Abstract
Background
Without virologic testing, HIV-infected African children starting antiretroviral (ARV)-therapy are at risk for undetected virological failure and the development of ARV-resistance. We sought to determine the prevalence of early virologic failure (EVF), to characterize the evolution of ARV-resistance mutations, and to predict the impact on second-line therapy.
Methods
The prevalence of EVF (HIV-RNA >400 copies/mL on sequential visits after 6 months of therapy) was identified among 120 HIV-infected Ugandan children starting ARV-therapy. ARV-mutations were identified by population sequencing of HIV-1 pol in sequential archived specimens. Composite discrete genotypic susceptibility scores (dGSS) were determined for second-line ARV-regimens.
Results
EVF occurred in 16 (13%) children and persisted throughout a median (IQR) 938 (760-1066) days of follow-up. M184V and non-nucleoside-reverse-transcriptase-inhibitor-associated mutations emerged within 6 months of EVF; thymidine-analog-mutations arose after 12 months. Worse dGSS scores correlated with increasing duration of failure (Spearman R = −0.47, p=0.001). Only 1 child met World Health Organization CD4 criteria for ARV-failure at the time of EVF or during the follow-up period.
Conclusions
A significant portion of HIV-infected African children experience EVF that would be undetected using CD4/clinical monitoring and resulted in the accumulation of ARV-mutations that could compromise second line therapy options.
Keywords: Resistance, Antiretroviral therapy, HIV, children, Africa
Introduction
There are over two million children living with HIV, 90% of whom are in Sub-Saharan Africa.1 While the number of children receiving antiretroviral (ARV) therapy continues to increase, 2 access to optimal laboratory monitoring with viral load testing remains limited. As a result, most African providers must make decisions about ARV therapy based on World Health Organization (WHO) immunologic and clinical guidelines,3-5 leaving HIV-infected children at risk for “unrecognized” virologic failure and the subsequent development of antiretroviral resistance. Cross sectional studies have demonstrated ARV resistance mutations in African children with virologic failure,6, 7 but data on evolution of ARV resistance– which resistance mutations arise first and after how much time with virologic failure – are limited. Such information is needed to evaluate the impact of unrecognized virologic failure and to inform optimal ARV management in this population.
Using archived specimens and data from a cohort of HIV-infected Ugandan children, our aims were to determine: 1) the prevalence of “early virological failure’ (EVF) defined as sustained HIV viremia beginning after six to nine months of ARV therapy, 2) the prevalence of ARV-resistance mutations after different durations of virologic failure and the discrete genotypic susceptibility scores (dGSS) of these isolates and 3) if cases of EVF would be identified using the 2010 WHO immunologic criteria for treatment failure.5
Methods
Study participants
We evaluated virologic responses in a cohort of HIV-infected Ugandan children in Kampala, Uganda.8 Children received acute and routine monthly care at the study clinic using standardized diagnostic protocols that assigned WHO stage. Plasma HIV RNA levels (range of detection 400 to 750,000 copies/mL, Roche Amplicor Version 1.5, Pleasanton, CA, USA) and CD4 cell counts and percentages were measured at 12 week intervals (FACS Calibur, Becton Dickinson, San Jose, CA) at a College of American Pathologists–certified laboratory; excess plasma was archived when available. Among the 300 children in the cohort, 35 were already receiving ARV therapy at the time of enrollment. ARV therapy was initiated in 114 additional children during cohort follow-up, using the CD4 and clinical criteria of the Ugandan national and World Health Organization 2006 guidelines3 (with addendum in 20084). For the present study, we included all children who were receiving ARV-therapy, had at least two plasma HIV RNA values, and had the first value in the 6-9 month period following ARV therapy initiation. History of perinatal nevirapine use by mother and/or infant was obtained by parental interview. A visual analog scale in which parents or guardians estimated adherence was determined at monthly visits9; the mean adherence over the first 6 months of therapy was estimated for each child.
Early Virologic Failure
EVF was defined as having plasma HIV RNA levels of >400 copies/ml on two successive visits, with the first occurring in the six to nine month period after ARV initiation.
ARV Resistance Mutation Testing
ARV resistance mutations were investigated using available banked plasma specimens. The duration of failure that preceded each specimen was estimated using three months from ARV initiation as the starting time point. A reverse transcriptase PCR-based population sequencing protocol8, 10 was adapted for use with non-subtype B virus using outer primers: CIPol Fin ( 5′-GGAAGAAATHTGTTGACTCAGATTGG-3′) and OutRT Bin(5′-CATTGCTCTCCAATTRCTGTGATA-3′); and inner primers CIPol Fin, 3RT Bin: 5′-CCCATCCAAAGRAATGGAGGTTCTTC-3′ in a hemi-nested format. PCR products were purified using the Qiagen Qiaquick PCR cleanup kit. Bi-directional cycle sequencing was performed on PCR products without additional cloning using ABI PRISM dye terminator chemistries and resolved on an ABI Automated Genetic Analyzer. The default ABI base caller was employed that typically identifies minority populations of 20 to 30% but the sequence data were additionally aligned and manually edited using Sequencher 4.9 (Genecodes, Ann Arbor MI) with HXB2 as the reference sequence. Genotypic resistance mutations were defined using the 2009 International AIDS Society-USA drug-resistance mutation guidelines and Stanford HIVdb (http://hivdb.stanford.edu).11-14 Mixtures were designated with standard ambiguity codes when detected in both forward and reverse directions at approximately 20% or greater minor variant.
