Abstract
Background
Mast cells (MCs) have a central role in the induction of allergic inflammation, such as seen in asthma, and contribute to the severity of certain autoimmune diseases, such as rheumatoid arthritis. The mast cell thus represents an important inflammatory cell, and one which has resisted therapeutic attempts to alter its role in disease.
Objective
Because bone marrow-derived stromal cells (BMSC, also known as mesenchymal stem cells or MSCs) have been reported to alter allergic inflammation in vivo, we chose to study the interaction between mouse BMSC and mouse bone marrow derived MCs.
Methods
MC degranulation, cytokine production and chemotaxis were evaluated in vitro following co-culture with BMSCs either in cell contact or a transwell. In addition, MC degranulation was assessed in vivo following administration of BMSCs in a model of passive cutaneous anaphylaxis and a peritoneal degranulation assay. Mechanisms of MC suppression by BMSCs were determined through use of inhibitors or antibodies to COX1, COX2, nitric oxide, indoleamine 2, 3-dioxygenase, EP1-4 receptors, TGF-β and IL-10. Lastly, we utilized either BMSCs or MCs deficient in COX1, COX2 or EP1-4 receptors to confirm the mechanisms of inhibition of MC function by BMSCs.
Results
We discovered that BMSCs will effectively suppress specific MC functions in vitro as well as in vivo. When MCs are cocultured with BMSCs to allow cell-to-cell contact, BMSCs suppressed MC degranulation, proinflammatory cytokine production, chemokinesis and chemotaxis. Similarly, MC degranulation within mouse skin or the peritoneal cavity was suppressed following in vivo administration of BMSCs. Further, we found that these inhibitory effects were dependent on up-regulation of COX2 in BMSCs; and were facilitated through the activation of EP4 receptors on MCs.
Conclusion and Clinical Relevance
These observations support the concept that BMSCs have the ability to suppress mast cell activation and therefore could be the basis for a novel cell based therapeutic approach in the treatment of MC driven inflammatory diseases.
Keywords: Stromal cell, mesenchymal stem cell, EP4 receptor, allergy, COX2
Introduction
Bone marrow stromal cells (BMSCs, also known as Mesenchymal Stem Cells or MSCs) are multipotent progenitor cells which may be isolated from the bone marrow by adherence and culture expansion. These cells have the natural ability to differentiate into bone, fat or cartilage. Recently, there is a renewed interest in BMSCs due to their immunomodulatory properties in vitro as well as in vivo. Intravenously injected BMSCs have been shown to have a beneficial effect in a variety of animal models of disease with an inflammatory component; and to prevent graft versus host disease in humans [1-5]. We have successfully utilized BMSCs to therapeutically treat Th2-mediated allergic responses in a mouse model of asthma [6]. While clinical trials using BMSCs as cellular therapy are currently ongoing for treatment of Th1 inflammatory diseases, such as Crohn's disease [7], our data suggests that also targeting Th2-dominant allergic diseases with BMSC treatment has the potential to restore immune balance and therefore may provide a novel therapeutic approach for diseases with a Th2 bias.
The interactions between BMSCs and T cells [8-11] and B cells [9, 12] have been well established. There is also emerging data suggesting that BMSCs have the ability to modulate cells of the macrophage/monocyte/dendritic cell lineage, including their differentiation, maturation and activation [11, 13-17]. MCs represent an important component of the immune system that are involved many allergic and Th2-mediated inflammatory diseases. MCs are derived from hematopoietic progenitor cells and influence both innate and adaptive immunity. They are well documented to be a critical effector cell in acute allergic reactions and to release histamine as well as cytokines and chemokines that influence innate and acquired immune responses [18]. Since mast cells are critical effector cells in allergic inflammation, they represent an important cell type to therapeutically target using the immunomodulatory properties of BMSCs. Further, there are currently no data in the literature regarding the possible interactions between MCs and BMSCs; and how these interactions might influence MC activation. We therefore initiated in vitro and in vivo experiments to study the immunomodulatory effects BMSCs on MC function.
Materials and Methods
Mice
C57BL/6, B6.Cg-KitW-sh, COX1-/- and COX2-/- mice were obtained from Jackson Laboratories at 4-6 weeks of age (Bar Harbor, ME). Bone marrows from EP1-4-/- mice were a generous gift of Dr. Beverly H. Koller. Mice were allowed food and water ad libitum; and were used experimentally between 6-8 weeks of age. All animal use procedures were in accordance with an institutional Animal Care and Use Committee.
Cell Culture
Bone Marrow-Derived MCs
Mouse bone marrow-derived MCs (MC) were derived and cultured from femoral marrow cells of C57Bl/6 mice (Jackson Laboratories, Bar Harbor, ME). Cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 25 mM HEPES, 1.0 mM sodium pyruvate, nonessential amino acids (BioSource International, Camarillo, CA), 0.0035% 2-ME and 300 ng/ml recombinant mouse IL-3 (PeproTech, Rocky Hill, NJ). MCs were used following 4-6 weeks of culture at 37°C and 5% CO2.
Bone Marrow Stromal Cells
Mouse BMSCs were collected from femora and tibiae of mice under aseptic conditions. Cells were cultured in Alpha-MEM with 20% FBS, 1% glutamine and 1% penicillin/streptomycin. Macrophages were depleted from cultures using magnetic cell sorting with CD11b/CD45 beads (Miltenyi Biotec, Auburn, CA). All BMSCs were shown to lack the hematopoietic lineage markers CD45, CD11b and Gr-1 (BD Biosciences) by FACS analysis. BMSCs were also shown to be able to differentiate into osteogenic and adipogenic lineages in vitro.
