Abstract
Ubiquitous environmental agents [e.g. polynuclear aromatic hydrocarbons (PAH) and their nitrated derivatives (NO2-PAH)] that are known to induce mammary cancer in rodents are regarded as potential human risk factors for inducing analogous human cancers. Although 6-nitrochrysene (6-NC) is less abundant than other NO2-PAH in the environment, it is the most potent mammary carcinogen in the rat; its carcinogenic potency is not only higher than that of the carcinogenic PAH, benzo[a]pyrene (B[a]P), but also of the well-known carcinogenic heterocylic aromatic amine, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Studies in rats and in vitro assays have indicated that 6-NC can be activated by simple nitroreduction leading to the formation of 6-hydroxylaminochrysene (N-OH-6-AC); this metabolite yielded N-(deoxyguanosin-8-yl)-6-aminochrysene (N-[dG-8-yl]-6-AC) and 5-(deoxyguanosin-N2-yl)-6-aminochrysene (5-[dG-N2-yl]-6-AC. These lesions are likely to cause mutations if they are not removed by cellular defense mechanisms before DNA replication occurs. However, nothing is known about the susceptibility of these adducts to nucleotide excision repair (NER), the major cellular repair system that removes bulky adducts. In order to address this issue, we synthesized the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC lesions and site-specifically inserted these lesions into 135-mer DNA duplexes. These constructs were incubated with NER-competent nuclear extracts from human HeLa cells. The efficiency of repair of these lesions were ~ 8 times less efficient than in the case of the well known and excellent substrate of NER, the intrastrand cross-linked cis-diaminodichloroplatinum II adduct in double-stranded DNA (cis-Pt), but similar to N2-dG adducts derived from the (+)-bay region diol epoxide of B[a]P [(+)-cis-B[a]P-N2-dG and (+)-trans-B[a]P-N2-dG]. The results support the hypothesis that the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC lesions may be slowly repaired and thus persistent in mammalian tissue which could, in part, account for the potent tumorigenic activity of 6-NC in the rat mammary gland.
Introduction
Breast cancer is second only to lung cancer as the leading cause of cancer-related deaths in American women (1); the etiology of most breast cancers remains obscure. Humans are continuously exposed to various amounts of chemicals that have been shown to have carcinogenic/mutagenic properties in experimental systems (2). It has been estimated that, in addition to genetic disposition, a significant portion of cancer incidence in the USA is related to environmental factors and lifestyle (3–8). Chronic exposure to traces of chemical carcinogens in the diet, in polluted air or in tobacco smoke, can be important in the etiology of breast cancer in the presence of host factors that favor the multi-step process of carcinogenesis (2, 9, 10). Ubiquitous environmental agents that are known inducers of mammary cancer in rodents must be regarded as potential human risk factors for inducing analogous human cancers. Examples of environmental carcinogens are polynuclear aromatic hydrocarbons (PAH) and their nitrated derivatives (NO2-PAH), as well as the food-derived heterocyclic aromatic amines (HAA). The presence of bulky DNA adducts in human breast tissues and blood, support the notion that environmental carcinogens play an important role in the etiology of breast cancer (9–14).
NO2-PAH have been detected in numerous environmental sources, including diesel engine emissions, combustion emissions from kerosene heaters, gas fuel, liquid petroleum, airborne particulates, coal fly ash, and aircraft engine emissions, as well as in certain foods and beverages (15, 16). In 2003, the World Health Organization Report concluded that NO2-PAH are probably human carcinogens (17). 6-Nitrochrysene (6-NC), a representative compound of the class of NO2-PAH, is the most active “parent” compound ever tested in the newborn mouse assay; it is much more active than chrysene and other known mono-nitrochrysene isomers (18). The carcinogenic potency of 6-NC in the rat mammary gland is not only higher than that of B[a]P and its bay region diol epoxide but also of the well-known HAA, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) (19–22). The discovery of hemoglobin adducts derived from 6-NC clearly demonstrates that humans are exposed to this environmental carcinogen (17). The remarkable carcinogenic activity of 6-NC in the rat mammary gland, its environmental occurrence, the ability of human liver, lung, and breast tissues to convert 6-NC into active metabolites that can readily react with DNA by forming covalent adducts suggest that 6-NC probably contributes to the development of human breast cancer (17).
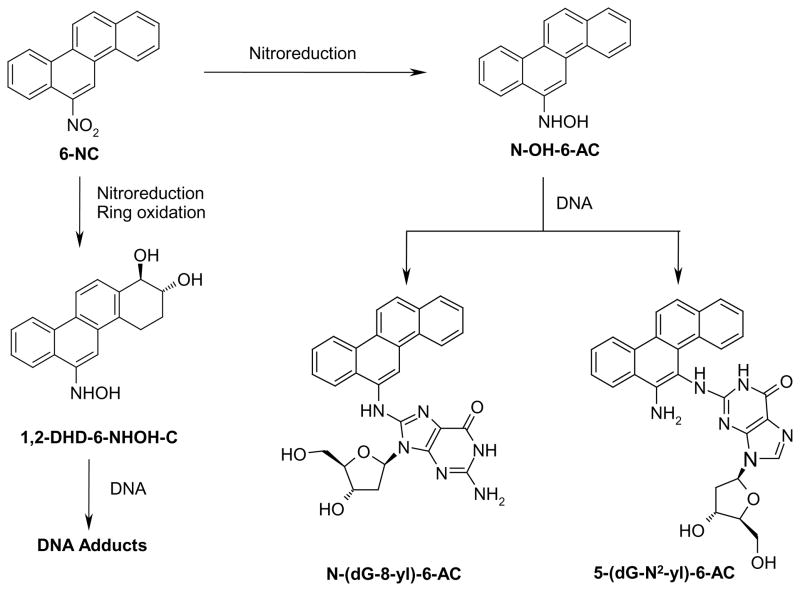
It is well established that 6-NC can be metabolically activated by two pathways (Scheme 1). The first pathway involves simple nitroreduction to form the corresponding 6-hydroxylaminochrysene (N-OH-6-AC); the latter is known to yield DNA adducts (Scheme 1) and their chemical structures have been characterized (23, 24). The second pathway involves a combination of ring oxidation and nitroreduction yielding a reactive electrophile, trans-1,2-dihydroxy-1,2-dihydro-6-hydroxylaminochrysene (1,2-DHD-6-NHOH-C) that primarily leads to the formation of a major adduct that has been characterized in our laboratory (25). These DNA lesions are likely to cause mutations if they are not removed by cellular defense mechanisms before DNA replication occurs. However, nothing is known about the susceptibility of these adducts to nucleotide excision repair (NER), the major cellular repair system that removes bulky DNA lesions (26, 27). In order to address this issue, in the present study we synthesized N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC lesions and site-specifically inserted these into 135-mer DNA duplexes. These constructs were incubated with NER-competent nuclear extracts from human HeLa cells. HeLa cells are widely used in the NER field because of their excellent repair activities and because they are standard cells used by many, if not most, workers in this area of research (28, 29). The efficiencies of repair of these lesions derived from 6-NC were compared to the repair of the cross-linked cis-Pt and N2-dG adducts derived from the (+)-bay region diol epoxide of B[a]P, the [(+)-cis-B[a]P-N2-dG and (+)-trans-B[a]P-N2-dG] that have been previously studied; cis-Pt and (+)-cis-B[a]P-N2-dG lesions, but not the (+)-trans-B[a]-N2-dG adduct, are known to be excellent substrates of the human NER apparatus (28, 29). The results suggest that the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC lesions may be more persistent in mammalian tissues because of slow DNA repair which may contribute to the potent tumorigenic activity of 6-NC.