Prediction of ARV susceptibility
To generate discrete genotypic susceptibility scores (dGSS) scores we utilized the HIVdb(http://hivdb.stanford.edu), which predicts the activity of individual and combinations of ARV medications for a given isolate by evaluating specific mutations with a database of genotypic-phenotypic and genotypic-clinical correlations.11-13 Because the data underlying dGSS assignments are disproportionately influenced by observations made on subtype B virus we opted to use a more conservative dichotomous designation of fully susceptible (1) or not fully susceptible (0) and omitted the category of “intermediate” susceptibility in this analysis. Three-drug regimens could yield combined scores of 0 (no fully susceptible drugs) to 3 (three fully susceptible drugs). dGSS scores were calculated for each subject according to : 1) current regimen and 2) the standard Ugandan second-line regimen of didianosine (DDI), abacavir (ABC), and ritonavir boosted lopinavir (LPVr).
WHO Clinical and Immunologic Criteria
Per the 2010 WHO guidelines, the immunologic definition of treatment failure is “characterized by a drop in the CD4 to values at or below the age-dependent values,” or “a failure of the CD4 count to rise above these threshold values,” which are “CD4 count of ≤200 cells/mm3 or %CD4+ ≤10% for a child more than 2 years to less than 5 years of age and CD4 count of ≤100 cells/mm3 for a child 5 years of age or more.”5 CD4 data from all visits for each child starting at the time of EVF and ending on the date of the last available archived plasma sample were evaluated to determine WHO ARV-switch eligibility.
Statistics
Median demographic and laboratory measures were compared between EVF and non-EVF children using the non-parametric Kruskall-Wallis Test. Susceptibility scores were tested for a correlation with increasing time of virologic failure using the Spearman rank correlation test.
Results
A total of 120 HIV-infected children with a median (IQR) age of 5.4 (3.2 to 8.0) years had initiated ARV therapy and were included in this study. The children received nevirapine (NVP) or efavirenz (EFV), plus a combination of lamivudine (3TC) with either zidovudine (ZDV) or stavudine (D4T) as follows: 19 (16%) on NVP/3TC/ZDV, 17 (14%) on NVP/3TC/D4T, 73 (61%) on EFV/3TC/ZDV, and 7(6%) on EFV/3TC/ZDV; four (3%) children initially received ABC/ZDV/3TC because of concurrent anti-tuberculosis therapy and then changed to NVP/ZDV/3TC after 154-237 days of ARV therapy.
Early Virologic Failure
EVF occurred in 16 (13%) children. Children with EVF were younger than those without EVF, with median (IQR) age of 2.5 (2.2-3.0) years compared to 6.2 (3.7-8.2) years, respectively (p<0.001). At the time of EVF, the median (IQR) plasma HIV RNA level (IQR) was 4.9 (4.2-5.1) log10 (copies/ml). Most (15 of 16) children with EVF had sustained viremia throughout the follow-up period (median 938 days, IQR: 760-1066 days) with plasma HIV RNA levels > 400 c/ml [median: 4.7 log10(c/ml), IQR:4.3-5.0 log10(c/ml)]. One child developed undetectable plasma HIV RNA by day 349. Of note, there were six additional children that had a single plasma HIV RNA level of > 400 (c/ml) in the six to nine month period after ARV-initiation, but because subsequent levels were < 400 (c/ml) they did not meet the definition of EVF. Baseline (pre-ARV) CD4 and HIV RNA measures were only available for the subset of children who had initiated ARV therapy after joining the cohort, including children with (7/14) and without (89/106) EVF; among them, children with EVF had higher median CD4 count (623 vs 235, p=0.04) and plasma HIV RNA level (5.4 vs 5.1 log10(copies/ml), p<0.01) but similar CD4 percentages (6.5 vs. 9.0, p=0.21). Monthly adherence data was available from at least 2 visits for 13 children with EVF and 102 without; the mean reported adherence was greater than 99% in both groups.
ARV-Resistance Mutations
Genotypic analysis was successful in 40 samples from 14 of 16 children with EVF (Tables 1a and 1b). M184V mutations were detected as early as 1.5 months of failure and at nearly every time point (39 of 40) tested. Thymidine-analog-mutations (TAMs) were not detected until after 12 months of failure and were frequently present as mixtures of wild-type and mutant variants. Mutations conferring resistance to NVP and EFV were present in the majority of children starting as early as after one to six months of failure. Mutations predicted to confer resistance to etravirine (E138A, Y181C, G190A) were present in five of the nine children with samples available in the first six months of failure; two of the seven children with samples in the seven to 12 month range had two etravirine associated mutations. Of note, the mothers of subjects 3 and 4 had a history of single dose nevirapine use for perinatal transmission prevention, but whether either child received nevirapine around birth was unknown. No history of ARV use by either mother or child was reported for the other subjects.
