassay platform was used for measurements of Aβ, tau, and p-tau. Over 50 studies have demonstrated clinical sensitivity and specificity for these biomarkers at greater than 80% each.[23] Routine laboratory measurements of CSF included total protein, glucose, and cell counts.
Magnetic Resonance Imaging
Image acquisition, quality control, image correction, and phantom based scaling methods are described in detail in the ADNI website (http://www.loni.ucla.edu/ADNI/Data/ADNI_Data.shtml). Raw imaging data were downloaded from the ADNI site by Dr. Anders Dale and colleagues at the Department of Neurosciences and Radiology, University of California, San Diego. Phantom scans were used to correct for gradient nonlinearities, followed by image intensity normalization. Cerebral and subcortical segmentation was performed based on 3D T1-weighted MRI volumes, using an automated whole-brain segmentation procedure for obtaining delineations of different neuroanatomical structures, including hippocampus, amygdala, thalamus, cerebellum, putamen, globus pallidus, whole brain and all ventricles.[24, 25] Neuroanatomical labels are assigned to each voxel based on probabilistic information estimated from an atlas, thus allowing estimation of volumetric measures of each anatomical structures. The accuracy of this procedure has been shown to be comparable to that of manual labeling and sensitive to subtle changes in AD [24] and normal aging.[26] These methods are publicly available through the FeeSurfer package.[27] The volumetric measures so acquired, were then uploaded to the ADNI website for public access.
Total ventricular volume and total brain volume were the primary regions of interest. Total brain volume represents a summary measure of total brain parenchyma including the cerebrum, basal ganglia, diencephalon, and cerebellum. An additional measure of total intracranial volume was obtained to control for head size variability between subjects. This measure was intended to be insensitive to cerebral atrophy and thus to reflect the intracranial volume regardless of age or disease progression. Total intracranial volume was thus derived by combining the masks for grey matter, white matter, and CSF obtained from the segmentation procedures above to form a binary mask. To account for spatial discontinuation, this binary mask was repeatedly smoothed with a Gaussian kernel to produce a connected uniform mask extending to but excluding the skull.
Statistical Analyses
Pearson correlation coefficients were examined comparing ventricle to whole brain volumes. For the entire sample, there was a significant relationship between the two volume measurements (r = .17, p < 0.005). Significant positive correlations between ventricle and whole brain volumes were seen in all three subject groups, suggesting a common relationship to overall head size (see Table 1).
Table 1.
Ventricle and whole brain volume correlations: Pearson correlation coefficients and (N)
| All subjects | APOE ε4 positive subjects | APOE ε4 negative subjects | |
|---|---|---|---|
| Controls | .03 * (87) | .15 * (21) | .01 (66) |
| MCI | .03 (136) | .02 (74) | .04 * (62) |
| AD | .07 * (65) | .11 * (44) | .03 (21) |
p < .05
To adjust for inter-subject differences in brain size, ventricle/whole brain volume ratio was derived as a secondary independent variable. Correction procedures are often necessary in volumetric MRI studies to account for such difference between individuals; however, there is no clearly preferred method. The ventricle/whole brain volume ratio has been shown to have more robust relationships with neuropsychological performance than either volume alone in a study of dementia and elderly control subjects.[28] In another study, ventricle/whole brain volume ratio performed better than ventricle/total intracranial volume ratio and better than uncorrected ventricular volume in distinguishing dementia subjects from elderly controls.[29]
A series of multivariable linear regression models were then analyzed using stepwise subtraction of independent variables with significance levels of p>0.05. The CSF biomarkers were the dependent variables in these models. Independent variables included ventricle and whole brain parenchymal volumes, as well as age. Total intracranial volume was also included as an additional covariate to account for head size variability between subjects. After accomplishing these analyses, the same analyses were repeated with ventricle/ brain parenchymal volume ratio substituting for the separate ventricle and brain parenchymal volumes, and the two set of results were compared. To assess whether ventricle volume may be simply a proxy for lateral ventricle expansion secondary to early degeneration and atrophy of medial temporal structures, significant relationships between biomarkers and hippocampal and entorhinal cortex volumes were examined as well. Hippocampal atrophy has been shown to be a stronger predictor of progression from MCI to AD than whole brain volume.[30]
Group analyses according to APOE genotype were defined according to presence or absence of at least one APOE ε4 allele. Statistical analyses were performed using Stata SE, version 10, software. Graphs were created using the same software.