Scheme 1.
Metabolic activation of 6-NC via the nitroreduction pathway leading to the formation of N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC lesions which are poor substrates of human nucleotide excision repair (NER) as shown in this work.
Materials and Methods
Caution
6-NC and its known metabolites described here are mutagenic in bacterial and mammalian systems as well as tumorigenic in rodents. Therefore, appropriate safety procedures must be followed when working with these compounds.
Preparation of N-OH-6-AC
This compound was synthesized following literature methods (25, 30). Briefly, to a stirred suspension of palladium/carbon (5%, 70 mg) in freshly distilled THF (15 mL) under N2 at −3 °C was added a solution of 6-NC (50 mg, 0.183 mmol) in THF (4 mL), followed immediately by hydrazine hydrate (N2H4 . H2O) (90 μL, 1.8 mmol). The reaction was monitored by thin layer chromatography (TLC) using benzene as an eluent, and after ~10 min. was quickly filtered using 0.22 μ PTFE syringe filter into ice-cold 0.1M sodium acetate buffer solution (pH 6.0 20 mL). The mixture was extracted with cold ether (40 mL), organic phase separated and dried (MgSO4). The solvent was removed under reduced pressure (protecting the vessel from light) at 0°C, yielded 38 mg (80%) of N-OH-6-AC as an off-white solid. Analysis by HPLC (Nucleosil C18 column, 5μ, 250 × 4.6 mm), using methanol:water mixture (82:18) in the isocratic mode at a flow rate of 1 mL/min); under these conditions, the product exhibited a retention time of 10 min with a purity level of 82%. The 1H NMR (Bruker Avance 500 MHz spectrometer) spectrum of this product was consistent with that previously reported (23). This compound was used for oligonucleotide adduction without further purification. The TLC were obtained using aluminum-supported pre-coated silica gel plates (EM industries, Gibbstown, NJ). In the column chromatography experiments, silica gel (60–200 mesh) was utilized.
Preparation of modified oligonucleotides
The oligo-2′-deoxyribonucleotide 5′-CTCTCGCTTCC-3′ was purchased from Integrated DNA Technologies. N-OH-6-AC (15 mg) was dissolved in tetrahydrofuran (THF) (1 mL) and added to a 50 mM sodium phosphate buffer solution (pH = 5.0, 15% THF, 85% buffer solution) containing the oligonucleotide (1 mM) and incubated overnight (molar ratio [N-OH-6-AC]/[DNA oligo] = 10/1). The covalently modified oligo-2′-deoxyribonucleotides were separated from the unmodified oligo-2′-deoxyribonucleotides using an Agilent 1200 Series Rapid Resolution LC System equipped with a 7.0 × 305 mm PRP-1 HPLC column (Hamilton Company). The modified oligonucleotides were purified using the same HPLC system with a 250 mm × 4.6 mm Microsorb-MV C18 column (Varian, Inc.) utilizing a 14–24% acetonitrile/triethylammonium acetate (100 mM) solution gradient in 60 min. The purities of oligonucleotides containing site-specific N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC are demonstrated using high resolution gel electrophoresis methods (Supporting Information). A small fraction of these modified oligonucleotides were digested to the nucleoside level using a combination of phosphodiesterase I and II exonucleases for use in detailed LC-MS/MS analyses as described in detail elsewhere (31).
Mass spectrometry (LC-MS/MS) Assays
The end products of N-OH-6-AC reaction with oligo-2′-deoxyribonucleotides after enzymatic digestion were identified using an Agilent 1100 Series capillary LC/MSD Ion Trap XCT equipped with an electrospray ion source. In typical ion trap experiments 8 μL of the sample solutions were injected into a narrow bore Zorbax SB-C8 column (50 × 1 mm i. d.) and eluted with an isocratic mixture of methanol and water (70 : 30) with 0.1% formic acid as the mobile phase, at a flow rate of 0.25 mL/min. The mass spectra were recorded in the negative mode. The nebulizer gas pressure was 40 psi, the dry gas flow rate was 8.0 L/min, and the dry temperature was set at 350°C. Mass spectra were obtained by averaging over the area of a particular total ion chromatogram peak and the background intensity was then subtracted.
Preparation of oligonucleotide substrates for Nucleotide Excision Repair assays
Briefly, the first step is to purify and re-purify the modified 11-mer oligonucleotides by repeated reversed-phase HPLC methods, and to isolate and characterize the products as already described. The purity was further verified using 32P-endlabeled 11-mer oligonucleotides containing the lesions and denaturing 12% polyacrylamide gel. The purified 11-mer oligonucleotides 5′-CTCTCG*CTTCC containing the single G* = N-(dG-8-yl)-6-AC or 5-(dG-N2-yl)-6-AC lesions were 32P-labeled at the 5′-end and incorporated into 135-mer oligonucleotides by standard ligation methods as described elsewhere (28). These internally and radioactively labeled and carcinogen-modified 135-mers were then purified using 12% denaturing polyacrylamide gels, and subsequently annealed with their fully complementary 135-mer strands by heating the solutions to ~90°C for 2 min and cooling overnight to 4°C. The 135-mer duplexes were then incubated with the cell extracts.
We compared the efficiencies of NER of N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC containing oligonucleotide duplexes with those containing a single intrastrand cross-linked lesion derived from the binding of cisplatin II guanine in DNA in the sequence context 5′-GAGCTCTTCTTAATTAACTCG^TG^CACACTACATCAC (cis-Pt sequence) where the G^ indicates the cross-linked lesions. This cross-linked adduct is an excellent substrate of NER in human cell extracts (28, 39). Here it was used as the positive control and reference standard in each cell extract experiment in order to compensate for differences in NER activities of different cell extracts. In this manner, good reproducibility was obtained in assessing the NER efficiencies of the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC using cell extracts prepared on different days as described previously (34).
