Mucolipidosis II mice, as well as patients with this disorder, exhibit extensive vacuolization of their exocrine gland cells. We now demonstrate that the vacuoles are enlarged autolysosomes containing undigested cytoplasmic material that accumulate secondary to deficient lysosomal degradative function. A model to explain this finding is proposed.
Abstract
We previously reported that mice deficient in UDP-GlcNAc:lysosomal enzyme GlcNAc-1-phosphotransferase (mucolipidosis type II or Gnptab −/− mice), the enzyme that initiates the addition of the mannose 6-phosphate lysosomal sorting signal on acid hydrolases, exhibited extensive vacuolization of their exocrine gland cells, while the liver, brain, and muscle appeared grossly unaffected. Similar pathological findings were observed in several exocrine glands of patients with mucolipidosis II. To understand the basis for this cell type–specific abnormality, we analyzed these tissues in Gnptab −/− mice using a combined immunoelectron microscopy and biochemical approach. We demonstrate that the vacuoles in the exocrine glands are enlarged autolysosomes containing undigested cytoplasmic material that accumulate secondary to deficient lysosomal function. Surprisingly, the acid hydrolase levels in these tissues ranged from normal to modestly decreased, in contrast to skin fibroblasts, which accumulate enlarged lysosomes and/or autolysosomes also but exhibit very low levels of acid hydrolases. We propose that the lysosomal defect in the exocrine cells is caused by the combination of increased secretion of the acid hydrolases via the constitutive pathway along with their entrapment in secretory granules. Taken together, our results provide new insights into the mechanisms of the tissue-specific abnormalities seen in mucolipidosis type II.
INTRODUCTION
Mucolipidosis type II (MLII, inclusion-cell disease or I-cell disease) is an autosomal recessive disorder due to deficiency of UDP-GlcNAc:lysosomal enzyme GlcNAc-1-phosphotransferase (GlcNAc-1-phosphotransferase), the first enzyme involved in the synthesis of the mannose 6-phosphate (Man-6-P) signal, which allows the specific targeting of lysosomal acid hydrolases from the trans-Golgi network (TGN) to lysosomes (Kornfeld and Sly, 2000; Pohl et al., 2009; Cathey et al., 2010; Kollmann et al., 2010). The fibroblasts of MLII patients have been shown to hypersecrete most of their newly synthesized acid hydrolases via the constitutive secretory pathway, thereby accounting for the deficiency of acid hydrolases in these cells and, at least partially, for the high level of these same hydrolases in the extracellular fluids of the patients (Leroy and Demars, 1967; Lightbody et al., 1971; Tondeur et al., 1971; Wiesmann et al., 1971a, 1971b; Hickman and Neufeld, 1972; Leroy et al., 1972; Aula et al., 1975; Martin et al., 1975). At the morphological level, the most striking alterations have been found in these fibroblasts and other cell types of mesenchymal origin, which develop a lysosomal storage phenotype characterized by the accumulation of phase-dense inclusions that gave rise to the term “inclusion-cell disease” (Leroy and DeMars, 1967; Kenyon and Sensenbrenner, 1971; Aula et al., 1975; Martin et al., 1975; Nagashima et al., 1977). By contrast, other cell types exhibit few, if any, morphological alterations, including most cells of the hepatic parenchyma, CNS, and muscle (Kenyon et al., 1973; Martin et al., 1975, 1984; Nagashima et al., 1977). Interestingly, a more recent report described striking vacuolization at a novel site: the serous secretory cells of the pancreas and salivary glands of several infants with MLII (Elleder and Martin, 1998). In that study, the origin of the vacuoles was not explained and the acid hydrolase content of these tissues was not determined. During the course of studying mice mutant in the gene Gnptab, which encodes the α/β catalytic subunits of GlcNAc-1-phosphotransferase (MLII mouse model), we observed similar vacuolization in the exocrine secretory cell types (Gelfman et al., 2007; Vogel et al., 2009). This finding provided the opportunity to investigate the nature and cause of this pathological alteration.