RESULTS
Demographic information for the study sample is provided in Table 2. The sample included 87 control subjects, 136 MCI subjects, and 65 AD subjects. Global cognitive impairment for the AD subjects was mild, with a mean MMSE of 23.7 ± 2.0, compared to 27.1 ± 1.8 for MCI subjects and 29.1 ± 1.1 for controls.
Table 2.
Demographic data subclassified by presence of APOE ε4 genotype
| Normal Older Controls | Mild Cognitive Impairment | Alzheimer’s Disease | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ε 4− | ε 4+ | All | ε 4− | ε 4+ | All | ε 4− | ε 4+ | All | |
| Sample size: N | 66 | 21 | 87 | 62 | 74 | 136 | 21 | 44 | 65 |
| Sex: female N (proportion) | 30 (.45) | 15 (.71) | 45 (.52) | 47 (.52) | 42 (.57) | 89 (.65) | 11 (.52) | 25 (.57) | 36 (.55) |
| Age: years mean (S.D.) | 75.9 (5.3) | 75.4 (6.3) | 75.8 (5.5) | 75.8 (8.1) | 73.2 (6.7) | 74.4 (7.4) | 75.0 (9.2) | 74.9 (6.8) | 74.9 (7.6) |
| Education: years mean (S.D) | 15.7 (2.7) | 15.7 (3.6) | 15.7 (2.9) | 15.9 (2.9) | 15.8 (2.9) | 15.9 (2.9) | 16.9 (2.5) | 14.5 (3.6) | 15.3 (3.5) |
| MMSE: score mean (S.D.) | 29.1 (1.0) | 28.9 (1.2) | 29.1 (1.1) | 27.0 (1.9) | 27.2 (1.8) | 27.1 (1.8) | 23.6 (1.9) | 23.7 (2.0) | 23.7 (2.0) |
Tau, Aβ, and ventricular volume
Mean measurements for the biomarkers and MRI volumes are provided in Table 3. Similar summary data from a cross-sectional study of 399 subjects using ADNI data have been previously presented.[31] As expected, CSF Aβ levels declined and tau levels rose progressively among normal, MCI, and AD groups. Ventricular volumes increased and brain volumes declined progressively among normal, MCI, and AD groups.
Table 3.
CSF biomarkers and MRI volumes subclassified by diagnosis and presence of APOE ε4 genotype
| Normal Older Controls | Mild Cognitive Impairment | Alzheimer’s Disease | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ε 4− | ε 4+ | All | ε 4− | ε 4+ | All | ε 4− | ε 4+ | All | |
| CSF Aβ1–42 (pg/ml. ± S.D.) | 220.7 (47.9) | 156.9 (48.5) | 205.6 (55.1) | 187.5 (59.3) | 143.0 (40.9) | 163.7 (54.9) | 170.0 (52.3) | 131.0 (27.2) | 143.0 (40.8) |
| CSF Tau (pg/ml. ± S.D.) | 66.3 (25.9) | 80.4 (40.2) | 69.6 (30.3) | 86.2 (47.2) | 118.4 (67.3) | 103.5 (60.9) | 124.9 (68.7) | 120.1 (52.3) | 121.6 (57.6) |
| CSF P-Tau181 (pg/ml. ± S.D.) | 22.5 (11.1) | 32.3 (21.0) | 24.8 (14.6) | 29.7 (16.3) | 40.5 (18.0) | 35.5 (18.0) | 41.5 (22.1) | 41.7 (18.8) | 41.6 (19.8) |
| CSF Protein (g/dl. ± S.D.) | 46.0 (18.8) | 39.8 (21.1) | 44.3 (19.6) | 45.0 (20.7) | 38.2 (18.5) | 41.6 (19.8) | 47.3 (21.3) | 47.6 (34.3) | 47.5 (29.7) |
| Ventricular volume (mean ml. ± S.D.) | 37.3 (19.2) | 36.0 (22.3) | 37.0 (20.0) | 46.1 (23.4) | 44.0 (19.6) | 45.0 (21.3) | 48.0 (21.7) | 49.1 (22.4) | 48.7 (22.0) |
| Whole brain volume (mean ml. ± S.D.) | 997.1 (99.1) | 999.8 (97.7) | 997.9 (98.4) | 998.3 (110.0) | 993.5 (109.7) | 995.6 (109.7) | 946.0 (110.1) | 955.3 (92.8) | 952.3 (98.6) |
| Ventricles/brain volume (proportion ± S.D.) | .038 (.019) | .036 (.020) | .037 (.020) | .046 (.022) | .045 (.020) | .045 (.021) | .051 (.023) | .051 (.022) | .051 (.023) |
For the entire sample, ventricles (t = −2.90; p = 0.004) and age (t = 2.29; p = 0.02) were significantly related to CSF Aβ. Similar results were found for ventricle/brain ratio (t = −2.94; p = 0.004) and age (t=2.39; p = 0.02). Tau was significantly related to whole brain volume (t = −2.70; p = 0.007) but not ventricle volume or age. There was a trend toward relationship between p-tau and whole brain (t = −1.82; p = 0.07). CSF total protein was not significantly associated with any brain or ventricular volume in any of the analyses.