Table 1a.
Nucleoside Reverse Transcriptase Inhibitor (NRTI) Associated Mutations
| Months of Failure* | ||||||
|---|---|---|---|---|---|---|
| Subject | 1-6 months | 7-12 months | 13-18 months | 19-24 months | 25-30 months | NRTI^ |
| 1 | - | - | D67N, A62V, M184V | D67N, M184V | D67N, A62V, T215Y, M184V | D4T/3TC |
| 2 | - | - | V75I, M184V | V75I, M184V | - | D4T/3TC |
| 3† | - | - | - | - | M184V | D4T/3TC |
| 4† | M184V | - | - | M184V | K65R, delT69##, M184V | D4T/3TC |
| 5 | M184V | M184V | M184V | T215Y, M184V | M41L, T215Y, M184V | D4T/3TC |
| 6 | M184V | K70R/K, M184V | K70R, K219E, M184V | - | D4T/3TC | |
| 7 | M184V | - | T215Y, M184V | - | T215F, M184V | ZDV/3TC |
| 8 | - | - | T215F, M184V | T215F, M184V | T215F, M184V | ZDV/3TC |
| 9 | M184V | - | - | M184V | - | ZDV/3TC |
| 10 | K65R | M184V | M184V | - | - | ZDV/3TC |
| 11 | M184V | M184V | M184V | - | - | ZDV/3TC |
| 12 | - | M184V | M184V | D67N/D, K70R/K, K219E/K, M184V | - | ZDV/3TC° |
| 13 | M184V | Y115F, M184V | M184V | - | - | ZDV/3TC/ABC# |
| 14 | M184V | M184V | M184V | - | - | ZDV/3TC/ABC# |
|
| ||||||
| M184V | 8/9 (89%) | 7/7 (100%) | 11/11 (100%) | 7/7 (100%) | 6/6 (100%) | |
| ≥1 TAM~ | 0/9 (0%) | 1/7 (0%) | 4/11 (36%) | 4/7 (57%) | 4/6 (67%) | |
| ≥2 TAM~ | 0/9 (0%) | 0/7 (0%) | 1/11 (9%) | 1/7 (0%) | 2/6 (33%) | |
estimated to start 90 days after ARV initiation;
patients also received nevirapine unless otherwise noted;
sample not available;
Thymidine analog mutations (TAMs) are underlined 14;
perinatal maternal use of single dose nevirapine was reported;
patient received efavirenz in lieu of nevirapine;
patients initially received ZDV/3TC/ABC due to concurrent anti-tuberculosis therapy and then changed to ZDV/3TC/NVP at 7 months.
Table 1b.
Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) Associated Mutations
| Months of Failure* | ||||||
|---|---|---|---|---|---|---|
| Subject | 1-6 months | 7-12 months | 13-18 months | 19-24 months | 25-30 months | NNRTI^ |
| 1 | - | - | K101E, Y181I | K101E, Y181I | K101E, V108I/V, Y181I | NVP |
| 2 | - | - | none | none | - | NVP |
| 3† | - | - | - | - | Y181C, V179T, K103N | NVP |
| 4† | K103N | - | - | K103N | delT69##, V90I, Y181C, K103N | NVP |
| 5 | Y181C | Y181C | Y181C | Y181C, V108I | Y181C | NVP |
| 6 | Y181C, V108I | Y181C, V108I | Y181C, V108I | - | - | NVP |
| 7 | K103N | - | K103N | - | K103N | NVP |
| 8 | - | - | A98G, K103N | K103N | K103N | NVP |
| 9 | none | - | - | none | - | NVP |
| 10 | Y181C, V108I | K103N, V108I | K103N, V108I | - | - | NVP |
| 11 | G190A | G190A | K101H, G190A | - | - | NVP |
| 12 | - | V179D, Y188L | V179D, Y188L | V179D, Y188L | - | EFV |
| 13 | E138A | E138A, G190A, K103N | E138A, G190A | - | - | NVP# |
| 14 | none | G190A | G190A | - | - | NVP# |
|
| ||||||
| ≥1 ETV-m’ | 5/9 (55%) | 6/7 (86%) | 8/11 (73%) | 3/7 (43%) | 4/6 (67%) | |
| ≥2 ETV-m’ | 0/9 (0%) | 2/7 (29%) | 3/11 (27%) | 2/7 (29%) | 3/6 (50%) | |
| NVP/EFV-m | 6/9 (67%) | 7/7 (100%) | 10/11 (90%) | 5/7 (71%) | 6/6 (100%) | |
estimated to start 90 days after ARV initiation;
patients received either D4T or ZDV plus 3TC unless otherwise noted;
sample not available; “none” tested but none detected.