Preparation of cell extracts
The HeLa cells (American Type Culture Collection GM00637, Coriell Institute for Medical research, Camden, NJ) were grown in culture using standard methods. Briefly, the cells were harvested by trypsinization followed by centrifugation at 1,000 rpm for 5 min at 4 ºC. The cell pellets were washed with cold PBS twice and then resuspended in lysis buffer (10 mM Tris, pH 8.0, 1 mM EDTA, and 5 mM DTT) with protease inhibitor cocktail (Sigma P8340) and incubated on ice for 20 min. The cell pellets were then homogenized and mixed with gentle stirring in four volumes of sucrose-glycerol buffer (50 mM Tris, pH 8.0, 10 mM MgCl2, 2 mM DTT, 25 % sucrose, and 50% glycerol) at 4 °C. A saturated ammonium sulfate solution was then added, followed by ultracentrifugation at 41,000 rpm for 3 hr at 2 °C. Ammonium sulfate (0.33 g/mL) was then added slowly to the supernatant, followed by centrifugation at 11,000 rpm for 30 min at 4 °C. The pellet was then re-suspended in dialysis buffer (25 mM HEPES-KOH, pH 7.9, 0.1 M KCl, 12 mM MgCl2, 1 mM EDTA, 17% glycerol, 2 mM DTT, and 2 mM ATP) and dialyzed against fresh buffer. The dialysate was then centrifuged (10 min at 4 °C). The protein concentration of the supernatant was determined by the method of Bradford using a BioRad protein assay kit. Five femtomoles of the 135-mer duplexes were then added to 25 μL aliquot of the cell extract and incubated for up to one hour at 37 °C.
Nucleotide Excision repair assays
The modified 135-mer sequences (or the unmodified control oligonucleotide 135-mer duplexes) were incubated in the cell extracts (60 – 80 μg protein in 80 μL of aliquots of the cell extracts) for specified amounts of time (up to 40 – 45 min). The oligonucleotide excision products and intact DNA sequences were desalted by precipitation with 80% (v/v) methanol, and subjected to electrophoresis in denaturing 12% (w/v) polyacrylamide gel. The gels were analyzed by autoradiography using a Storm 840 phosphorimager. The NER dual incisions yielded short oligonucleotide fragments 24 – 32 nt in length containing the 32P-label and the lesion that are easily resolved from the 135-mer unreacted oligonucleotides. The yield of dual incision products was determined from densitometry tracings of autoradiographs by summing the total radioactivity in the 24–32 oligonucleotide region and by dividing it by the total radioactivity in the same lane (unreacted 135-mers and 24 – 32 incision products) as described in more detail elsewhere (34).
Results
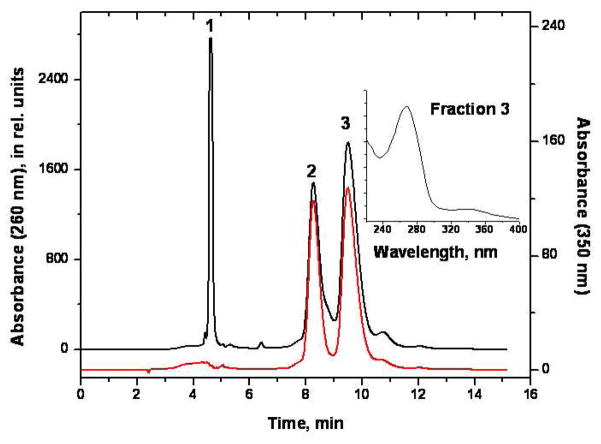
The modified oligonucleotides were generated by incubating the N-OH-6-AC derived from nitroreduction of 6-NC with the oligonculeotide 5′-CTCTCGCTTCC as described above. The reaction mixture was then subjected to reversed phase HPLC experiments to separate the modified oligonucleotides from the unmodified ones. A typical elution profile is depicted in Figure 1. The material eluting at 4.6 min (Peak 1) is the unmodified oligonucleotide. The two prominent fractions eluting at 8.3 min (Peak 2) and 9.5 min (Peak 3) exhibited UV absorption spectra above 300 nm that are characteristic of the 5-(dG-N2-yl)-6-AC and N-(dG-8-yl)-6-AC nucleoside adducts as shown earlier (23–25). Mass spectrometric methods that have been established during the past two decades, to detect DNA adducts of environmental, dietary, and endogenous genotoxic agents (35) were used to verify the structures of the lesions G*. The oligonucleotides 5′-CTCTCG*CTTCC were enzymatically digested to the nucleoside level (dG*). The LC-MS/MS characteristics were then compared to those exhibited by an authentic N-(dG-8-yl)-6-AC nucleoside adduct dGSt* that was synthesized as described in detail elsewhere (23–25).
Figure 1.
Reversed phase HPLC purification of the products of reaction of the oligonucleotide 5′-CTCTCGCTTCC-3′ with N-OH-6-AC monitored at 260 nm (black trace) and at 350 nm (red trace) where only the aromatic chrysenyl residue is light-absorbing. The absorbances are shown in relative units and the absorbance at 350 nm is ~ 6.7 times smaller than at 260 nm. Elution conditions: 14-to-24% acetonitrile/100 mM TEAA aqueous buffer solution (pH 7.0) in 60 min; C-18 Microsorb column (Varian). Reaction conditions for producing the modified oligonucleotides were: 15%THF, 85% (V/V) 20 mM sodium phosphate buffer, the N-OH-6-AC concentration was 15 mg/mL, and the concentration of the oligonucleotide was 2 mg/ml. The absorption spectrum of fraction 3 eluting at 9.3 min is shown in the inset (the absorption spectrum of fraction 2 eluting at 8.3 min is similar). The material eluting at 4.6 min (fraction 1) is the unmodified oligonucleotide. As shown in Figure 1, fractions 2 and 3 were found to contain the oligonucleotides 5′-CTCTCG*CTTCC with G* = 5-(dG-N2-yl)-6-AC and N-(dG-8-yl)-6-AC, respectively.