MC Degranulation and Cytokine Production
IgE-mediated MC degranulation was determined by measuring release of intracellular pre-formed β-hexosaminidase (β-hex) 30 min following challenge with antigen. For these experiments, MCs were seeded at 5×104 cells/well in 96 well flat bottom plates; and were sensitized with 100 ng/ml mouse IgE anti-dinitrophenyl (DNP) antibody (Sigma-Aldrich) for 24 h for degranulation experiments. MC degranulation was induced by the addition of 100 ng/ml dinitrophenylated human serum albumin (DNP-HSA) (Sigma-Aldrich, St. Louis, MO). Thirty min (at 37°C) after addition of DNP, we measured ß-hex release as described (Dastych et al. 1999), using p nitrophenyl-N-acetyl-β-D-glucopyranoside (8 mM; Sigma-Aldrich) as a substrate for ß-hex. For experiments where MCs and BMSCs were co-cultured, we added BMSCs at a ratio of 100:1, 10:1 or 1:1 twenty-four h prior to stimulation of MC degranulation. The reaction between ß-hex and p nitrophenyl-N-acetyl-β-D-glucopyranoside was stopped after 90 min with 0.2 M glycine. Optical density was measured at 405 nm using a GENios ELISA plate reader (ReTirSoft, Inc. Toronto, Ontario, Canada). β-Hex release was expressed as the percentage of total cell content after subtracting background release from unstimulated cells. Cell content of ß-hex from unstimulated and antigen challenged cells was determined by lysing cells with 0.1% Triton X-100.
For cytokine measurements, the cells were plated as above, but the cytokines were measured in cell culture supernatants 12 h following addition of 100 ng/ml DNP-HSA; MCs alone were used as a positive control. Additionally, cytokines were measured in supernatants of BMSCs exposed to IgE and DNP-HSA to account for any cytokine production from the BMSC population in the co-cultures. Ninety-six well transwell plates (Corning, Lowell, MA) were utilized in cytokine measurement experiments to determine the contribution of a contact dependent mechanism, and the contribution of soluble mediators in the genesis of BMSC mediated immunosuppression. For these experiments, BMSC were coated onto the bottom chamber of the transwell system, while MCs were added to the upper chamber. IgE sensitized MCs were then stimulated by the addition of DNP-HSA followed by collection of supernatants from the upper chamber 12 h later. In addition, conditioned medium from BMSC cultures was added to standard BMMC cultures followed by IgE sensitization and challenge 24 h following addition of the conditioned medium in the MC cultures. For all cytokine measurements, we used mouse TNF-α/TNFSF1A Quantikine ELISA kits (R&D Systems). In addition to the co-culture experiments described above, we utilized the following inhibitors and antibodies within the co-cultures system to determine a mechanism by which BMSCs suppress TNF-α production by MCs: 5 μM indomethacin (Cayman Chemicals), 1 μM NS-398 (Sigma), 1 μM SC560 (Sigma), 1 mM L-NAME (Sigma), 1 mM methyl-D-tryptophane (Sigma), 10 μg/ml IL-10 neutralizing Ab (Pierce), and 10 μg/ml TGF-β neutralizing Ab (R&D Systems).
Passive Cutaneous Anaphylaxis (PCA) and Peritoneal Degranulation Assay (PDA)
The immunomodulatory effects of murine BMSCs on in vivo MC degranulation were determined by monitoring both the PCA reaction and peritoneal degranulation. The PCA reaction measures changes in vascular permeability, as determined by local tissue extravasation of i.v. administered Evans blue dye that is induced by release of vasodilatory mediators following MC degranulation. For the PCA experiments, C57BL/6 mice (6-8 weeks of age) (n=6 mice per group) received intradermal injections of 1 μg mouse monoclonal IgE anti-DNP (Sigma-Aldrich) in 25 μl PBS in the right ear to sensitize tissue MCs, followed by intradermal injection of 0.5×106 BMSC in 25 μl PBS in the same ear 24 h later. The left ear served as a negative control and received an intradermal injection of PBS. Positive control mice received only an injection of IgE anti-DNP in the right ear and PBS in the left ear. Thirty min after BMSC injection, mice were challenged with antigen by intravenous injection into the tail vein with 0.5 mg/ml DNP-HSA which was resuspended in 1 % Evan's blue in 100 μl saline. The mice were euthanized by CO2 asphyxiation 30 min after injection of antigen and Evan's blue, and the ears were excised and incubated in 200 μl formamide at 55°C for 24 h to extract the Evan's blue dye from the tissue. The total content of Evan's blue that was extracted from each ear was quantitated by spectrophotometric analysis at 620 nm. The net Evan's blue was determined by subtraction of the amount of Evan's blue in the IgE positive ear or BMSC- treated ear minus the PBS treated ear. Comparison was made between IgE/antigen positive control mice and mice that received IgE/antigen and a local administration of BMSCs.