patients also received either ZDV/3TC or D4T/3TC unless otherwise noted;
mutations with potential impact on etravirine (ETV-m) are underlined14;
perinatal maternal use of single dose nevirapine was reported;
patients initially received ZDV/3TC/ABC due to concurrent anti-tuberculosis therapy and then changed to ZDV/3TC/NVP at 7 months; NVP/EFV-m: mutations that would affect NVP and EFV.14
Discrete genotypic susceptibility scores (dGSS)
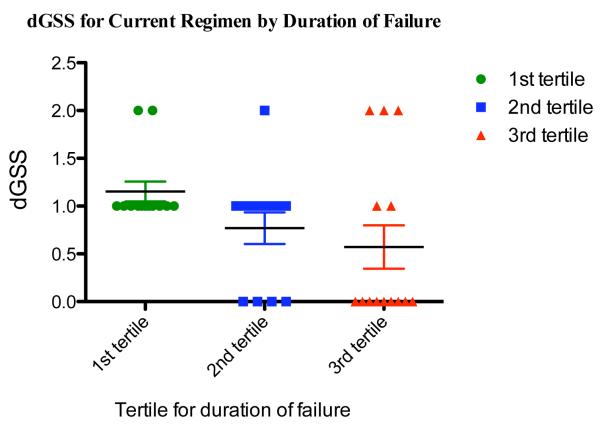
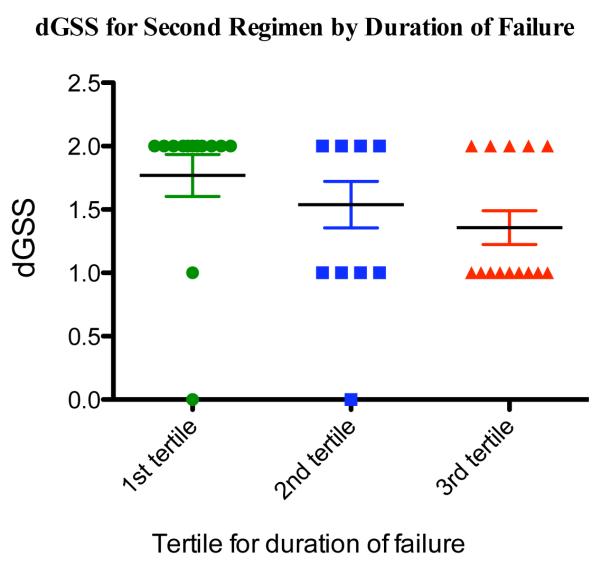
Predicted resistance to the current regimens increased significantly with duration of failure (Spearman R = −0.50, p=0.001) (Figure 1a). Predicted resistance to what would be the standard second-line ARV regimen (ABC/DDI/LPVr) also increased significantly over duration of failure (Spearman R = −0.47, p=0.001). Notably, nine of 14 children (64%) had only one active second line drug predicted by the third tertile of failure (19-28 months).
Figure 1a. Composite Genotype Sensitivity Scores (dGSS) for Current Regimen by Duration of Failure.
Figure 1a Caption. Results were divided into tertiles by duration of failure ((9.4 months); dGSS scores indicate the number of fully active drugs predicted using detected mutations and the child’s current regimen. (http://hivdb.stanford.edu);11 bars are means +/− standard errors.
CD4 Outcomes and WHO Criteria
CD4 count and percentages in the six to nine month period after ARV therapy initiation were similar between those with and without EVF, respectively: median (IQR) 926 (730-1024) vs. 746 (472-1102) (p=0.10) cells/ul and 23(16-28) vs. 24(20-30) (p=0.30). During the median 903 days (range: 382-1254 days) of follow-up, only 1 of 16 child (Subject 1, Tables 1a and 1b) met the 2010 WHO CD4 criteria for treatment failure developing CD4% below age specific cutoffs after initial recovery (at 266 days of ART). In the other 15 children, CD4 counts and/or percentages remained above WHO defined thresholds.
Discussion
We found that 13% of HIV-infected Uganda children starting ARV therapy experienced persistent virological failure after only 6 months. While this rate is lower than that reported in clinical programs of South Africa (16%),15 Thailand (16%)16, Kenya (33%)17 and Mali (44%),7 it underscores the fact that even with the exceptional access to care and adherence counseling provided by our research study, a significant number of HIV-infected children will experience virological failure at this early time point. It should also be noted that we utilized a stringent definition that required two consecutive detectable plasma HIV RNA levels in order to distinguish persistent failure from transient blips or delayed response to therapy, unlike studies which define virological failure by detectable viremia at a single time-point.