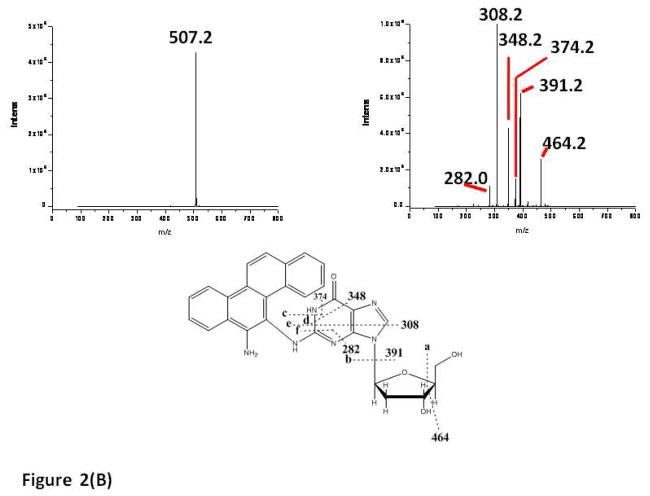
Data acquisition was performed by monitoring the product ion [M-H]− (m/z 507) in the negative mode and typical MS/MS fragmentation patterns of the modified nucleoside dG* (Peak 3, Figure 1) are shown in Figure 2A (left panel). The product ion spectra of the standard nucleoside adduct dGSt* (Figure S1, Supplementary Information) are exactly the same as those of dG*(Peak 3). A major fragmentation pathway of the dG* (Peak 3) adduct involves the loss of the deoxyribose group (116 Da) due to scission of bond b (cf structure in Figure 2A), which yields an ion of m/z 391 as shown in Figure 2A (middle panel). In addition to the loss of the deoxyribose group, previous work has shown that the dissociation pattern of deprotonated guanine (36) contains three major ion fragment products. These fragments result from the elimination of ammonia (NH3, −17 Da, Figure 2A, bond c), isocyanic acid (HNCO, −43 Da) and cyanamide (HNCNH, −42 Da). In our case, the dG* (Peak 3) nucleoside exhibits major MS/MS fragments at m/z 374 (due to ammonia elimination, as shown in Figure 2A, middle and right panels), and m/z 346 possibly resulting from the subsequent elimination of CO (−28 Da, bond d and Figure 2A, right panel) (36–38). The m/z 267 fragment may be due to the disruption of the guanine bonds as shown in Figure 2A, bond e, and right panel. These results are in excellent agreement with the fragmentation patterns of the authentic standard dGSt* (Figure S1).
Figure 2.
LC-MS/MS of the nucleoside adducts obtained from the enzymatic digestion of the modified oligonucleotide 5′-CTCTCG*CTTCC: (A) fraction 3 in Figure 1, dG*(3) = N-(dG-8-yl)-6-AC), and (B) fraction 2 in Figure 1, dG*(2) = 5-(dG-N2-yl)-6-AC).
The MS/MS fragmentation patterns of the modified nucleoside dG* (Peak 2, Figure 1) is clearly very different from those exhibited by dG* (Peak 3) and are shown in Figure 2B. In addition to the loss of the deoxyribose group (m/z 391) in this case, there are major MS/MS fragments at m/z 374 (due to ammonia elimination, scission of bond c in Figure 2B), m/z 348 (due to isocyanic acid elimination, bond d), m/z 308 (bond e) and m/z 282 (bond f) are possibly due to the disruption of guanine bonds. As discussed below, this fragmentation pattern is consistent with an N2-adducted guanine residue. This interpretation is also consistent with the observation that both the 5-[dG-N2-yl]-6-AC and N-[dG-8-yl]-6-AC adducts are formed in similar proportions when N-OH-6--AC is reacted with DNA in vitro and in vivo, and that the 5-[dG-N2-yl]-6-AC nucleoside adduct eluted before the N-[dG-8-yl]-6-AC adduct in reversed phase HPLC experiments (23–25).
Before incorporating the modified 11-mer oligonucleotides into 135-mer duplexes using ligation methods, the purities of the 32P-endlabeled modified 11-mers were verified by high-resolution gel electrophoresis; an example is now shown in Supporting Information. The 11-mers are then ligated with other oligonucleotides to form 135-mer duplexes that are also gel-purified. The 135-mer duplexes were then eluted from the gels, re-annealed, and incubated with the cell extracts.
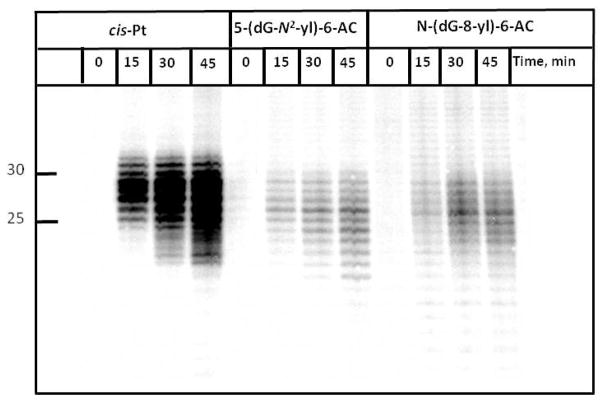
Typical patterns of NER dual incision products obtained by incubating 135-mer duplexes containing the 5′-…..CTCTCG*CTTCC…. sequence in HeLa cell extracts are shown in Figure 3. We selected HeLa cells for our studies because the extracts exhibit high NER activities and have been used in numerous previous studies of NER of bulky lesions (28, 29, 34, 36, 39). Prominent 24 – 32-mer oligonucleotide fragments appear and their intensities increase with incubation time (Figure 3). In the case of N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC lesions, NER is significantly weaker than in the case of the cis-Pt sample (Figure 3). We emphasize that earlier studies have shown that this cross-linked adduct is an excellent substrate of the human NER apparatus (28, 39).
Figure 3.
A typical autoradiogram obtained after the incubation of the modified 135-mer duplexes at the indicated time intervals (minutes). The numbers on the left indicate the sizes of the oligonucleotides expressed as numbers of nucleotides per oligonucleotide fragment. The ‘O min’ time points represent experiments in which the substrates were incubated with heat-deactivated cell extracts. Unmodified DNA substrates do not exhibit any NER bands in the 24–34-mer oligonucleotide region of the gel (data not shown).