A second method was used to measure MC degranulation within the peritoneal cavity of mice. For the PDA experiments, the resident peritoneal MCs in C57BL/6 mice (n=6 mice/group) were sensitized i.p. with 1 μg monoclonal IgE anti-DNP (Sigma) followed 24 h later by i.p. challenge with DNP-HSA. To determine the degree of MC degranulation following challenge, the peritoneal cavity was irrigated with PBS and the irrigation fluid collected to measure β-hex release as described above. The reaction between ß-hex in the peritoneal fluid and p nitrophenyl-N-acetyl-β-D-glucopyranoside was stopped after 90 min with 0.2 M glycine. Optical density (OD) was measured at 405 nm using a GENios ELISA plate reader (ReTirSoft, Inc.). For PDA experiments, β-hex release is expressed as OD values. To determine the effects of BMSCs on peritoneal mast cell degranulation, an additional set of mice received 1 × 106 BMSCs 1 h prior to challenge with DNP-HSA. We used B6.Cg-KitW-sh (MC-deficient) mice in these experiments to establish that the observed response is MC specific; and that the β-hex measured in the peritoneal cavity is is a result of MC degranulation.
To study EP receptor function in vivo we used specific receptor antagonists (Cayman Chemical) at different doses (EP1 antagonist SC-51322, 3mg/kg; EP2 antagonist AH 6809, 1mg/kg; EP4 antagonist GW627368X, 1mg/kg). All antagonists were administered as a single injection in 200 uL PBS at the time of BMSC injection.
Quantitative PCR
MCs were co-cultured with BMSC at a ratio of 100:1, 10:1 or 1:1. Following 24 h co-culture, MCs were stimulated by aggregation of FcεRI with antigen as described above. The non-adherent MC population was washed off (between 2-13% of mast cell were adherent and not washed off as assessed by morphology), and thus separated from the BMSC population. Total RNA from MCs was collected using a Qiagen Rneasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer's instructions. RNA was reverse-transcribed using the Quantitect reverse transcription kit (Qiagen). Quantitative real-time PCR was performed using a Quantitech SYBR Green PCR Kit (Qiagen) and the ABI PRISM 7500 Detection system (Applied Biosystems, Foster City, CA) to obtain cycle threshold (Ct) values for target and internal reference cDNA levels. Gene specific primers for TNF-α were obtained from Qiagen. Target cDNA levels were normalized to GAPDH, an internal reference using the equation 2-[ΔCt], where ΔCt is defined as Ct target – Ct internal reference. Values shown were derived from the average of 3 independent experiments.
Chemokinesis and chemotaxis
We cultured MCs in the presence of BMSCs for 24 h, after which we placed only the MCs in a 96 well microchemotaxis plate with 8 μm pore size (Neuroprobes, Gaithersburg, MD) as described[19]. The lower chamber either contained only PBS (assay for spontaneous migration or chemokinesis) or contained stem cell factor (SCF) as an attractant (chemotaxis). Cells from the lower well were collected and counted by flow cytometry. Chemotaxis of MC to stem cell factor (SCF) was examined either alone or in the presence of BMSC. MC chemotaxis was measured following a 24 h co-culture with BMSC. MCs were plated on the chemotaxis filters at 30,000 cells/well and allowed to settle for 10 min. The filter was assembled with the lower plate filled with PBS/0.1% BSA, with or without 100 ng/ml SCF and placed at 37°C for 60 min. The lower well content (30 μl of PBS/0.1% BSA with transmigrated cells) was collected, and the total number of migrated cells was determined by counting the total events of unlabeled MCs by allowing the total volume of cells to flow through the flow cytometer (FACS Caliber, Becton Dickinson). Each condition was performed in triplicate and 2 experiments were averaged.
Statistics
Data are summarized as mean ± SEM. Student's t-test or two-way ANOVA were performed using GraphPad Prism version 4.00 for Macintosh (GraphPad Software, San Diego, California). The statistical significance value was set at P<0.05.
Results
BMSCs suppress MC degranulation and cytokine production
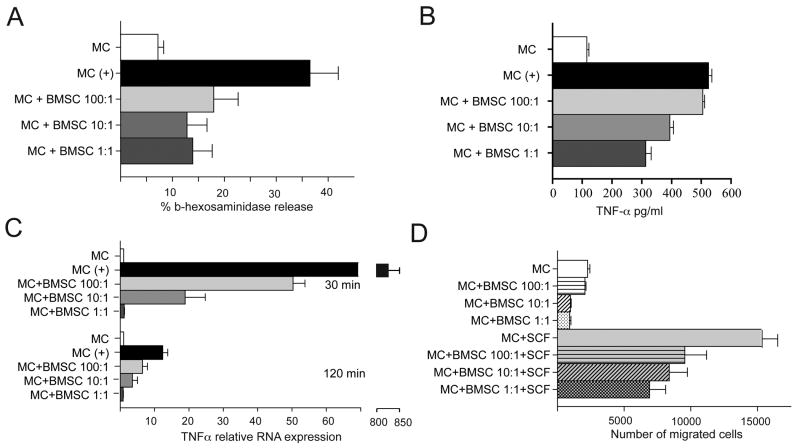
To determine if BMSCs could alter MC function, we first co-cultured mast cells with BMSCs at ratios from 1:1 to 1:100. We found that BMSCs significantly decreased degranulation of MCs as measured by release of β-hex, even at culture ratios of 100 MCs to 1 BMSC (Fig.1A). Similarly, co-culture of MCs with BMSCs for 24 h significantly reduced MC derived TNF-α 12 h following challenge with antigen in a dose dependent manner (Fig.1B). Since we established a decrease in the protein level of TNF-α in the medium of co-cultured MCs and BMSCs, we next verified that this observation was associated with a concomitant decrease in TNF-α mRNA synthesis within the MCs co-cultured with BMSCs. Our data revealed that MCs transcribed significantly less TNF-α mRNA when they were in contact with BMSCs at both 30 and 120 min following challenge with antigen. Lastly, we measured TNF-α expression by BMSCs co-cultured with MCs in a transwell system to exclude the possibility that BMSCs are not indirectly activated by MC mediators, however, we did not observe any TNF-α production by BMSCs (data not shown). Thus, BMSCs do have the capacity to down-regulate certain MC responses.