With early and persistent viremia, children with EVF would be predicted to be at high risk for the rapid development of ARV resistance mutations. In fact, we found that all children with EVF had at least one reverse transcriptase mutation at the first time point tested, including all nine children who had banked samples available within the first six months of failure. Clinical programs in Mali, Cotes d’Ivoire, and Mozambique have reported slightly lower prevalence, with resistance mutations found in 22/30 (73%),7 27/38 (71%)18 and 77/84 (93%)19 failing children, respectively. Studies of Thai children have reported RT mutation prevalence as high as 97% and 98% among failing children.16, 20
As expected, M184V was the most common NRTI-associated mutation, emerging as early as after 1.5 months of failure and persisting at nearly every time point tested (38 of 40). M184V has been shown to emerge in high prevalence among children and adults failing on 3TC-containing regimens in developed countries21; cross-sectional studies have likewise reported a high prevalence of M18V among African (63-69%)6, 7 and Thai (84-85%)16, 20 children. In contrast to M184V, TAMs generally arise after long periods of virological failure 22. A recent study of Ugandan adults found no TAMs after a median 320 days of virological failure.23 Our data, although limited by small numbers, suggests the same for Ugandan children, with no TAMs detected until after 12 months of failure. Germanaud et al also found a low prevalence of TAMs, with only 3 of 30 HIV-infected Malian children demonstrating a single TAM after 6 months of ARV therapy. 7 Taken together, these data suggest that early detection of virologic failure could prevent the development of multiple TAMs and thereby preserve the activity of thymidine analog ARVs for use in second line regimens.
We found a high rate of NNRTI-associated mutations, with six of nine patients demonstrating at least one NNRTI-associated mutation within six months of failure, comparable to what was reported (70%) in Mozambique.7 Notably, we isolated mutations that would confer resistance to etravirine as early as after six months of virologic failure. Etravirine was designed for use in patients who had failed first generation NNRTI’s, retaining activity in the context of K103N 24-26. When used with darunanavir as in the DUET trial,27, 28 etravirine can serve to spare newer classes of ARVs such as integrase inhibitors. However, because etravirine loses activity in the presence of multiple mutations,29 persistent failure in the context of nevirapine or efavirenz can compromise its future use, as we document here.
While there have been several cross sectional studies demonstrating ARV-mutations in African children failing therapy6, 7, 19, we used longitudinal specimens to show that mutations threatening the efficacy and reliability of second line regimens increase over time in individual patients. A limitation our study is that we used HIVdb modeling, which is based primarily on data from HIV-subtype B viruses. However, our findings underscore the need for controlled clinical studies of second line ARV regimens in African children, particularly where access to virologic monitoring will remain limited. Data from Ugandan adults suggest protease-inhibitor-based second line regimens can result in good outcomes30 even in the context of NRTI-associated resistance mutations, but there are no such data for African children.
Perhaps our most concerning finding was that the WHO immunologic criteria for treatment failure detected virologic failure in only 1 of 16 children (6%). The WHO criteria are understandably conservative in order to prevent the premature switch of ARV therapy in settings where access to second line regimens is limited but this comes at the potential expenses of delaying ARV therapy changes for patients who need it. In adults, the WHO criteria for immunologic failure have been shown to be insensitive measures for virologic outcomes 31-35 and a high prevalence of ARV resistance mutations has been documented in HIV-infected adults by the time they progress to meet the WHO criteria for ARV therapy switch 36. A recent report showed that similarly, pediatric WHO guidelines offer an insensitive measure of virologic failure.37 We confirm that finding in this study, and additionally show that the HIV-infected African children with ongoing “undetected” virologic failure develop ARV-mutations that could compromise future therapeutic options.
It is noteworthy that the majority of children with EVF had good immunologic outcomes. Indeed, the reason that 15 of the 16 children did not meet WHO immunologic criteria for treatment failure was because they maintained high CD4 counts and percentages. The development of drug resistance mutations can result in alterations in viral fitness38-40. Such effects on viral fitness have been credited with preservation of immunologic benefits of therapy despite the emergence of resistance and fitness changes may explain, in part, long durations of virologic failure without CD4 cell losses.41-44 Additionally, interactions between mutations may affect the net susceptibility of resistant virus to drugs. For HIV subtype B, the M184V, L74V and Y181C mutations partially re-sensitize TAM containing viruses to thymidine analogues 45, 46. It is possible that these mutational interactions are responsible for preservation of partial inhibitory activity in the context of HIV subtype A and D infections as well. On the other hand, if compensatory mutations that restore viral fitness and virulence accumulate over time or mutations conferring multidrug resistance emerge, it is likely that the salutary effects of the first line drugs will abate for most if not all patients over time. Of note, children who developed EVF had higher pre-treatment CD4 counts; we believe this was a result of their younger median age because CD4 counts peak in infancy and naturally decline with age.47, 48 For this reason, and in accordance with World Health Organization Guidelines, we used the mores age-stable measure of CD4% to determine treatment eligibility and outcomes in children under age 5 years.
There are several possible explanations for the virologic failure in these children. While the adherence reported by visual analogue scale was extremely high, studies have shown that measures relying on caretaker report can overestimate adherence in children.47. Furthermore, even slight alterations in adherence can lead to virological failure and the development of resistance;49 that we identified no wild type virus indeed suggests that children with EVF were partially adherent. Because of small numbers and limited access to pre-ARV data and specimens we were not able to evaluate risk factors for EVF or the development of resistance.