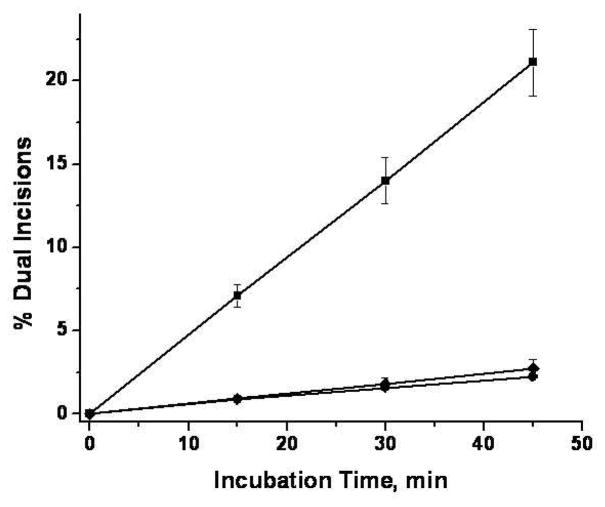
Densitometric analysis of typical autoradiographs of the type shown in Figure 3 were analyzed in order to quantitatively compare the efficiency of repair of the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC lesions relative to the efficiencies of the cis-Pt positive control sample. The results of four independent experiments are plotted in Figure 4. The time course of the dual incisions is linear at least for incubation times of up to 45 min. These results suggest that the slopes of these straight lines reflect the differences in the initial rates of dual incisions of these two substrates. The efficiencies are 8 ± 1 times greater for the cis-Pt than the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC lesions derived from the 6-NC. The efficiencies of repair of these two latter lesions are similar.
Figure 4.
Time course of formation of dual incision products in HeLa cell extracts. The time points are the averages of four different experiments conducted with different extracts from HeLa cells. Squares: cis-Pt; diamonds: N-(dG-8-yl)-6-AC; circles: 5-(dG-N2-yl)-6-AC.
Discussion
The main DNA lesions are formed by the 6-NC nitroreduction pathway are N-[dG-8-yl]-6-AC and 5-[dG-N2-yl]-6-AC (23–25). The mass-isomeric N-[dG-8-yl]-6-AC and 5-[dG-N2-yl]-6-AC adducts, as well as other dG-8 and dG-N2 lesions derived from other carcinogens and alkylating agents (40–44), display different and characteristic fragmentations of the guanyl moiety. Some of these differences in fragmentation patterns of covalently modified guanine residues may be used to distinguish between isomeric dG-C8 and dG-N2 adducts. For example, loss of an exocyclic nitrogen atom from a nucleoside base has been used to provide evidence for substitution through the exocyclic amine of the base rather than at other locations such as dG-C8. The MS/MS fragmentation patterns (Figures 2A and 2B) of reversed phase HPLC fractions 3 and 2 (Figure 1) are quite different: the de-ammoniation is the only dissociation pathway shared by these two adducts. Isotopic labeling of the pyrimidine ring nitrogens clearly demonstrated that ring opening occurs prior to decomposition with loss of identity of N1 and exocyclic N2 in protonated (37) and deprotonated (36) guanine. We thus concluded that fraction 2 (Figure 1) corresponds to the 5-(dG-N2-yl)-6-AC adduct. This conclusion is supported by the relative elution order of these two guanosine lesions and previous observations that the N-[dG-8-yl]-6-AC and 5-[dG-N2-yl]-6-AC adducts are major covalent products formed when N-OH-6-AC was incubated with native DNA (23).
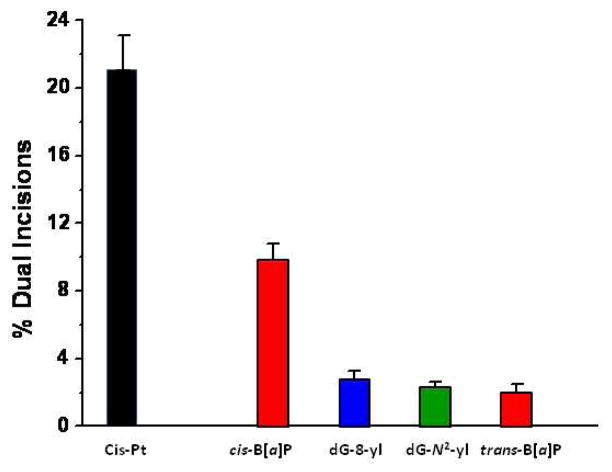
The N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC lesions are clearly relatively poor substrates of human nucleotide excision repair proteins in human HeLa cell extracts. It is interesting to quantitatively compare the repair efficiencies of these lesions with those of the previously reported adducts derived from the reactions of the highly tumorigenic (+)-7R,8S,9S,10R bay region diol epoxide of benzo[a]pyrene ((+)-anti-BPDE) that gives rise to both (+)-trans- and (+)-cis-B[a]P-N2-dG adducts that have been previously reported (28, 29). The (+)-trans-N2-dG adduct in double-stranded DNA is characterized by a minimally distorting minor groove conformation in double-stranded DNA (45), while the (+)-cis-stereoisomeric DNA adduct gives rise to a highly distorting base-displaced intercalative conformation (46). Consistent with the extent of structural distortions, the (+)-cis adduct is repaired more efficiently by a factor of 5 – 8 than the (+)-trans-adduct (28, 29), the latter being the major reaction product of (+)-anti-BPDE with DNA in vitro and in vivo. The relative efficiency of NER in HeLa cell extracts is greater by a factor 2.0 ± 0.2 for cis-Pt- than in the case of the (+)-cis-B[a]P-N2-dG-duplexes (an example is shown in Supporting Information). The relative efficiencies of the cis-Pt, N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC adducts studied in this work and the (+)-cis- and (+)-trans-B[a]P-N2-dG adducts studied previously (28, 29, 36) are compared in Figure 5. The ratios of NER efficiencies for the cis-Pt: (+)-cis-B[a]P-N2-dG: N-(dG-8-yl)-6-AC: 5-(dG-N2-yl)-6-AC: (+)-trans-B[a]P-N2-dG adducts are ~ 100: 48: 13: 12:10. Thus, the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC adducts are approximately as resistant to human NER and are as poorly repaired in human cell extracts as the highly genotoxic (+)-trans-B[a]P-N2-dG adduct.
Figure 5.
Comparisons of relative NER efficiencies (dual incision rates) of the 5-(dG-N2-yl)-6-AC and N-(dG-8-yl)-6-AC lesions in 5′-…CTCTCG*CTTCC with those of the cis-Pt positive control standard and N2-dG adducts derived from benzo[a]pyrene diol epoxide (the base-displaced intercalated (+)-cis- and the minor groove (+)-trans-anti-B[a]P-N2-dG adduct) in 5′-....CCATCG*CTACC... sequence context in double-stranded DNA. The B[a]P-N2-dG data is derived from previous publications (28, 29), while the relative NER responses of the (+)-cis-B[a]P-N2-dG lesions with cis-Pt adducts have been compared here (Supporting Information) and also in (28). We note that the sequence contexts in which the the 6-AC-derived and B[a]PDE derived guanine adducts are embedded in similar five-base sequence contexts 5-..TCG*CT...