Figure 1. In vitro studies of the interactions between MCs and BMSCs.
A: Different ratios (1:1; 1:10 and 1:100) of IgE sensitized MCs and BMSCs were cultured together for 24 h prior to IgE specific antigen challenge. β-hex release was used as a marker of MC degranulation. BMSCs attenuate MC degranulation in all ratios tested.
B: Different ratios (1:1; 1:10 and 1:100) of IgE sensitized MCs and BMSCs were cultured together for 24 h prior to IgE specific antigen challenge for 12 h. TNF-α release was measured by ELISA. The BMSCs decreased the amount of released TNF-α in a ratio dependent manner.
C: The experiment in B was repeated to measure TNF-α mRNA levels at two time-points (30 and 120 min) following antigen challenge. Similar to the levels of TNF-α protein, mRNA synthesis also decreased in response to the presence of BMSCs at both time-points in a ratio dependent manner.
D: The migration of MCs was affected by the presence of BMSCs in the culture with increasing numbers of BMSCs within the co-culture, the spontaneous (upper four columns) as well as SCF induced migration (chemokinesis and chemotaxis, respectively) of MCs were significantly reduced.
In all Figures:*=p<0.05; **=p<0.01 and ***=p,0.001
BMSCs inhibit MC functions in vitro
BMSCs significantly reduce MC chemotaxis
Once we observed that BMSCs affect the degranulation and cytokine synthesis of MCs, we determined if BMSCs might also influence the ability of MCs to migrate towards a stimulus. Cells collected from the lower well of a microchemotaxis plate were counted by flow cytometry. When the lower chamber in the chemotaxis plate contained only PBS, there was significantly less migration of MCs (Fig. 1D). Similarly, when the lower chamber contained SCF as an attractant, the number of MCs that migrated was significantly decreased when the MCs had been co-cultured with BMSCs, as compared to MCs that had not been co-cultured with BMSCs (Fig.1 D). Thus, MCs cultured in the presence of BMSCs for 24 h were significantly impaired in their ability to migrate.
MC activation in vivo is impaired following administration of BMSCs
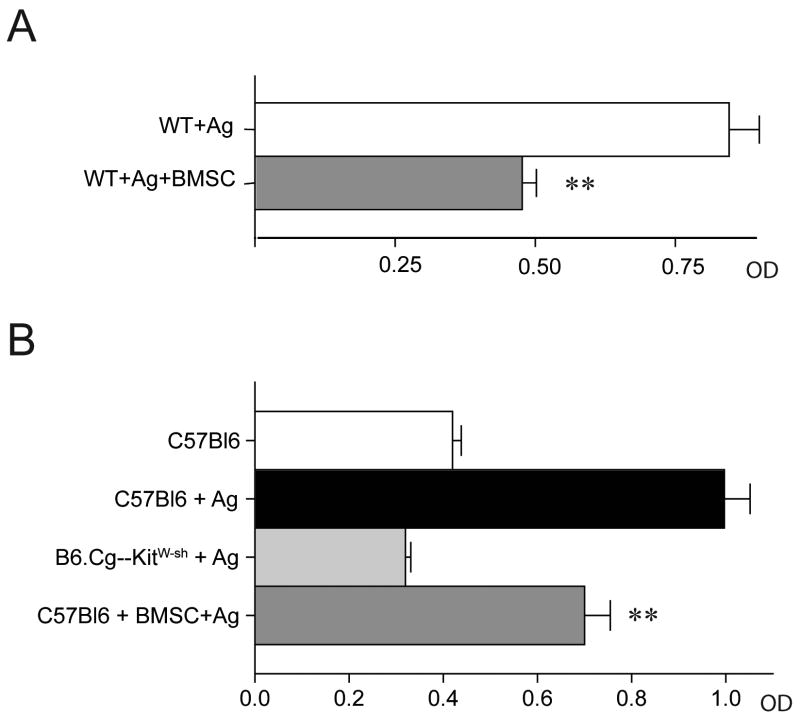
We next wanted to examine whether mast cell behavior could be altered by BMSCs in vivo. To assess this possibility, we used two separate in vivo assays of mast cells responsiveness. Using a PCA model, when antigen-specific IgE was injected intradermally into the ear followed by systemic antigen challenge 24 h later, we found that the increased vascular permeability resulting from MC activation was significantly reduced when BMSCs were administered (Fig.2A). In the PDA assay, where MCs in the peritoneal cavity are sensitized with antigen-specific IgE and then challenged with antigen, we found a significant decrease in the amount of β-hex in the peritoneal fluid when BMSCs were present (Fig.2B). To confirm that the change in β-hex levels was due to MC degranulation, we repeated the experiment using B6.Cg-KitW-sh MC deficient mice. In these experiments, we did not observe any increase in β-hex release within the peritoneal cavity following challenge with antigen, consistent with the conclusion that the β-hex measured in the C57BL/6 mice is indeed derived from MCs (Fig. 2B).