Tau, Aβ, and ventricular volume by diagnostic group
The models containing significant variables (p < 0.05) for diagnostic subgroups are shown in Table 4. The ventricle/brain volume ratio (VBR) proved to be an equivalent or more significant predictor than ventricular volume alone. Furthermore, the ratio served as a correction factor for head size (which was not recorded in the ADNI dataset). Therefore, the models containing the VBR are shown in the table rather than the ventricle volumes.
Table 4.
Summary of multiple regression models by disease category and APOE ε4 genotype
| N | F | Probability > F |
R2 | CSF Biomarker |
Region | t | p | |
|---|---|---|---|---|---|---|---|---|
| Controls | ||||||||
| ε 4+ | 21 | (1,19)=5.95 | .025 | .24 | A-beta | Ventricles/brain | −2.44 | .025 |
| ε 4− | 66 | (2,63)=4.24 | .02 | .12 | Tau | Ventricles/brain | −2.54 | .01 |
| Age | 2.05 | .04 | ||||||
| All | 87 | (2,84)=4.34 | .02 | 0.09 | Tau | Ventricles/brain | −2.32 | .02 |
| Age | 2.45 | .02 | ||||||
| MCI | ||||||||
| All | 133 | (1,131)=8.24 | .005 | 0.06 | Tau | Brain | −2.87 | .005 |
| All | 133 | (1,135)=4.40 | .04 | 0.03 | P-tau | Brain | −2.10 | .04 |
| AD | ||||||||
| ε 4+ | 43 | (1,41)=11.95 | .001 | 0.23 | Tau | Ventricles/brain | −3.46 | .001 |
Controls
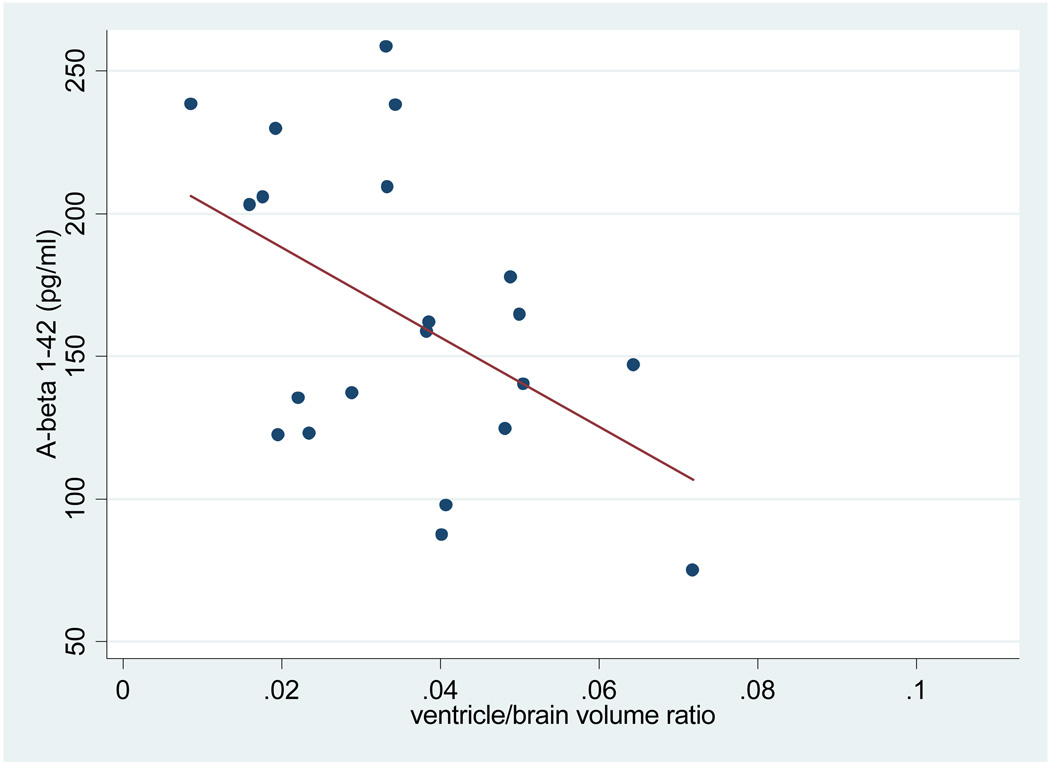
VBR was significantly associated with Aβ, but not age, in the APOE ε4 positive cognitive healthy controls. VBR was not significantly associated with Aβ for cognitively healthy controls who were APOE ε4 negative, or for the control group as a whole. Aβ was also not associated with whole brain volume for the group as a whole or as a function of APOE genotype. The relationship between Aβ levels and VBR in the APOE ε 4 positive healthy controls is shown in Figure 1, with a scatter plot of data points relative to the regression line.