Genrally, the efficiency of NER depends on a subtle manner on the conformations of the B[a]P-N2-dG lesions and the extent of structural distortions that they cause in the modified DNA duplexes (28). We are unable to establish the NMR solution structure of the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC adducts because the reaction of the active intermediate N-OH-6-AC – prepared from nitroreduction of 6-NC - with oligonucleotides resulted in a very low yield, thus making it difficult to generate sufficient amounts of these adducts for NMR analysis; this will be the focus of our future studies using improved methodologies for synthesizing oligonucleotides with site-specific N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC adducts. However, since the exocyclic amino group of guanine protrudes into the minor groove, it is possible that the 5-(dG-N2-yl)-6AC adduct is also positioned in the minor groove like the (+)-trans-anti-B[a]P-N2-dG adduct. Such an adduct conformation would not strongly perturb the quality of Watson-Crick hydrogen bonding of base pairs adjacent to these lesions (28) which would be consistent with the low efficiency of NER (Figure 5). The N-(dG-8-yl)-6-AC adduct is also a poor substrate of NER. Since the site of attachment, the (C8)-dG group is positioned in the major groove of B-DNA with the glycosidic bond of G* remaining in the anti-orientation, the integrity of the Watson-Crick hydrogen bonding might also not be dramatically affected by the lesion, thus accounting for its low activity as an NER substrate. Other C8-dG adducts derived from 2-acetylaminofluorene (AAF-C8-dG) (47) and PhIP (48) are known to adopt base-displaced intercalative adduct conformations and thus significantly distort the normal structural parameters of double-stranded DNA. It is interesting to note that both of these lesions are good substrates of NER with efficiencies identical (Geacintov et al, unpublished results) to those of the (+)-cis-B[a]P-N2-dG adduct (Figure 5). It thus seems likely that the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC adducts do not adopt a base-displaced intercalative conformation because of its relative resistance to repair. On the other hand, it can adopt a conformation in which the aromatic chrysenyl ring is positioned in the major groove with all Watson-Crick hydrogen bonds remaining intact, similar to the conformations adopted by the adduct derived from 2-aminofluorene (AF-C8-dG) in double-stranded DNA (47). This is in contrast to base displaced intercalative conformations of AAF-C8-dG adducts in which the hydrogen bonds at the site of the lesion are completely ruptured (47). Our hypothesis is therefore that the resistance of the N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC adducts to human NER proteins is similar to that of the biologically significant, potentially tumor-initiating (+)-trans-B[a]P-N2-dG adduct because both are positioned in the major or minor grooves, respectively, without significant distortions of Watson-Crick hydrogen bonding. Since both adducts are present in the mammary glands of rats exposed to 6-NC (25, 49), it will be interesting to determine whether N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC adducts are resistant to repair in vivo as they are in mammalian cell extracts in vitro. Furthermore, it remains to be determined the susceptibility of repair of DNA adducts derived from 1,2-DHD-6-NHOH-C (25) resulting from nitroreduction and ring oxidation of 6-NC (Scheme 1) that yields the 5-(dG-N2-yl)-1,2-DHD-6-AC adduct.. Such experiments are presently in progress.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute grant CA35519 (KELB) and the Pennsylvania Department of Health (Tobacco CURE grant). We thank Dr. J.M. Egly (CNRS, Strasbourg, France) for providing the cis-Pt sequence.
Abbreviations
- NO2-PAH
nitropolynuclear aromatic hydrocarbons
- 6-NC
6-nitrochrysene
- N-OH-6-AC
6-hydroxylaminochrysene
- 1,2-DHD-6-NC
trans-1,2-dihydroxy-1,2-dihydro-6-nitrochrysene
- 1,2-DHD-6-NHOH-C
trans-1,2-dihydroxy-1,2-dihydro-6-hydroxylaminochrysene
- N-(dG-8-yl)-6-AC
N-(deoxyguanosin-8-yl)-6-aminochrysene
- 5-(dG-N2-yl)-6-AC
5-(deoxyguanosin-N2-yl)-6-aminochrysene
Footnotes
Supporting Information Available:
Figures S1-S5. LC-MS/MS characteristics of the authentic N-(dG-8-yl)-6-AC (dGSt*) nucleoside adduct prepared as described (cf references 23–25) are depicted (Figure S1). The full gel of Figure 3 is depicted (Figure S2). The purities of oligonucleotides containing site-specific N-(dG-8-yl)-6-AC and 5-(dG-N2-yl)-6-AC used in this work are demonstrated using high resolution gel electrophoresis methods (Figure S3), and the relative NER response of the cis-Pt and and (+)-cis-anti-B[a]P-N2-dG adducts in HeLa cell extracts are compared (Figure S4). The structures of (+)anti-BPDE, (+)-trans-anti-B[a]P-N2-dG and (+)-cis-anti-B[a]P-N2-dG adducts are shown in Figure S5.