Figure 2. Suppression of MC degranulation by BMSCs in vivo.
A: Passive cutaneous anaphylaxis (PCA) was performed using systemically administered Evans blue as a marker of tissue permeability within the mouse ear. Administration of BMSCs to the IgE sensitized mouse ear prior to challenge with IgE-specific antigen significantly decreased the amount of Evans blue leakage into the tissues as quantified by optical density.
B: In vivo MC degranulation was also examined by challenging IgE sensitized peritoneal MCs with antigen (PDA) following i.p. administration of either PBS or BMSCs. MC degranulation was quantified by measuring an increase in peritoneal β-hex release following challenge as shown in the top 2 bars. In the bottom 2 bars, an increase in β-hex release was not observed in MC deficient mice, while i.p. administration of BMSCs significantly decreased the amount of β-hex release in C57Bl/6 mice which the MCs were sensitized and challenged with antigen.
BMSCs suppress MC degranulation in vivo
Upregulation of COX2 by BMSCs mediates the suppression of MC activation via the EP4 receptor
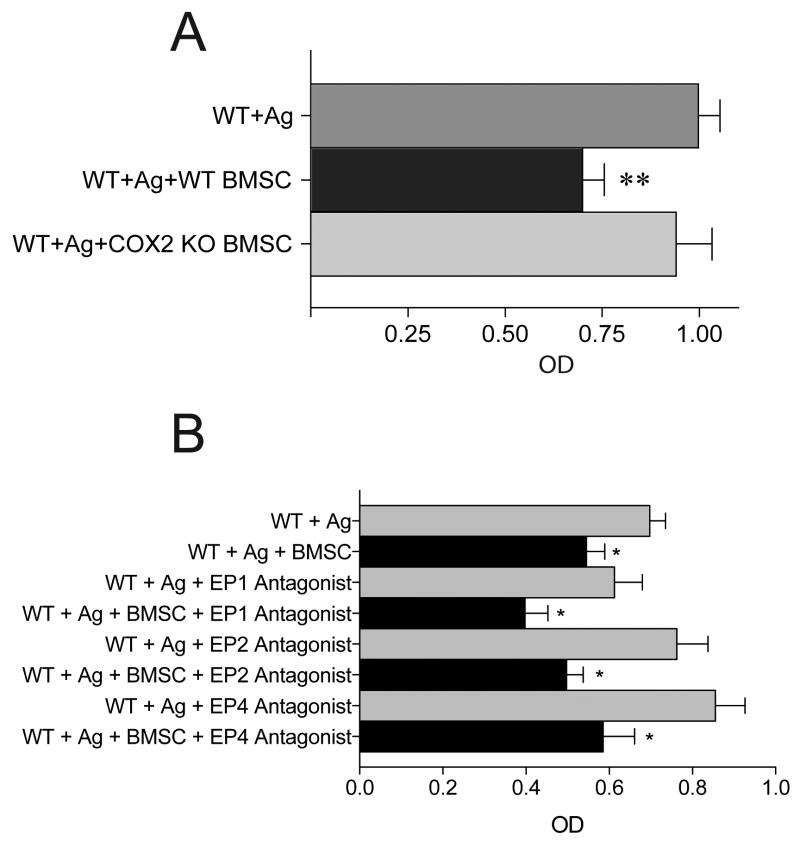
Next we set out to identify a mechanism of action with regards to the ability of BMSCs to suppress MC function. For these experiments, we used MC-derived TNF-α production as an endpoint. Following the addition of a variety of pharmacological inhibitors (COX1/2 inhibitors, IDO inhibitor, NO inhibitor), blocking antibodies (anti-TGF-α and anti-IL-10) to our co-cultures or use of BMSC conditioned media or a transwell system, we measured TNF-α release by MCs. We found that BMSCs were still effective at suppressing MC derived TNF-α production in transwell system or in the presence of COX1 inhibitor, NO inhibitor, IDO inhibitor and blocking antibodies to IL-10 and TGF-β. Further, the BMSC conditioned media did not have an effect on MC TNF-α production suggesting that presence of MCs either in contact with BMSCs or in a transwell system are required to elicit the suppressive effect of BMSCs. When we added indomethacin (a COX1/COX2 non-selective inhibitor) or a specific COX2 inhibitor (NS-398), the suppressive effect mediated by BMSCs was eliminated (Fig.3A). To determine which cell population was targeted by the COX2 inhibitors for suppression of TNF-α release by MCs, we repeated the in vitro co-cultures using either BMSCs or MCs that were deficient in COX2. The suppressive effect was eliminated when COX2 deficient BMSCs were used, but persisted when COX2-deficient MCs were used in the co-culture system (Fig.3B). Since COX2 will induce PGE2 production, which exerts its effects through binding to the EP1-4 receptors, we next examined the involvement of each of these individual receptors in the MC population. Once again, we used TNF-α production as a marker of MC response. Data revealed that when MCs were derived from EP1, EP2 or EP3-deficient mice, they remained responsive to the suppressive effects of the BMSCs. However, when EP4 deficient MCs were used in the co-culture, the BMSCs were unable to suppress TNF-α production by MCs (Fig.3C). Lastly, we confirmed that COX2 mRNA is upregulated upon co-culture of BMSCs and MCs. As shown in Figure 3D, we observed a significant upregulation of COX2 mRNA in BMSCs 4 hr following co-culture. This upregulation was observed in both the contact and transwell co-cultures, however, the expression of COX2 was much greater when the cells were co-cultured in contact. Finally, to test the mechanism of suppressive effect in vivo, we repeated the PDA experiments and found that BMSCs derived from COX2-/- mice were unable to suppress β-hex release by MCs in vivo (Fig.4A). Surprisingly we could not confirm the importance of EP4 receptor in vivo using EP receptor antagonists (Fig. 4B). Pre-treating the mice with different EP receptor antagonists prior to BMSC injection and antigen challenge could not eliminate the suppressive effect observed in vivo.