In this article, we demonstrate that the vacuolization of the acinar cells of the pancreatic and salivary glands of Gnptab −/− mice (or MLII mice) seen by light microscopy represents the accumulation of numerous, large autolysosomes, involved in crino-, micro-, and macroautophagy. Interestingly, enzymatic assays revealed that these glands have only a modest decrease in acid hydrolase content, in contrast to the major deficiency exhibited by the skin fibroblasts derived from the mutant mice, in which we also detected an accumulation of enlarged lysosomes and/or autolysosomes by electron microscopy. On the basis of our findings and the current understanding of the special role of the Man-6-P recognition system in secretory cells, we propose that the lack of Man-6-P signals on the acid hydrolases results in their entrapment within exocrine gland secretory granules as well as increased secretion and, consequently, in lysosomal dysfunction. These results suggest that hypersecretion of acid hydrolases via the constitutive secretory pathway may not be the only cause for the development of lysosomal insufficiency in mucolipidosis II mice and patients.
RESULTS
Vacuolization of the acinar cells of the salivary glands and pancreas of Gnptab −/− mice represents accumulation of autolysosomes
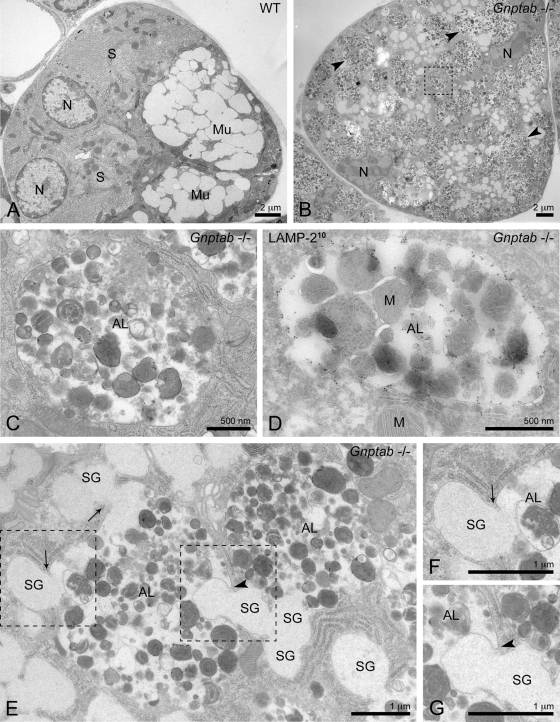
The acini of the exocrine salivary glands, which consist of mucous- and serous-type secretory cells, exhibited extensive vacuolization in Gnptab −/− mice when examined by light microscopy (Gelfman et al., 2007; Vogel et al., 2009). To gain insight into the underlying pathology, we analyzed the submandibular salivary gland of these mice by electron microscopy (EM). Both mucous- and serous-type secretory cells were readily observed in the wild-type mice (Figure 1A). By contrast, the overall architecture of the submandibular salivary gland of the Gnptab −/− mice was so grossly disrupted that it greatly hampered the identification of the serous cells in the EM sections (Figure 1B). The mucous-type secretory cells, however, could be recognized by their characteristic electron lucent secretory granules (average diameter size ∼800 nm). These cells were packed with large (up to ∼4 μm in diameter) membrane-bound vacuoles that contained heterogeneous material (Figure 1B arrowheads, higher magnification in Figure 1C), which accounted for the extensive vacuolization observed by light microscopy. Detailed morphological analysis of these vacuoles in Gnptab −/− mucous cells showed that they often contained material reminiscent of undigested cytoplasmic organelles, particularly mitochondria (Figure 1D), suggesting a defect in the autophagic pathway.