References
- 1.Jemal A, Smigal R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Semin Cancer Biol. 2004;14:473–486. doi: 10.1016/j.semcancer.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 3.WHO. The World Health Report. Geneva: World Health Organization; 1997. [Google Scholar]
- 4.Willett WC. Diet, nutrition, and avoidable cancer. Environ Health Perspect. 1995;103(Suppl 8):165–170. doi: 10.1289/ehp.95103s8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen LA, Rose DP, Wynder EL. A rationale for dietary intervention in postmenopausal breast cancer patients: an update. Nutr Cancer. 1993;19:1–10. doi: 10.1080/01635589309514231. [DOI] [PubMed] [Google Scholar]
- 6.Doll R. Nature and nurture: possibilities for cancer control. Carcinogenesis. 1996;17:177–184. doi: 10.1093/carcin/17.2.177. [DOI] [PubMed] [Google Scholar]
- 7.Lipworth L. Epidemiology of breast cancer. Eur J Cancer Prev. 1995;4:7–30. doi: 10.1097/00008469-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Wynder EL, Cohen LA, Muscat JE, Winters B, Dwyer JT, Blackburn G. Breast cancer: weighing the evidence for a promoting role of dietary fat. J Natl Cancer Inst. 1997;89:766–775. doi: 10.1093/jnci/89.11.766. [DOI] [PubMed] [Google Scholar]
- 9.El-Bayoumy K. Environmental carcinogens that may be involved in human breast cancer etiology. Chem Res Toxicol. 1992;5:585–590. doi: 10.1021/tx00029a001. [DOI] [PubMed] [Google Scholar]
- 10.Morris JJ, Seifter E. The role of aromatic hydrocarbons in the genesis of breast cancer. Med Hypotheses. 1992;38:177–184. doi: 10.1016/0306-9877(92)90090-y. [DOI] [PubMed] [Google Scholar]
- 11.Gammon MD, Wolff MS, Neugut AI, Eng SM, Teitelbaum SL, Britton JA, Terry MB, Levin B, Stellman SD, Kobat GC, Hatch M, Senie R, Berkowitz G, Bradlow HL, Garbowski G, Maffeo C, Montalvan P, Kemeny M, Citron M, Schnabel F, Schuss A, Hajdu S, Vinceguerra V, Niguidula N, Ireland K, Santella RM. Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood. Cancer Epidemiol Biomarkers Prev. 2002;11:686–697. [PubMed] [Google Scholar]
- 12.Gammon MD, Santella RM, Neugut AI, Eng SM, Teitelbaum SL, Paykin A, Levin B, Terry MB, Young TL, Wang LW, Wang Q, Britton JA, Wolff MS, Stellman SD, Hatch M, Kabat GC, Senie R, Garbowski G, Maffeo C, Montalvan P, Berkowitz G, Kemeny M, Citron M, Schnabel F, Schuss A, Hajdu S, Vinceguerra V. Environmental toxins and breast cancer on Long Island. I. Polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol Biomarkers Prev. 2002;11:677–685. [PubMed] [Google Scholar]
- 13.Perera FaRA. Correspondence re: Gammon et al. Environmental toxins and breast cancer on Long Island I Polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol Biomarkers Prev. 2003;12:75–76. [PubMed] [Google Scholar]
- 14.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 15.WHO International Agency for Research on Cancer. Diesel and gasoline engine exhausts and some nitroarenes. IARC Monogr Eval Carcinog Risks Hum. 1989;46:1–458. [PMC free article] [PubMed] [Google Scholar]
- 16.Fu PP, Herreno-Saenz D. Nitropolycyclic aromatic hydrocarbons: a class of genotoxic environmental pollutants. Environ Carcinogen Ecotoxicol Rev. 1999;C17:1–43. [Google Scholar]
- 17.Kielhorn JW, Mangelsdorf U. The World Health Organization, Environmental Health Criteria. Vol. 229 2003. IA Report: Selected Nitro- and Nitro-oxy-polycyclic aromatic Hydrocarbons. [Google Scholar]
- 18.El-Bayoumy K, Desai D, Upadhyaya P, Amin S, Hecht SS. Comparative tumorigenicity of nitrochrysene isomers in newborn mice. Carcinogenesis. 1992;13:2271–2275. doi: 10.1093/carcin/13.12.2271. [DOI] [PubMed] [Google Scholar]
- 19.Hecht SS, El-Bayoumy K, Rivenson A, Amin S. Potent mammary carcinogenicity in female CD rats of a Fjord Region diol epoxide of benzo[c]phenanthrene compared to a Bay Region diol epoxide of benzo[a]pyrene. Cancer Res. 1994;54:21–24. [PubMed] [Google Scholar]
- 20.El-Bayoumy K, Rivenson A, Upadhyaya P, Chae YH, Hecht SS. Induction of mammary cancer by 6-nitrochrysene in female CD rats. Cancer Res. 1993;53:3719–3722. [PubMed] [Google Scholar]
- 21.El-Bayoumy K, Chae YH, Upadhyaya P, Rivenson A, Kurtzke C, Reddy B, Hecht SS. Comparative tumorigenicity of benzo[a]pyrene, 1-nitropyrene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine administered by gavage to female CD rats. Carcinogenesis. 1995;16:431–434. doi: 10.1093/carcin/16.2.431. [DOI] [PubMed] [Google Scholar]
- 22.El-Bayoumy K, Desai D, Boyiri T, Rosa J, Krzeminski J, Sharma AK, Pittman B, Amin S. Comparative tumorigenicity of the environmental pollutant 6-nitrochrysene and its metabolites in the rat mammary gland. Chem Res Toxicol. 2002;15:972–978. doi: 10.1021/tx020019a. [DOI] [PubMed] [Google Scholar]
- 23.Delclos KB, Miller DW, Lay JO, Jr, Cascino DA, Walker RP, Fu PP, Kadlubar FF. Identification of C8-modified deoxyinosine and N2- and C8-modified deoxyguanosine as major products of the in vitro reaction of N-hydroxy-6-aminochrysene and 6-aminochrysene with DNA and the formation of these adducts in isolated rat hepatocytes with 6-nitrochrysene and 6-aminochrysene. Carcinogenesis. 1987;8:1703–1709. doi: 10.1093/carcin/8.11.1703. [DOI] [PubMed] [Google Scholar]
- 24.Chae YH, Delclos KB, Blaydes B, El-Bayoumy K. Metabolism and DNA binding of the environmental colon carcinogen 6-nitrochrysese in rats. Cancer Res. 1996;56:2052–2058. [PubMed] [Google Scholar]
- 25.El-Bayoumy K, Sharma AK, Lin JM, Krzeminski J, Boyiri T, King LC, Lambert G, Padgett W, Nesnow S, Amin S. Identification of 5-(deoxyguanosin-N2-yl)-1,2-dihydroxy-1,2-dihydro-6-aminochrysene as the major DNA lesion in the mammary gland of rats treated with the environmental pollutant 6-nitrochrysene. Chem Res Toxicol. 2004;17:1591–1599. doi: 10.1021/tx049849+. [DOI] [PubMed] [Google Scholar]
- 26.Gillet LC, Scharer OD. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev. 2006;106:253–276. doi: 10.1021/cr040483f. [DOI] [PubMed] [Google Scholar]
- 27.Reardon JT, Sancar A. Nucleotide excision repair. Prog Nucleic Acid Res Mol Biol. 2005;79:183–235. doi: 10.1016/S0079-6603(04)79004-2. [DOI] [PubMed] [Google Scholar]