Figure 3. Identifying a mechanism of MC suppression.
A. In the co-culture system a variety of inhibitor and blocking antibodies were used to test if they prevent the immunosuppressive effect of BMSCs on MCs' TNF-α production. The suppressive effect of BMSCs was not altered when a COX1 inhibitor, IDO inhibitor, NO inhibitor or antibodies to TGF-β and IL-10 were applied to the co-culture. However, when indomethacin (a COX1 and COX2 inhibitor) or a specific COX2 inhibitor (NS-398) were used the immuno-suppressive effect of the BMSCs was eliminated.
B. Identifying the cellular source of COX2 in the system. Using COX1 and COX2 deficient cells (either MCs or BMSCs) in the co-culture system, TNF-α production was measured following antigen stimulation of MCs. Exclusively, the use of COX2 KO BMSC in the co-culture eliminated the effect suggesting that BMSC upregulate COX2 synthesis.
C. Screening for the responsible receptor in the MC. MCs lacking the individual EP receptors (EP1-4) was used to determine their effect on TNF-α production in the co-culture system with BMSCs. BMSCs were still able to suppress MC derived TNF-α production when the EP1-3 receptors were absent. When EP4 KO MCs were used in the culture system, the immunosuppressive effect of BMSCs was eliminated.
D. Upregulation of COX2 in BMSC following co-culture with MCs. COX2 mRNA levels were measured by quantitative PCR at 2, 4, and 6 hr following co-culture with MCs in either contact dependent or a transwell system. COX2 mRNA was significantly upregulated in BMSCs 4 hr following co-culture with MCs. While BMSC COX2 mRNA expression was increased in the transwell system, we observed a greater increase in COX2 expression when the cells were in contact.
Mechanism of BMSC driven inhibition of MC function (in vitro)
Figure 4. In vivo confirmation of COX2 mediated mechanism.
A. The peritoneal assay depicted in Figure 2 was repeated using COX2 KO BMSCs followed by challenging the sensitized resident MCs with antigen. In contrast to wild-type BMSCs, COX2 deficient BMSCs failed to suppress β-hex release by peritoneal MCs.
B. The peritoneal assay depicted in Figure 2 was repeated using EP1-4 receptor antagonists delivered to the peritoneal cavity at the same time as injection of BMSCs which was followed 24 hrs later by challenging the sensitized resident MCs with antigen and measuring β-hex release. In contrast to injection of COX2 deficient BMSCs as shown in Figure 4A, addition of EP receptor antagonists did not abrograte the suppressive effect of BMSCs.
N=4 mice/group and was repeated 2 times.
Mechanism of BMSC driven inhibition of MC function (in vivo)
Discussion
There are now a number of studies which report that BMSCs are capable of suppressing T and B cells, dendritic cells and NK cells in vitro, and ameliorating inflammatory responses in vivo [3-5, 20, 21]. The majority of these studies explored how BMSCs could be used to treat graft versus host disease (GVHD) or inflammatory diseases mediated by autoimmune pathology. More recent studies have explored if BMSCs could influence innate immune functions; and if these interactions could provide any benefit in host defense [20, 22]. Indeed, we have demonstrated that BMSCs provide protection against the development of sepsis and more recently have reported that BMSCs can modulate disease pathology in an allergic model of ragweed-induced asthma [6, 23].
MCs have long been recognized for their role in the genesis of allergic inflammation [18], thereby providing an ideal cell target in which the immunomodulatory effects of BMSCs may be beneficial. Despite the central effector role of MCs in allergic inflammation, no studies are yet available to address if BMSCs interact with MCs and could thereby influence allergic inflammation.
In this study we address this question, and provide evidence that mouse BMSCs co-cultured with MCs in vitro effectively suppress MC activation, including FcεRI-mediated degranulation, TNF-α production and SCF induced migration. Using a classic PCA model and a model of peritoneal MC degranulation, we found that in accordance with our in vitro data, BMSCs also attenuate MC activation in vivo. As such, these in vivo models demonstrate the potential of BMSCs to reduce the severity of MC initiated allergic diseases.
The suppression of MC activation by BMSCs is greatest when the cells are in contact as compared to when the cells are co-cultured in a transwell system (Fig. 3A). Further, BMSC conditioned media does not have the ability to suppress MC activation. The lack of an effect with conditioned media suggests that there is cross-talk required between the MC and BMSC to initiate the suppressive effect elicited by the BMSC. These findings are similar to what is reported with the attenuation of mast cell degranulation by bronchial epithelial cells [24]. Yang, et al demonstrated that after 16 h of co-culture with bronchial epithelial cells, human lung MCs have significantly attenuated response to IgE-mediated activation. Similar to our data, the effect of bronchial epithelial cells was contact dependent [24]. In contrast, it has been reported that several other cell types may promote MC survival and proliferation. Hollins, et al have shown that airway smooth muscle promotes lung MC survival, proliferation and activation primarily through a physical interaction between membrane bound SCF and it's receptor on MCs [25]. In addition, it has been recently demonstrated that generation of cord blood-derived MCs can be enhance when co-cultured with a bone-marrow stomal cell line [26]. It remains to be determined what effects BMSCs may have on MC proliferation and maturation.