Figure 1.
Aβ levels and ventricle/brain volume in APOE ε4 positive healthy controls
In contrast with findings for Aβ, both VBR and age were significantly associated with CSF tau levels in the APOE ε4 negative cognitively healthy controls. Yet, tau levels were not significantly associated with VBR for either APOE ε4 positive healthy controls or the group as a whole. There was no interaction between age and MRI volumetric variables.
There was not a significant relationship between either Aβ or tau and total hippocampal or entorhinal volumes among APOE ε4 positive cognitively healthy controls. A significant relationship was found between tau and right hippocampal volume in APOE ε4 negative controls (P<.05) but not for left hippocampal volume or entorhinal cortex in either hemisphere. Smaller right hippocampal volume was associated with greater CSF tau among the healthy controls.
Mild Cognitive Impairment Subjects
Significant associations were found between whole brain volume and both CSF tau and CSF p-tau for the MCI group as a whole. However, VBR was not significantly associated with either tau or p-tau for MCI patients. Furthermore, neither whole brain nor VBR were associated with any biomarkers (Aβ, tau, p-tau) as a function of APOE ε4 status among people with MCI.
Alzheimer Subjects
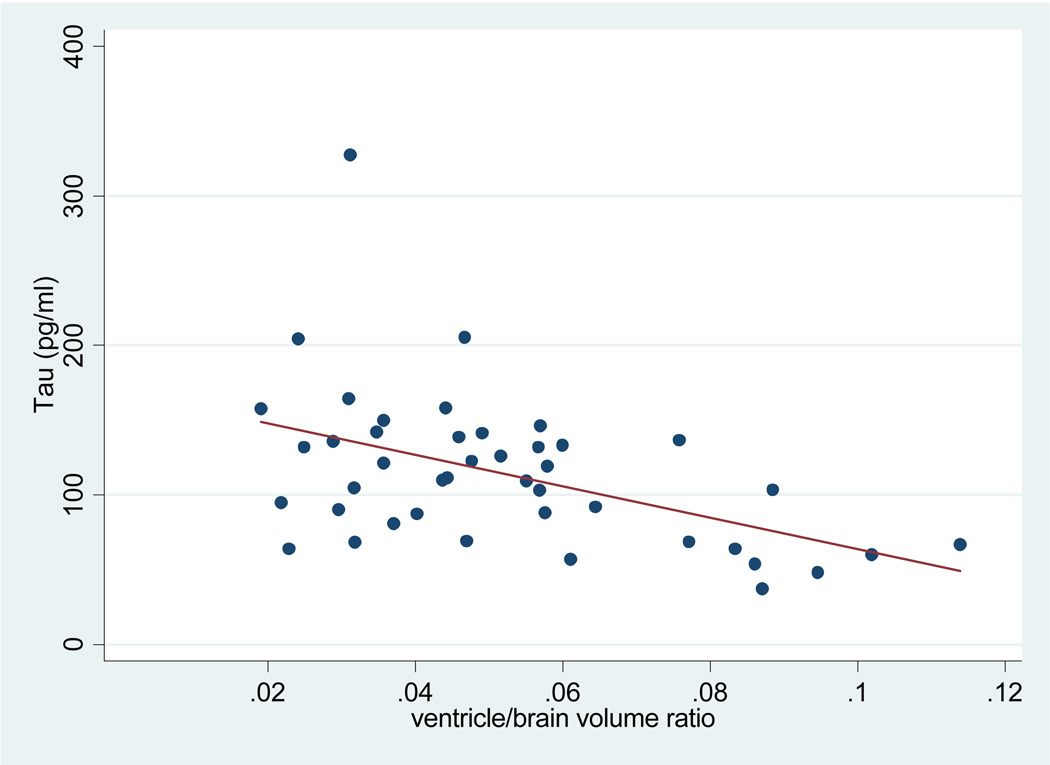
VBR was significantly associated with tau levels among APOE ε4 positive patients with AD (see Figure 2). In contrast, VBR was not associated with Aβ in APOE ε4 positive subjects.