- 28.Mocquet V, Kropachev K, Kolbanovskiy M, Kolbanonovskiy A, Tapias A, Cai Y, Broyde S, Geacintov NE, Egly JM. The human DNA repair factor XPC-HR23B distinguishes stereoisomeric benzo[a]pyrenyl-DNA lesions. EMBO J. 2007;26:2923–2932. doi: 10.1038/sj.emboj.7601730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hess MT, Gunz D, Luneva N, Geacintov NE, Naegeli H. Base pair conformation-dependent excision of benzo[a]pyrene diol epoxide-guanine adducts by human nucleotide excision repair enzymes. Mol Cell Biol. 1997;17:7069–76. doi: 10.1128/mcb.17.12.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guttenplan JB, Zhao ZL, Kosinska W, Norman RG, Krzeminski J, Sun YW, Amin S, El-Bayoumy K. Comparative mutational profiles of the environmental mammary carcinogen, 6-nitrochrysene and its metabolites in a lacI mammary epithelial cell line. Carcinogenesis. 2007;28:2391–2397. doi: 10.1093/carcin/bgm142. [DOI] [PubMed] [Google Scholar]
- 31.Joffe A, Mock S, Yun BH, Kolbanovskiy A, Geacintov NE, Shafirovich V. Oxidative generation of guanine radicals by carbonate radicals and their reactions with nitrogen dioxide to form site specific 5-guanidino-4-nitroimidazole lesions in oligodeoxynucleotides. Chem Res Toxicol. 2003;16:966–973. doi: 10.1021/tx025578w. [DOI] [PubMed] [Google Scholar]
- 32.Jung Y, Lippard SJ. Direct cellular responses to platinum-induced DNA damage. Chem Rev. 2007;107:1387–1407. doi: 10.1021/cr068207j. [DOI] [PubMed] [Google Scholar]
- 33.Cheng SC, Hilton BD, Roman JM, Dipple A. DNA adducts from carcinogenic and noncarcinogenic enantiomers of benzo[a]pyrene dihydrodiol epoxide. Chem Res Toxicol. 1989;2:334–40. doi: 10.1021/tx00011a011. [DOI] [PubMed] [Google Scholar]
- 34.Kropachev K, Kolbanovskii M, Cai Y, Rodriguez F, Kolbanovskii A, Liu Y, Zhang L, Amin S, Patel D, Broyde S, Geacintov NE. The sequence dependence of human nucleotide excision repair efficiencies of benzo[a]pyrene-derived DNA lesions: insights into the structural factors that favor dual incisions. J Mol Biol. 2009;386:1193–203. doi: 10.1016/j.jmb.2008.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turesky RJ, Vouros P. Formation and analysis of heterocyclic aromatic amine–DNA adducts in vitro and in vivo. J Chromatogr B: Anal Technol Biomed LifeSci. 2004;802:155–166. doi: 10.1016/j.jchromb.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 36.Buterin T, Meyer C, Giese B, Naegeli H. DNA quality control by conformational readout on the undamaged strand of the double helix. Chem Biol. 2005;12:913–22. doi: 10.1016/j.chembiol.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Sultan J. Collision induced dissociation of deprotonated guanine: Fragmentation of pyrimidine ring and water adduct formation. Int J Mass Spectrom. 2008;273(1–2):58–68. [Google Scholar]
- 38.Gregson JM, McCloskey JA. Collision-induced dissociation of protonated guamine. Int J Mass Spectrom Ion Processes. 1997:165–166. 475–485. [Google Scholar]
- 39.Riedl T, Hanaoka F, Egly JM. The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J. 2003;22:5293–303. doi: 10.1093/emboj/cdg489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paehler A, Richoz J, Soglia J, Vouros P, Turesky R. Analysis and quantification of DNA adducts of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in liver of rats by liquid chromatography/electrospray tandem mass spectrometry. Chem Res Toxicol. 2002;15:551–561. doi: 10.1021/tx010178e. [DOI] [PubMed] [Google Scholar]
- 41.Wolf SM, Vouros P. Application of capillary liquid chromatography coupled with tandem mass spectrometric methods to the rapid screening of adducts formed by the reaction of N-acetoxy-N-acetyl-2-aminofluorene with calf thymus DNA. Chem Res Toxicol. 1994;7:82–88. doi: 10.1021/tx00037a013. [DOI] [PubMed] [Google Scholar]
- 42.Gangl ET, Turesky RJ, Vouros P. Determination of in vitro- and in vivo-formed DNA adducts of 2-amino-3- methylimidazo[4,5-f]quinoline by capillary liquid chromatography/microelectrospray mass spectrometry. Chem Res Toxicol. 1999;12:1019–1027. doi: 10.1021/tx990060m. [DOI] [PubMed] [Google Scholar]
- 43.Bessette EE, Goodenough AK, Langouet S, Yasa I, Kozekov ID, Spivack SD, Turesky RJ. Screening for DNA adducts by data-dependent constant neutral loss-triple stage mass spectrometry with a linear quadrupole ion trap mass spectrometer. Anal Chem (Washington, DC, United States) 2009;81(2):809–819. doi: 10.1021/ac802096p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiarelli MP, Lay J., Jr Differentiation of Isomeric C8- and N2-deoxyguanosine adducts of 2-acetylaminofluorene by fast-atom bombardment and tandem mass spectrometry. J Am Soc Mass Spectrom. 1994;5:58–63. doi: 10.1016/1044-0305(94)85037-2. [DOI] [PubMed] [Google Scholar]
- 45.Cosman M, de los Santos C, Fiala R, Hingerty BE, Singh SB, Ibanez V, Margulis LA, Live D, Geacintov NE, Broyde S, Patel DJ. Solution conformation of the major adduct between the carcinogen (+)-anti-benzo[a]pyrene diol epoxide and DNA. Proc Natl Acad Sci USA. 1992;89:1914–1918. doi: 10.1073/pnas.89.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cosman M, de los Santos C, Fiala R, Hingerty BE, Ibanez V, Luna E, Harvey R, Geacintov NE, Broyde S, Patel DJ. Solution conformation of the (+)-cis-anti-[BP]dG adduct in a DNA duplex: intercalation of the covalently attached benzo[a]pyrenyl ring into the helix and displacement of the modified deoxyguanosine. Biochemistry. 1993;32:4145–55. doi: 10.1021/bi00067a001. [DOI] [PubMed] [Google Scholar]
- 47.Patel DJ, Mao B, Gu Z, Hingerty BE, Gorin A, Basu AK, Broyde S. Nuclear magnetic resonance solution structures of covalent aromatic amine-DNA adducts and their mutagenic relevance. Chem Res Toxicol. 1998;11(5):391–407. doi: 10.1021/tx9702143. [DOI] [PubMed] [Google Scholar]
- 48.Brown K, Hingerty BE, Guenther EA, Krishnan VV, Broyde S, Turteltaub KW, Cosman M. Solution structure of the 2-amino-1- methyl-6-phenylimidazo[4,5-b]pyridine C8-deoxyguanosine adduct in duplex DNA. Proc Natl Acad Sci U S A. 2001;98:8507–12. doi: 10.1073/pnas.151251898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyiri T, Guttenplan J, Khmelnitsky M, Kosinska W, Lin JM, Desai D, Amin S, Pittman B, El-Bayoumy K. Mammary carcinogenesis and molecular analysis of in vivo CII gene mutations in the mammary tissue of female transgenic rats treated with the environmental polloutant 6-nitrochrysene. Carcinogenesis. 2004;25:637–643. doi: 10.1093/carcin/bgh040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.