FIGURE 1:
Autolysosomes accumulate in the submandibular salivary gland of Gnptab −/− mice. (A) Electron micrograph showing a low-magnification overview of a wild-type (WT) mouse submandibular salivary gland acinus, composed of mucous and serous cells. (B) Overview of a Gnptab −/− submandibular salivary gland acinus. The overall architecture is disrupted by the massive accumulation of large vacuoles with diameter sizes up to ∼4 μm (arrowheads). Mucous-type secretory cells are recognized by the presence of the electron lucent secretory granules (average diameter size ∼800 nm). (C) Higher magnification of a vacuole from (B), which is surrounded by a single membrane and contains undegraded material. Area of magnification is indicated by the box in (B). (D, E) Further characterization of the vacuoles that accumulate in the Gnptab −/− submandibular salivary gland shows the presence of the lysosomal membrane protein LAMP-2 in their limiting membrane by immunogold labeling (D, 10-nm gold particles), mitochondria inside their lumen (D), fusion with (E, arrows) and engulfment (E, arrowhead) of mucous secretory granules (crinophagy and microautophagy events, respectively). Taken together, these observations define these vacuoles as autolysosomes. Higher magnifications of the (F) crinophagy and (G) microautophagy events shown in (E). Areas of magnification are indicated by the boxes in (E). AL, autolysosome; M, mitochondrion; Mu, mucous cell; N, nucleus; S, serous cell; SG, secretory granule.
Macroautophagy (hereafter referred to as autophagy), which is the most prevalent type of autophagy in most tissues, starts with the sequestration of cargo destined for degradation in a large double-membrane vesicle, the autophagosome. The fusion of the autophagosome with a lysosome gives rise to an autolysosome, where degradation of the cargo takes place (Cao and Klionsky, 2007). Recent studies of the lysosomal storage disorders “multiple sulfatase deficiency” and “mucopolysaccharidosis type IIIA” in mice reported the accumulation of autophagosomes in mouse embryonic fibroblasts and macrophages, resulting from defective autophagosome–lysosome fusion (Settembre et al., 2008). We therefore asked whether a similar block in autophagy might be occurring in the Gnptab −/− submandibular gland. The large vacuoles filled with undegraded cytoplasmic material that accumulate in the Gnptab −/− mucous cells were surrounded by a single membrane (Figure 1, C and D) and, as shown in Figure 1D, LAMP-2 was readily detected by cryoimmunogold EM in their limiting membrane. These observations indicate that they represent autolysosomes formed by fusion of autophagic compartments with lysosomes. In this regard, these results differ from the accumulation of autophagosomes reported in the other lysosomal storage disorders.
Interestingly, we observed numerous fusion profiles of mucous granules with the autolysosomes, consistent with ongoing crinophagy, an autophagic process by which secretory granules directly fuse with lysosomes or autolysosomes (Figure 1, E and F, arrows) (Smith and Farquhar, 1966). We also found mucous secretory granules that were being engulfed by autolysosomes, a process described as microautophagy (Figure 1, E and G, arrowhead) (Cuervo, 2004).