To identify the molecular pathways involved in such a suppressive effect by BMSCs, we employed specific inhibitors as well as cells isolated from specific knock-out mice. Based on our initial screening experiments using inhibitors, we identified COX2 as a crucial intermediate in the BMSC driven suppression of MCs. As both BMSCs and MCs upregulate COX2 in response to stimuli, we repeated our experiments using co-culture systems where either MCs or BMSCs lacked the COX2 gene. Based on these studies we identified that the upregulation of COX2 is occurring within the BMSC population; and is critical regulator of their suppressive action on MCs. Further, MC derived COX2 did not prove essential.
Our next question was whether COX2 upregulation mediates the suppression of MCs by BMSC derived PGE2 by acting through EP receptors expressed on MCs. To explore this hypothesis, we employed MCs deficient for one of the four known receptors for PGE2: EP1, EP2, EP3 or EP4. Through the use of EP deficient MCs, we demonstrate that lack of EP4 in MCs abrogated the suppressive effect elicited by BMSCs in vitro while the presence of EP1, EP2, or EP3 was not critical. To determine whether EP4 is critical to BMSC directed suppression of mast cell activation in vivo, we used antagonists specific to EP1, EP2, EP3 and EP4 receptors. However, when we probed the importance of EP receptors in vivo using these receptor antagonists we could not confirm our in vitro data. EP4 receptor antagonists did not prevent BMSCs from inhibiting mast cell degranulation. In fact neither of the receptor antagonists could hinder the BMSC suppressive phenotype. These seemingly contradicting data between in vitro and in vivo findings could be explained in several ways. First, in vitro we utilized mast cells deficient for EP receptors while in vivo we pretreated wild-type mice with specific EP receptor antagonists. It's possible that pharmacologic inhibition of mast cell EP receptors is not as efficient as complete deletion of the genes using transgenic technology. It's also possible that in vivo, and in particular within the peritoneal cavity, there are other cell types (macrophages, neutrophils, etc.) that influence either direct interactions between BMSCs and mast cells or influence the pharmacological intervention used to study the importance of EP receptors considering these cell types also express EP receptors. In fact, we have shown that PGE2 released from BMSCs acting through EP2 and EP4 receptors modulates macrophage phenotype in a mouse model of sepsis [23] which in turn could influence mast cell activation and pharmacological intervention. Lastly, the fact that EP4 antagonists did not prevent the suppression of mast cell degranulation in vivo further highlights that ability of these cells to adjust to their environment and lead to suppression of immune responses.
The effect of PGE2 on MCs varies with the model system employed. Several studies suggest that the presence of PGE2 is inhibitory to MC activation [27-31]. Other data reported that addition of PGE2 potentiates activation of MCs stimulated with IgE and antigen [32-36]. In our experiments, we examined the cellular interactions between MCs and BMSCs and our results are consistent with the conclusion that PGE2 derived from a cellular source (e.g. BMSCs) inhibits MC activation. In this regard they are most consistent with published data showing that PGE2 inhibits lung mast cells degranulation and the resultant inflammation and Th2 cytokine production [28, 30, 31].
When BMSCs are administered into the circulation they will eventually end up in the microvascular bed of different organs. Since mast cells are primarily located in the perivascular space of vessels BMSCs will inevitably interact with them when leaving the vascular space during the process of homing. It's also well described that BMSCs preferentially home to sites of active inflammation, responding to a wide variety of inflammatory signals. In an anaphylactic setting mast cells predominate such an inflammatory environment hence there is an increased chance for them to communicate with and to be suppressed by injected BMSCs. Based on our data we suggest that BMSC infusion (either systematic or local) could effectively suppress immunologic responses mediated by mast cells in the setting of anaphylaxis. There is also an increasing amount of data emphasizing the importance of mast cells in the coordination of autoimmune diseases [37]. In many cases the presence of mast cells is critical to orchestrate autoimmune inflammation as shown in animal models of multiple sclerosis, rheumatoid arthritis or bullous pemphigoid [37]. Since BMSCs are known to be able to attenuate autoimmune inflammation [38], we hypothesize that BMSC/mast cell interactions could –at least in part- explain this suppressive phenotype and guide us in a better understanding of BMSC immune biology.
In summary, we have shown that BMSCs have the ability to influence the biology of MCs by limiting their activation and migration. Our data additionally document that these effects are mediated through prostaglandin pathways. These observations support the conclusion that BMSCs should be explored as a cell-based therapy in the treatment of MC directed allergic inflammation.
Acknowledgments
The authors would like to thank Dr. Beverly H. Koller for supplying the EP1-4 KO mice. This research was supported by the Division of Intramural Research programs of NIDCR and NIAID.