Figure 2.
Tau levels and ventricle/brain volume in APOE ε4 positive AD patients
Aβ and tau were not significantly related to whole brain volume among the AD patients. There was no significant relationship between Aβ and tau and volume measurements of either hippocampus or entorhinal cortex, among APOE ε4 positive control and AD subjects. There was no significant effect of total intracranial volume when entered as a covariate in any of the models above.
DISCUSSION
As expected, a significant negative relationship between ventricular volume and CSF Aβ levels existed for the ADNI cohort as a whole. AD patients exhibited greatest ventricular volumes and lowest CSF Aβ levels, healthy controls had the smallest ventricles and highest CSF Aβ levels, whereas MCI patients fell in between on both of these measures. This finding is consistent with widely accepted thinking regarding the relationship between CSF Aβ levels and neuropathological findings in AD.[22]
We also expected that this relationship would be strongest for patients with AD, but surprisingly found the opposite relationship when only the AD group was considered. This distinction between the relationships observed when the entire cohort is analyzed versus when AD patients alone were considered is somewhat paradoxical, but may point to important issues regarding the relationships between biomarkers and AD pathogenesis. Specifically, the neuropathology occurring at different stages of AD may produce a different relationship between CSF Aβ and ventricular volume when only people with preclinical or well-established AD are considered.
Overall these results suggest the possibility that Aβ sequestration in brain occurs very early in the prodromal stage of AD, and that by the time people reach the stages of MCI and AD, CSF Aβ dynamics are less operative, and tau sequestration is more active. Another possible explanation is that dementia patients most likely to have a significant association between Aβ and ventricular volume, those with symptoms and radiological signs of NPH, were excluded from the ADNI cohort.
As predicted, CSF tau concentration was more significantly related to whole brain volume than ventricular volume in MCI subjects; however, we were surprised not to see a similar relationship in AD subjects. We suspect that a ceiling effect for this relationship is reached in pathologically well-established AD. To illustrate this point, among APOE ε4 positive subjects, CSF tau increased by 48% between normal and MCI groups, but only by 2% between MCI and AD groups, while VBR increased 25% between normal and MCI groups, and by 13% between MCI and AD groups.
Our findings partially replicate another recent study showing a relationship between elevated levels of CSF tau and ptau181 and whole brain volume in a mixed group of 21 subjects with very mild (CDR 0.5) AD and 8 subjects with mild (CDR 1.0) AD. Among 69 cognitively normal subjects CSF Aβ-42 was positively correlated with whole brain volume. Ventricle volume and VBR were not examined, however, limiting comparison with the present study.[32]
The most interesting finding in our study was the negative relationship between Aβ levels and ventricular volume in normals who were positive for the APOE ε4 allele. No significant relationship was found for APOE ε4 positive MCI subjects, suggesting that there is a transition state, when subjects at genetic risk for AD may be sequestering Aβ in the brain mainly during the prodromal stage of the disease. Along similar lines, amyloid deposition in brain has been shown by PiB amyloid PET imaging studies to occur frequently in cognitively normal elders, however, the causes and prognostic significance of such cases of early amyloid deposition are unknown.[33]
We propose that altered CSF-blood-brain barrier functions may account for these complex relationships. There is increasing evidence of blood-brain barrier compromise[34] as well as microvascular damage occurring early in AD.[22,35,36] Apolipoprotein E is essential for both blood-brain barrier integrity and for deposition of fibrillar Aβ. Both APOE and Aβ are ligands for low-density lipoprotein receptor-related protein 1 (LRP-1), a major transporter of Aβ out of brain, and all three proteins are located in plaques. Most plaques are in close proximity to the cerebral microvessels, leading to potentially complex interactions affecting clearance of Aβ. In AD, LRP-1 is downregulated at the blood-brain barrier, which is likely one of the mechanisms of reduced Aβ clearance from the brain.[37] There is evidence to suggest that APOE ε4 enhances vascular and parenchymal deposition of Aβ in the brain[38] and may influence both transport and permeability of the blood-brain barrier.[34,39]