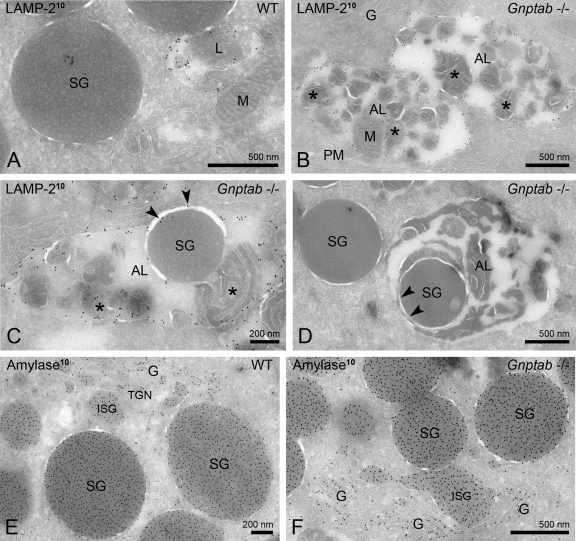
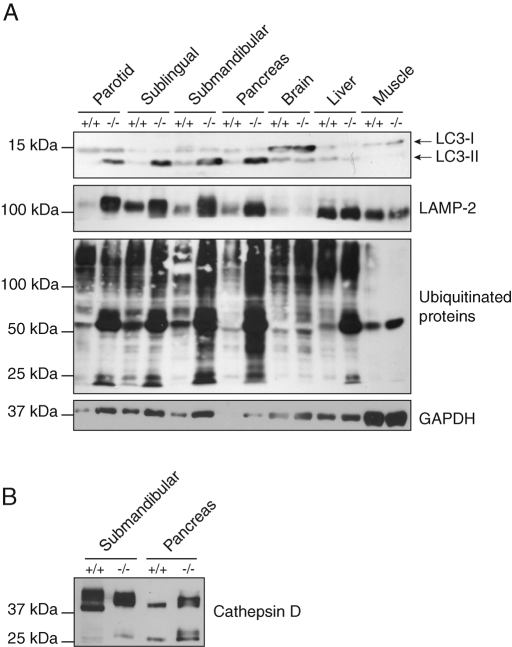
In addition to the salivary gland, the exocrine pancreas of the Gnptab −/− mice also exhibited a clear vacuolization under the light microscope (Gelfman et al., 2007; Vogel et al., 2009). EM analysis revealed that the acinar cells of this organ, which are serous-type secretory cells, were similarly affected by the loss of the Man-6-P targeting pathway. In the wild-type pancreas, LAMP-2 localized to normal sized lysosomes with a diameter of ∼300–600 nm (Figure 2A). By contrast, the Gnptab −/− serous cells exhibited an accumulation of large LAMP-2 positive compartments, which had an approximate diameter of 500 nm to 2 μm and were filled with undegraded cytoplasmic material (Figure 2, B-D). Mitochondrial remnants were regularly observed in the lumen of these compartments (Figure 2B) and undigested secretory granules were occasionally detected (Figure 2, C and D). Therefore, as in the salivary gland, these compartments could be identified as autolysosomes on the basis of their content and membrane protein composition. LAMP-2 negative autophagosomes were also occasionally observed (unpublished data). Consistent with the accumulation of LAMP-2 positive autolysosomes, the level of LAMP-2 as determined by Western blotting was markedly increased in the Gnptab −/− pancreas, as well as in the mutant parotid, sublingual, and submandibular salivary glands (Figure 3A). In contrast, the brain, liver, and muscle exhibited normal levels of LAMP-2 in the mutant mouse compared with wild-type (Figure 3A). Of note, the secretory granules in the Gnptab −/− pancreatic acinar cells appeared similar to those of the wild-type cells in shape and density (Figure 2, E and F) but were smaller in size (∼27% smaller compared with wild type).
FIGURE 2:
The acinar cells of Gnptab −/− mouse pancreas accumulate autolysosomes. (A–C) Ultrathin cryosections of wild-type and Gnptab −/− mouse exocrine pancreatic cells, immunogold labeled (10-nm gold particles) for LAMP-2. (A) Typical example of a LAMP-2 positive lysosome in wild-type (WT) cells. (B, C) Gnptab −/− pancreatic acinar cells accumulate numerous large LAMP-2 positive organelles that contain cytoplasmic material (asterisks), including (B) mitochondrial remnants and (C) secretory granules, which defines them as autolysosomes. The arrowheads in (C) show LAMP-2 labeling on the limiting membrane of the autolysosome, indicating that the secretory granule is enclosed within the autolysosome. (D) Another example of an autolysosome in Gnptab −/− pancreatic acinar cells that contains a secretory granule. The arrowheads indicate the membrane of the secretory granule, showing that the granule is inside the lumen of the autolysosome. (E, F) Immunogold labeling for amylase in (E) wild-type and (F) Gnptab −/− pancreas showing numerous secretory granules that appear similar in shape and density, albeit smaller in size in the Gnptab −/− cells. AL, autolysosome; G, Golgi apparatus; ISG, immature secretory granule; L, lysosome; M, mitochondrion; PM, plasma membrane; SG, secretory granule.