However, children with EVF were younger than those without and young age is associated with greater risk of ARV failure37. Two of four children receiving a triple-NRTI regimen because of concurrent anti-tuberculosis treatment developed failure which may reflect the limited potency of this regimen or reduced adherence among children treated simultaneously for tuberculosis and HIV. Some children may have carried viruses with pre-existing resistance mutations from unreported prior treatment. Isolates from one child demonstrated an NNRTI-associated mutation despite receiving a triple-NRTI regimen, strongly suggesting prior unreported exposure to an NNRTI. Two mothers reported ARV-use in a mother-to-child prevention regimen, but others may have declined to report it. Children could also have received unreported treatment from another clinic prior to enrollment in the cohort.
While our study was small, we found early virologic failure occurred in 13% of HIV-infected Ugandan children receiving ARV therapy and went undetected by WHO CD4 based monitoring in 15 of 16 children. The accumulation of ARV-resistance mutations in these children not only jeopardizes second-line therapy options for individual patients but could also contribute to the reservoir of circulating drug resistant virus. Our observations add to growing evidence from adults36 underscoring the urgent need to integrate affordable virologic monitoring into HIV-treatment programs in Africa.
Figure 1b. Composite Genotype Sensitivity Scores (dGSS) for Second Regimen by Duration of Failure.
Figure 1a Caption. Results were divided into tertiles of duration of failure (9.4 months); dGSS scores indicate the number of fully active drugs predicted using detected mutations and the standard second line regimen (didanosine, abacavir, and ritonavir boosted lopinavir). (http://hivdb.stanford.edu);11 bars are means +/− standard errors.
Acknowledgments
Financial Support: National Institute of Allergy and Infectious Diseases, National Institutes of Health, AI69502 and AI027763, A151982, A1060530.
Footnotes
Prior Presentation: Portions of these data were presented at the 14th (Montreal, Canada, Feb 8-11, 2009) and 15th (February 16-19, San Francisco, CA, 2010) Conferences on Retroviruses and Opportunistic Infections.
Potential Conflict of Interest: JKW has served on the Scientific Advisory Board of Abbott Laboratories.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.AIDS epidemic update - December 2009. Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO); 2009. [Google Scholar]
- 2.Progress Report 2009. World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
- 3.Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. World Health Organization; Geneva: 2006. [PubMed] [Google Scholar]
- 4.WHO Report of the WHO Technical Reference Group. Paper presented at: Paediatric HIV/ART Care Guideline Group Meeting; Geneva, Switzerland. April 10-11, 2008; WHO Headquarters; [Google Scholar]
- 5.Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach - 2010 revision. World Health Organization; Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 6.Gody JC, Charpentier C, Mbitikon O, et al. High prevalence of antiretroviral drug resistance mutations in HIV-1 non-B subtype strains from African children receiving antiretroviral therapy regimen according to the 2006 revised WHO recommendations. J Acquir Immune Defic Syndr. 2008 Dec 15;49(5):566–569. doi: 10.1097/QAI.0b013e318183acae. [DOI] [PubMed] [Google Scholar]
- 7.Germanaud D, Derache A, Traore M, et al. Level of viral load and antiretroviral resistance after 6 months of non-nucleoside reverse transcriptase inhibitor first-line treatment in HIV-1-infected children in Mali. J Antimicrob Chemother. 2010 Jan;65(1):118–124. doi: 10.1093/jac/dkp412. [DOI] [PubMed] [Google Scholar]
- 8.Kamya MR, Gasasira AF, Achan J, et al. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. Aids. 2007 Oct 1;21(15):2059–2066. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 9.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004 Mar-Apr;5(2):74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- 10.Strain MC, Letendre S, Pillai SK, et al. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol. 2005 Feb;79(3):1772–1788. doi: 10.1128/JVI.79.3.1772-1788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006 Jun 1;42(11):1608–1618. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee SY, Gonzales MJ, Kantor R, Betts BJ, Ravela J, Shafer RW. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003 Jan 1;31(1):298–303. doi: 10.1093/nar/gkg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson JA, Jiang H, Ding X, et al. Genotypic susceptibility scores and HIV type 1 RNA responses in treatment-experienced subjects with HIV type 1 infection. AIDS Res Hum Retroviruses. 2008 May;24(5):685–694. doi: 10.1089/aid.2007.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2009. Top HIV Med. 2009 Dec;17(5):138–145. [PubMed] [Google Scholar]