CSF protein concentration was consistently higher in AD compared to MCI and controls. This likely reflects enhanced blood-brain barrier permeability to albumin, but there was no correlation between blood-brain barrier function, measured as the CSF total protein level, and any brain or ventricular volume. This finding suggests that blood-brain barrier dysfunction is not directly related to brain atrophy. Aβ-induced disruptions of blood-brain barrier and choroid plexus permeability and transport would be expected to destabilize interstitial and CSF dynamics (and ventricle size) thereby impairing brain metabolism and blood flow.[40, 41]
Enhancement of vascular amyloid deposition by APOE ε4 in arachnoid granulations may have a role in reducing Aβ clearance from brain via CSF circulation. This may account for the observation of increased APOE ε4 allele frequency in NPH patients with dementia [42] and a role for hydrocephalus in the pathogenesis of AD in some patients. Alternatively APOE ε4 may serve as just a marker of earlier onset and more severe AD pathology and not be directly involved in the mechanisms of Aβ clearance via CSF. In further support of a hydrocephalic mechanism for AD is a recent report of Aβ42 and hyperphosphorylated tau pathology occurrence in a kaolin-induced hydrocephalus model of the aged rat.[43, 44]
Little is known about compartmentalization of tau in the course of AD, but this data suggests that as neurodegeneration becomes established by cascading pathogenic events, tau becomes sequestered at a later time in those with well-established disease. Tau sequestration in AD may be related to similar mechanisms described previously for Aβ, which occur much earlier than tau in the pathogenic cascade.
Finally, among APOE ε4 negative controls we found a negative relationship between CSF tau and ventricular volume. In this group, age and hippocampal volume were also associated with tau levels, suggesting that age–related atrophy rather than APOE genetic mechanisms may be driving this relationship. Since this group of subjects likely includes many who would never go on to develop AD, the relevance of this relationship to our understanding of biomarkers for AD is limited.
The results of this study should be interpreted with great caution for a number of reasons. The measure of ventricular volume is a global measure of the entire ventricular system. We cannot exclude the possibility that the ventricle volume is merely a proxy for brain atrophy in specific adjacent brain regions such as the medial temporal lobe, which could affect mainly the temporal horn. While the lack of relationship to brain volume in this area in our APOE ε4 positive subjects argues against this possibility, further analyses using segmented ventricle volumes [45] and ventricular shape data [46] could provide more definitive evidence for a primary role of ventricular pathology leading to Aβ deposition.
The analyses here were only cross-sectional, due to the limited availability of longitudinal CSF biomarker data in ADNI. Future studies examining sequential changes in biomarkers compared to brain and ventricular volumes in prodromal AD may shed more light on the mechanisms we propose based on baseline data.
Also to be noted, the sample size of 21 in the APOE ε4 positive control group is particularly small. While the relationship between ventricle/brain ratio and CSF Aβ is one of the most interesting observations, these results need verification from studies involving larger samples of older cognitively normal subjects.
Experimental evidence using animal models of hydrocephalus and APOE may shed light on the exact nature of these relationships. If indeed altered CSF clearance mechanisms in the prodromal stage of AD caused by interaction of APOE ε4 and epithelial/vascular function in the choroid plexus and/or arachnoid villi leads to sequestration of Aβ in the brain, setting off a cascade of pathologic events, then efforts to interrupt these mechanisms may prove fruitful in disease prevention.
While this exploration of the ADNI data provides evidence of a potential hydrocephalic mechanism early in AD for some patients as well as a potential explanation of amyloid deposition in NPH, we were unable to examine actual CSF production, which is reduced in aging and AD,[13,47]and how this too may affect Aβ, tau, or other brain-derived proteins. These are rich areas of potential future research.
An alternative explanation to the proposed obstructive hydrocephalic mechanism is that enlarged ventricles relative to the rest of brain tissue reflect central atrophy involving white matter volume changes which are more dramatic in APOE ε4 carriers. This could be explored further by examining volumetric measurements of white matter on MRI in comparison to CSF biomarkers.
Ventricular volume [45,48] and VBR change [49] in aging, MCI, and AD is emerging as an important biological indicator of disease progression. As previously mentioned, VBR has been shown to be a more robust correlate of cognitive function in AD and MCI than other whole brain measures. The reason for this significant relationship is not well understood but deserves further investigation, as VBR may be a useful biomarker outcome for early disease intervention and prevention trials, particularly for those at genetic risk due to APOE ε4 genotype.