FIGURE 3:
Steady-state levels of autophagy markers and lysosomal proteins in different tissues of wild-type and Gnptab −/− mice. (A) The levels of LAMP-2, LC3-II, and ubiquitinated proteins are greatly increased in the salivary and pancreatic glands of Gnptab −/− mice. Western blotting of the autophagy marker LC3 reveals that the level of the phosphatidylethanolamine-conjugated form of the protein, LC3-II, is highly increased in the parotid, sublingual, and submandibular salivary glands, as well as in the pancreas of the Gnptab −/− mice. An increase in the level of LAMP-2 is detected in the same organs, whereas the levels of both LC3-II and LAMP-2 are unchanged in the brain, liver, and muscle of the Gnptab −/− animals compared with wild-type levels. The immunoblot detection of ubiquitin shows that the levels of ubiquitinated proteins are modestly to strongly increased in the Gnptab −/− mouse salivary and pancreatic glands, while barely changed, if at all, in the brain and muscle of these mice compared with wild-type levels. A slight elevation of the level of ubiquitinated proteins is detected in the liver of the Gnptab −/− mice. The substrate of chaperone-mediated autophagy, GAPDH, is slightly increased in the exocrine secretory glands. (B) Cathepsin D levels as determined by Western blotting are similar in the Gnptab −/− submandibular salivary gland and slightly increased in the Gnptab −/− pancreas compared with wild-type levels. The mature enzyme is detected as a 39-kDa band, and partial processing to the heavy and light chain occurs in these tissues as shown by the presence of the 27-kDa band representing the heavy chain. Note that cathepsin D runs slightly slower in the Gnptab −/− tissues, which is due to differential processing of its N-linked glycans.
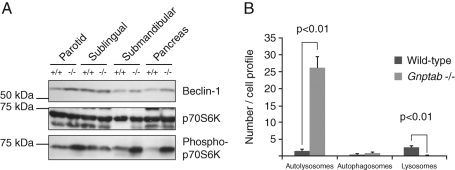
Gnptab −/− exocrine glands lack signs of a strong induction of macroautophagy
One mechanism that could lead to the accumulation of autolysosomes in the salivary and pancreatic glands of the Gnptab −/− mice would be an enhanced activation of the autophagy process that might overwhelm the lysosomal degradative capacity. To address this possibility, we determined the levels of Beclin-1 and the activity of the serine/threonine kinase mTOR (target of rapamycin) in the salivary glands and pancreas of the Gnptab −/− and wild-type mice. Beclin-1 and mTOR are key regulators in the process of macroautophagy (Pattingre et al., 2008). Increased levels of Beclin-1, which is part of a PI(3)-kinase complex, activate and up-regulate the process of autophagy through its interaction with hVps34 (Cao and Klionsky, 2007). The mTOR (kinase complex 1) is a central element in several sensing-signaling pathways that regulate cellular processes such as cell growth, nutrient import, protein synthesis, and autophagy (Pattingre et al., 2008). It has been reported that, under various stress conditions, the inhibition of mTOR results in up-regulation of autophagy (Pattingre et al., 2008; Jung et al., 2010). We analyzed the activity of mTOR in the Gnptab −/− tissues by measuring the phosphorylation level of one of its downstream targets, the ribosomal kinase S6K (p70S6K). Our analysis showed that the level of Beclin-1 was slightly elevated in the Gnptab −/− exocrine glands (with the exception of the sublingual gland), whereas the phosphorylation of p70S6K was highly increased in these same tissues compared with wild-type animals (Figure 4A). These data indicate that, while the process of autophagy may be stimulated via the Beclin-1 pathway in the mutant cells, at the same time it is being inhibited by the activation of the mTOR pathway. Taken together, these observations suggest that macroautophagy is not strongly enhanced in these tissues. In accordance with this observation, evaluation of the occurrence of autophagosomes in EM sections of Gnptab −/− and wild-type pancreas did not reveal a significant increase in the number of these organelles in the mutant mice compared with wild-type (1.1 ± 0.2 autophagosomes per Gnptab −/− cell profile compared with 0.6 ± 0.1 for wild-type, p = 0.11) (Figure 4B). In contrast, the marked increase in the number of autolysosomes in Gnptab −/− acinar cells (26.2 ± 3.1 per cell profile vs. 1.5 ± 0.6 for the wild-type cells), as well as the decrease in the number of lysosomes (0.1 ± 0.1 in Gnptab −/− vs. 2.7 ± 0.5 in wild-type), attained statistical significance (p < 0.01).