- 15.Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7:13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jittamala P, Puthanakit T, Chaiinseeard S, Sirisanthana V. Predictors of virologic failure and genotypic resistance mutation patterns in thai children receiving non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Pediatr Infect Dis J. 2009 Sep;28(9):826–830. doi: 10.1097/INF.0b013e3181a458f9. [DOI] [PubMed] [Google Scholar]
- 17.Wamalwa DC, Farquhar C, Obimbo EM, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007 Jul 1;45(3):311–317. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaix ML, Rouet F, Kouakoussui KA, et al. Genotypic human immunodeficiency virus type 1 drug resistance in highly active antiretroviral therapy-treated children in Abidjan, Cote d’Ivoire. Pediatr Infect Dis J. 2005 Dec;24(12):1072–1076. doi: 10.1097/01.inf.0000190413.88671.92. [DOI] [PubMed] [Google Scholar]
- 19.Vaz P, Chaix ML, Jani I, et al. Risk of extended viral resistance in human immunodeficiency virus-1-infected Mozambican children after first-line treatment failure. Pediatr Infect Dis J. 2009 Dec;28(12):e283–287. doi: 10.1097/INF.0b013e3181ba6c92. [DOI] [PubMed] [Google Scholar]
- 20.Puthanakit T, Jourdain G, Hongsiriwon S, et al. HIV-1 drug resistance mutations in children after failure of first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. HIV Med. 2010 Mar 25; doi: 10.1111/j.1468-1293.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- 21.Gibb DM, Walker AS, Kaye S, et al. Evolution of antiretroviral phenotypic and genotypic drug resistance in antiretroviral-naive HIV-1-infected children treated with abacavir/lamivudine, zidovudine/lamivudine or abacavir/zidovudine, with or without nelfinavir (the PENTA 5 trial) Antivir Ther. 2002 Dec;7(4):293–303. [PubMed] [Google Scholar]
- 22.Cozzi-Lepri A, Phillips AN, Martinez-Picado J, et al. Rate of accumulation of thymidine analogue mutations in patients continuing to receive virologically failing regimens containing zidovudine or stavudine: implications for antiretroviral therapy programs in resource-limited settings. J Infect Dis. 2009 Sep 1;200(5):687–697. doi: 10.1086/604731. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds SJ, Kityo C, Mbamanya F, et al. Evolution of drug resistance after virological failure of a first-line highly active antiretroviral therapy regimen in Uganda. Antivir Ther. 2009;14(2):293–297. doi: 10.1177/135965350901400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llibre JM, Santos JR, Puig T, et al. Prevalence of etravirine-associated mutations in clinical samples with resistance to nevirapine and efavirenz. J Antimicrob Chemother. 2008 Nov;62(5):909–913. doi: 10.1093/jac/dkn297. [DOI] [PubMed] [Google Scholar]
- 25.Andries K, Azijn H, Thielemans T, et al. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2004 Dec;48(12):4680–4686. doi: 10.1128/AAC.48.12.4680-4686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metzner KJ, Giulieri SG, Knoepfel SA, et al. Minority quasispecies of drug-resistant HIV-1 that lead to early therapy failure in treatment-naive and -adherent patients. Clin Infect Dis. 2009 Jan 15;48(2):239–247. doi: 10.1086/595703. [DOI] [PubMed] [Google Scholar]
- 27.Nadler JP, Berger DS, Blick G, et al. Efficacy and safety of etravirine (TMC125) in patients with highly resistant HIV-1: primary 24-week analysis. Aids. 2007 Mar 30;21(6):F1–10. doi: 10.1097/QAD.0b013e32805e8776. [DOI] [PubMed] [Google Scholar]
- 28.Cohen CJ, Berger DS, Blick G, et al. Efficacy and safety of etravirine (TMC125) in treatment-experienced HIV-1-infected patients: 48-week results of a phase IIb trial. Aids. 2009 Jan 28;23(3):423–426. doi: 10.1097/QAD.0b013e32831c5040. [DOI] [PubMed] [Google Scholar]
- 29.Vingerhoets J, Tambuyzer L, Azijn H, et al. Resistance profile of etravirine: combined analysis of baseline genotypic and phenotypic data from the randomized, controlled Phase III clinical studies. Aids. 2010 Feb 20;24(4):503–514. doi: 10.1097/QAD.0b013e32833677ac. [DOI] [PubMed] [Google Scholar]
- 30.Castelnuovo B, John L, Lutwama F, et al. Three-year outcome data of second-line antiretroviral therapy in Ugandan adults: good virological response but high rate of toxicity. J Int Assoc Physicians AIDS Care (Chic Ill) 2009 Jan-Feb;8(1):52–59. doi: 10.1177/1545109708328538. [DOI] [PubMed] [Google Scholar]
- 31.Badri M, Lawn SD, Wood R. Utility of CD4 cell counts for early prediction of virological failure during antiretroviral therapy in a resource-limited setting. BMC Infect Dis. 2008;8:89. doi: 10.1186/1471-2334-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD. Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. Aids. 2008 Oct 1;22(15):1971–1977. doi: 10.1097/QAD.0b013e32830e4cd8. [DOI] [PubMed] [Google Scholar]