References
- 1.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–21. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 2.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–34. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Nasef A, Ashammakhi N, Fouillard L. Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med. 2008;3:531–46. doi: 10.2217/17460751.3.4.531. [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 5.Sotiropoulou PA, Papamichail M. Immune properties of mesenchymal stem cells. Methods Mol Biol. 2007;407:225–43. doi: 10.1007/978-1-59745-536-7_16. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107:5652–7. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dryden GW., Jr Overview of biologic therapy for Crohn's disease. Expert Opin Biol Ther. 2009;9:967–74. doi: 10.1517/14712590903048909. [DOI] [PubMed] [Google Scholar]
- 8.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 9.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 10.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 11.Maccario R, Moretta A, Cometa A, Montagna D, Comoli P, Locatelli F, Podesta M, Frassoni F. Human mesenchymal stem cells and cyclosporin a exert a synergistic suppressive effect on in vitro activation of alloantigen-specific cytotoxic lymphocytes. Biol Blood Marrow Transplant. 2005;11:1031–2. doi: 10.1016/j.bbmt.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–90. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Zhang W, Yue H, Han Q, Chen B, Shi M, Li J, Li B, You S, Shi Y, Zhao RC. Effects of human mesenchymal stem cells on the differentiation of dendritic cells from CD34+ cells. Stem Cells Dev. 2007;16:719–31. doi: 10.1089/scd.2007.0065. [DOI] [PubMed] [Google Scholar]
- 14.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Guo Z, Jiang X, Zhu H, Li X, Mao N. Mesenchymal Stem Cells Alter Migratory Property of T and Dendritic Cells to Delay the Development of Murine Lethal Acute Graft-Versus-Host Disease. Stem Cells. 2008;26(10):2531–41. doi: 10.1634/stemcells.2008-0146. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–71. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 18.Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy. 2008;38:4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- 19.Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, Metcalfe DD. 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol. 2006;177:6422–32. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- 20.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 21.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169–79. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–83. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 23.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Wardlaw AJ, Bradding P. Attenuation of human lung mast cell degranulation by bronchial epithelium. Allergy. 2006;61:569–75. doi: 10.1111/j.1398-9995.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 25.Hollins F, Kaur D, Yang W, Cruse G, Saunders R, Sutcliffe A, Berger P, Ito A, Brightling CE, Bradding P. Human airway smooth muscle promotes human lung mast cell survival, proliferation, and constitutive activation: cooperative roles for CADM1, stem cell factor, and IL-6. J Immunol. 2008;181:2772–80. doi: 10.4049/jimmunol.181.4.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi M, Azuma H, Fujihara M, Hamada H, Ikeda H. Generation of a considerable number of functional mast cells with a high basal level of FcepsilonRI expression from cord blood CD34+ cells by co-culturing them with bone marrow stromal cell line under serum-free conditions. Scand J Immunol. 2007;65:581–8. doi: 10.1111/j.1365-3083.2007.01937.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan CL, Jones RL, Lau HY. Characterization of prostanoid receptors mediating inhibition of histamine release from anti-IgE-activated rat peritoneal mast cells. Br J Pharmacol. 2000;129:589–97. doi: 10.1038/sj.bjp.0703072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrerias A, Torres R, Serra M, Marco A, Roca-Ferrer J, Picado C, de Mora F. Subcutaneous prostaglandin E(2) restrains airway mast cell activity in vivo and reduces lung eosinophilia and Th(2) cytokine overproduction in house dust mite-sensitive mice. Int Arch Allergy Immunol. 2009;149:323–32. doi: 10.1159/000205578. [DOI] [PubMed] [Google Scholar]
- 29.Hogaboam CM, Bissonnette EY, Chin BC, Befus AD, Wallace JL. Prostaglandins inhibit inflammatory mediator release from rat mast cells. Gastroenterology. 1993;104:122–9. doi: 10.1016/0016-5085(93)90843-2. [DOI] [PubMed] [Google Scholar]
- 30.Kay LJ, Yeo WW, Peachell PT. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br J Pharmacol. 2006;147:707–13. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang XS, Wu AY, Leung PS, Lau HY. PGE suppresses excessive anti-IgE induced cysteinyl leucotrienes production in mast cells of patients with aspirin exacerbated respiratory disease. Allergy. 2007;62:620–7. doi: 10.1111/j.1398-9995.2007.01364.x. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172:1227–36. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 33.Gomi K, Zhu FG, Marshall JS. Prostaglandin E2 selectively enhances the IgE-mediated production of IL-6 and granulocyte-macrophage colony-stimulating factor by mast cells through an EP1/EP3-dependent mechanism. J Immunol. 2000;165:6545–52. doi: 10.4049/jimmunol.165.11.6545. [DOI] [PubMed] [Google Scholar]
- 34.Leal-Berumen I, O'Byrne P, Gupta A, Richards CD, Marshall JS. Prostanoid enhancement of interleukin-6 production by rat peritoneal mast cells. J Immunol. 1995;154:4759–67. [PubMed] [Google Scholar]
- 35.Nakayama T, Mutsuga N, Yao L, Tosato G. Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. J Leukoc Biol. 2006;79:95–104. doi: 10.1189/jlb.0405226. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen M, Solle M, Audoly LP, Tilley SL, Stock JL, McNeish JD, Coffman TM, Dombrowicz D, Koller BH. Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J Immunol. 2002;169:4586–93. doi: 10.4049/jimmunol.169.8.4586. [DOI] [PubMed] [Google Scholar]
- 37.Benoist C, Mathis D. Mast cells in autoimmune disease. Nature. 2002;420:875–8. doi: 10.1038/nature01324. [DOI] [PubMed] [Google Scholar]
- 38.Pistoia V, Raffaghello L. Potential of mesenchymal stem cells for the therapy of autoimmune diseases. Expert Rev Clin Immunol. 2010;6:211–8. doi: 10.1586/eci.09.86. [DOI] [PubMed] [Google Scholar]