Acknowledgments
Support acknowledgement: Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI; Principal Investigator: Michael Weiner; NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and through generous contributions from the following: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer’s Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the U.S. Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles.
Footnotes
Data used in the preparation of this article were obtained from the Alzheimer’s disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu\ADNI). As such, the investigators within the ADNI, other than Dr. Ott, contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. ADNI investigators include Dr. Ott, and a complete listing is available at: www.loni.ucla.edu\ADNI\Collaboration\ADNI_Authorship_list.pdf).
Reference List
- 1.Chong MS, Lim WS, Sahadevan S. Biomarkers in preclinical Alzheimer's disease. Curr Opin Investig Drugs. 2006;7:600–607. [PubMed] [Google Scholar]
- 2.Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, Morris JC, McKeel DW, Jr, Farlow M, Weitlauf SL, Quinn J, Kaye J, Knopman D, Arai H, Doody RS, DeCarli C, Leight S, Lee VM, Trojanowski JQ. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003;60:1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- 3.Formichi P, Battisti C, Radi E, Federico A. Cerebrospinal fluid tau, A beta, and phosphorylated tau protein for the diagnosis of Alzheimer's disease. J Cell Physiol. 2006;208:39–46. doi: 10.1002/jcp.20602. [DOI] [PubMed] [Google Scholar]
- 4.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann N Y Acad Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 6.Golomb J, Wisoff J, Miller DC, Boksay I, Kluger A, Weiner H, Salton J, Graves W. Alzheimer's disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry. 2000;68:778–781. doi: 10.1136/jnnp.68.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leinonen V, Alafuzoff I, Aalto S, Suotunen T, Savolainen S, Nagren K, Tapiola T, Pirttila T, Rinne J, Jaaskelainen JE, Soininen H, Rinne JO. Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Arch Neurol. 2008;65:1304–1309. doi: 10.1001/archneur.65.10.noc80013. [DOI] [PubMed] [Google Scholar]
- 8.Savolainen S, Paljarvi L, Vapalahti M. Prevalence of Alzheimer's disease in patients investigated for presumed normal pressure hydrocephalus: a clinical and neuropathological study. Acta Neurochir (Wien) 1999;141:849–853. doi: 10.1007/s007010050386. [DOI] [PubMed] [Google Scholar]
- 9.Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D. Alzheimer's disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2003;2:506–511. doi: 10.1016/s1474-4422(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarty A. Unifying concept for Alzheimer's disease, vascular dementia and normal pressure hydrocephalus - a hypothesis. Med Hypotheses. 2004;63:827–833. doi: 10.1016/j.mehy.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Wostyn P, Audenaert K, De Deyn PP. Alzheimer's disease-related changes in diseases characterized by elevation of intracranial or intraocular pressure. Clin Neurol Neurosurg. 2008;110:101–109. doi: 10.1016/j.clineuro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Johanson C, McMillan P, Tavares R, Spangenberger A, Duncan J, Silverberg G, Stopa E. Homeostatic capabilities of the choroid plexus epithelium in Alzheimer's disease. Cerebrospinal Fluid Res. 2004;1:3. doi: 10.1186/1743-8454-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D. Alzheimer's disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2003;2:506–511. doi: 10.1016/s1474-4422(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 14.Kalaria RN, Premkumar DR, Pax AB, Cohen DL, Lieberburg I. Production and increased detection of amyloid beta protein and amyloidogenic fragments in brain microvessels, meningeal vessels and choroid plexus in Alzheimer's disease. Brain Res Mol Brain Res. 1996;35:58–68. doi: 10.1016/0169-328x(95)00180-z. [DOI] [PubMed] [Google Scholar]
- 15.Stopa EG, Berzin TM, Kim S, Song P, Kuo-LeBlanc V, Rodriguez-Wolf M, Baird A, Johanson CE. Human choroid plexus growth factors: What are the implications for CSF dynamics in Alzheimer's disease? Exp Neurol. 2001;167:40–47. doi: 10.1006/exnr.2000.7545. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg GD, Mayo M, Saul T, Fellmann J, Carvalho J, McGuire D. Continuous CSF drainage in AD: results of a double-blind, randomized, placebo-controlled study. Neurology. 2008;71:202–209. doi: 10.1212/01.wnl.0000316197.04157.6f. [DOI] [PubMed] [Google Scholar]