- 33.Messou E, Gabillard D, Moh R, et al. Anthropometric and immunological success of antiretroviral therapy and prediction of virological success in west African adults. Bull World Health Organ. 2008 Jun;86(6):435–442. doi: 10.2471/BLT.07.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Oosterhout JJ, Brown L, Weigel R, et al. Diagnosis of antiretroviral therapy failure in Malawi: poor performance of clinical and immunological WHO criteria. Trop Med Int Health. 2009 Aug;14(8):856–861. doi: 10.1111/j.1365-3156.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 35.Moore DM, Awor A, Downing R, et al. CD4+ T-cell count monitoring does not accurately identify HIV-infected adults with virologic failure receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2008 Dec 15;49(5):477–484. doi: 10.1097/QAI.0b013e318186eb18. [DOI] [PubMed] [Google Scholar]
- 36.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. Aids. 2009 Jun 1;23(9):1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emmett SD, Cunningham CK, Mmbaga BT, et al. Predicting Virologic Failure Among HIV-1-Infected Children Receiving Antiretroviral Therapy in Tanzania: a Cross-Sectional Study. J Acquir Immune Defic Syndr. 2010 Mar 5; doi: 10.1097/QAI.0b013e3181cf4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goudsmit J, De Ronde A, Ho DD, Perelson AS. Human immunodeficiency virus fitness in vivo: calculations based on a single zidovudine resistance mutation at codon 215 of reverse transcriptase. J Virol. 1996 Aug;70(8):5662–5664. doi: 10.1128/jvi.70.8.5662-5664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrigan PR, Bloor S, Larder BA. Relative replicative fitness of zidovudine-resistant human immunodeficiency virus type 1 isolates in vitro. J Virol. 1998 May;72(5):3773–3778. doi: 10.1128/jvi.72.5.3773-3778.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Croteau G, Doyon L, Thibeault D, McKercher G, Pilote L, Lamarre D. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J Virol. 1997 Feb;71(2):1089–1096. doi: 10.1128/jvi.71.2.1089-1096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell TB, Schneider K, Wrin T, Petropoulos CJ, Connick E. Relationship between in vitro human immunodeficiency virus type 1 replication rate and virus load in plasma. J Virol. 2003 Nov;77(22):12105–12112. doi: 10.1128/JVI.77.22.12105-12112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deeks SG, Barbour JD, Grant RM, Martin JN. Duration and predictors of CD4 T-cell gains in patients who continue combination therapy despite detectable plasma viremia. Aids. 2002 Jan 25;16(2):201–207. doi: 10.1097/00002030-200201250-00009. [DOI] [PubMed] [Google Scholar]
- 43.Goetz MB, Leduc R, Wyman N, et al. HIV replication capacity is an independent predictor of disease progression in persons with untreated chronic HIV infection. J Acquir Immune Defic Syndr. 2010 Apr 1;53(4):472–479. doi: 10.1097/QAI.0b013e3181cae480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deeks SG, Wrin T, Liegler T, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med. 2001 Feb 15;344(7):472–480. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 45.Kuritzkes DR, Quinn JB, Benoit SL, et al. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine (3TC) versus zidovudine (ZDV) versus ZDV plus 3TC in previously untreated patients. Aids. 1996 Aug;10(9):975–981. doi: 10.1097/00002030-199610090-00007. [DOI] [PubMed] [Google Scholar]
- 46.Goldschmidt V, Marquet R. Primer unblocking by HIV-1 reverse transcriptase and resistance to nucleoside RT inhibitors (NRTIs) Int J Biochem Cell Biol. 2004 Sep;36(9):1687–1705. doi: 10.1016/j.biocel.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 47.Farley J, Hines S, Musk A, Ferrus S, Tepper V. Assessment of adherence to antiviral therapy in HIV-infected children using the Medication Event Monitoring System, pharmacy refill, provider assessment, caregiver self-report, and appointment keeping. J Acquir Immune Defic Syndr. 2003 Jun 1;33(2):211–218. doi: 10.1097/00126334-200306010-00016. [DOI] [PubMed] [Google Scholar]
- 48.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: The pediatric AIDS clinical trials group P1009 study. Journal of Allergy and Clinical Immunology. 2003;112(5):973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Bangsberg DR, Charlebois ED, Grant RM, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. Aids. 2003 Sep 5;17(13):1925–1932. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki K, Kaufmann GR, Mukaide M, et al. Novel deletion of HIV type 1 reverse transcriptase residue 69 conferring selective high-level resistance to nevirapine. AIDS Res Hum Retroviruses. 2001 Sep 1;17(13):1293–1296. doi: 10.1089/088922201750461366. [DOI] [PubMed] [Google Scholar]
- 51.Villena C, Prado JG, Puertas MC, et al. Relative fitness and replication capacity of a multinucleoside analogue-resistant clinical human immunodeficiency virus type 1 isolate with a deletion of codon 69 in the reverse transcriptase coding region. J Virol. 2007 May;81(9):4713–4721. doi: 10.1128/JVI.02135-06. [DOI] [PMC free article] [PubMed] [Google Scholar]