- 17.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, Trojanowski JQ, Toga AW, Beckett L. Ways toward an early diagnosis in Alzheimer's disease: The Alzheimer's Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Toga AW, Beckett L. The Alzheimer's disease neuroimaging initiative. Neuroimaging Clin N Am. 2005;15:869–872. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9 Suppl 1:173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LM. PENN biomarker core of the Alzheimer's disease Neuroimaging Initiative. Neurosignals. 2008;16:19–23. doi: 10.1159/000109755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der KA, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 25.Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23 Suppl 1:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 28.Bigler ED, Neeley ES, Miller MJ, Tate DF, Rice SA, Cleavinger H, Wolfson L, Tschanz J, Welsh-Bohmer K. Cerebral volume loss, cognitive deficit and neuropsychological performance: comparative measures of brain atrophy: I. Dementia. J Int Neuropsychol Soc. 2004;10:442–452. doi: 10.1017/S1355617704103111. [DOI] [PubMed] [Google Scholar]
- 29.Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Invest Radiol. 2001;36:539–546. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, Scheltens P, Vrenken H, Barkhof F. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR., Jr MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donahue JE, Johanson CE. Apolipoprotein E, amyloid-beta, and blood-brain barrier permeability in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:261–270. doi: 10.1097/NEN.0b013e31816a0dc8. [DOI] [PubMed] [Google Scholar]
- 35.Stopa EG, Butala P, Salloway S, Johanson CE, Gonzalez L, Tavares R, Hovanesian V, Hulette CM, Vitek MP, Cohen RA. Cerebral cortical arteriolar angiopathy, vascular beta-amyloid, smooth muscle actin, Braak stage, and APOE genotype. Stroke. 2008;39:814–821. doi: 10.1161/STROKEAHA.107.493429. [DOI] [PubMed] [Google Scholar]
- 36.Kalaria RN, Premkumar DR, Pax AB, Cohen DL, Lieberburg I. Production and increased detection of amyloid beta protein and amyloidogenic fragments in brain microvessels, meningeal vessels and choroid plexus in Alzheimer's disease. Brain Res Mol Brain Res. 1996;35:58–68. doi: 10.1016/0169-328x(95)00180-z. [DOI] [PubMed] [Google Scholar]
- 37.Donahue JE, Flaherty SL, Johanson CE, Duncan JA, III, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 38.Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer's disease. Front Biosci. 2003;8:s515–s521. doi: 10.2741/1085. [DOI] [PubMed] [Google Scholar]
- 42.Nacmias B, Tedde A, Guarnieri BM, Petruzzi C, Ortenzi L, Serio A, Amaducci L, Sorbi S. Analysis of apolipoprotein E, alpha1-antichymotrypsin and presenilin-1 genes polymorphisms in dementia caused by normal pressure hydrocephalus in man. Neurosci Lett. 1997;229:177–180. doi: 10.1016/s0304-3940(97)00449-7. [DOI] [PubMed] [Google Scholar]
- 43.Klinge PM, Heile A, Slone S, Johanson CE, Miller M, Duncan JA, Brinker T, Silverberg GD. Evidence of TAU pathology in kaolin-induced hydrocephalus model of the aged rat. Cerebrospinal Fluid Res. 2009;6:S37. [Google Scholar]
- 44.Klinge PM, Samii A, Niescken S, Brinker T, Silverberg GD. Brain amyloid accumulates in aged rats with kaolin-induced hydrocephalus. Neuroreport. 2006;17:657–660. doi: 10.1097/00001756-200604240-00020. [DOI] [PubMed] [Google Scholar]
- 45.Chou YY, Lepore N, Avedissian C, Madsen SK, Parikshak N, Hua X, Shaw LM, Trojanowski JQ, Weiner MW, Toga AW, Thompson PM. Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alzheimer's disease, mild cognitive impairment and elderly controls. Neuroimage. 2009;46:394–410. doi: 10.1016/j.neuroimage.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu A, Fennema-Notestine C, Dale AM, Miller MI. Regional shape abnormalities in Mild Cognitive Impairment and Alzheimer's disease. Neuroimage. 2009;45:656–661. doi: 10.1016/j.neuroimage.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johanson CE, Duncan JA, III, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R. Ventricular enlargement as a possible measure of Alzheimer's disease progression validated using the Alzheimer's disease neuroimaging initiative database. Brain. 2008;131:2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC, Liu Y, Toga AW, Becker JT. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007;21:14–24. doi: 10.1097/WAD.0b013e318032d2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]