FIGURE 4:
Analysis of mTOR activity and Beclin-1 levels in wild-type and Gnptab −/− exocrine tissues. (A) Western blot showing the levels of Beclin-1 and the mTOR target p70S6K (total and phosphorylated) in wild-type and Gnptab −/− salivary glands (parotid, sublingual, and submandibular) and pancreas. Equal amounts of protein were loaded, as reflected by the equal levels of total p70S6K in each lane. Note the very slight increase in the level of Beclin-1, and the more pronounced increase in the amount of phosphorylated p70S6K, in the −/− tissues compared with wild-type levels. (B) Graph representing the quantification of the number of autolysosomes, autophagosomes, and lysosomes present detected per cell profile by EM in the pancreas of wild-type and Gnptab −/− mice. The number of autolysosomes and lysosomes are significantly increased and decreased in Gnptab −/− (p < 0.01), respectively, whereas the number of autophagosomes is not changed.
It should be noted, however, that in the Gnptab −/− mucous-type secretory cells of the submandibular gland, engulfment of secretory granules by autolysosomes and direct fusion of secretory granules with these compartments (microautophagy and crinophagy, respectively) were often observed, suggesting that these processes may be stimulated even though the degree of macroautophagy appears not to be (Figure 1, E–G).
The acinar cells of Gnptab −/− mice exhibit decreased lysosomal degradative capacity
Our EM analysis showed that the acinar cells of the mutant mice submandibular and pancreatic glands accumulate autolysosomes that are filled with autophagic substrates, that is, secretory granule and mitochondrial remnants. By Western blot, we determined that this defect was accompanied by an increase in the level of LC3-II, which was also detected in the parotid and sublingual salivary glands of the mutant mice (Figure 3A). LC3-II is the phosphatidylethanolamine conjugated form of LC3-I that is formed upon recruitment of LC3-I from the cytosol onto the autophagosomal membrane during the process of autophagosome formation. After autophagosome closure, LC3-II on the surface of the autophagosome is released back into the cytosol, whereas the luminal pool of LC3-II is normally degraded after fusion of the autophagosomes with the lysosomes (Pattingre et al., 2008). Because no obvious induction of macroautophagy was detected in the mutant glands, and because our EM analysis showed that the fusion of autophagosomes with lysosomes is not blocked in those tissues, the increase in the level of LC3-II strongly suggests a delay in its degradation within autolysosomes.
We also observed a striking increase in ubiquitinated proteins in the Gnptab −/− secretory glands compared with wild-type, especially in the mutant submandibular salivary gland and pancreas (Figure 3A). It was recently proposed that impaired progression of autophagy delays the delivery in ubiquitinated substrates to the proteasome (Korolchuk et al., 2009), which could, at least in part, account for the increase of ubiquitinated proteins detected in the Gnptab −/− glands. In addition, because autophagy is a bulk degradation process, ubiquitinated proteins present in the cytosolic portions that are engulfed within forming autophagosomes would be expected to accumulate secondary to a defect of autolysosomal degradation. Finally, the Gnptab −/− glands displayed a slight increase in GAPDH, which is a substrate of chaperone-mediated autophagy, a process by which cytosolic proteins are directly translocated to the lysosomal lumen for degradation (Aniento et al., 1993) (Figure 3A). These findings are all consistent with a defect in the degradative function of the lysosomes.
To determine whether this decrease in lysosomal degradative capacity might be accounted for by a reduction in the acid hydrolase content in lysosomes, we measured the activity of a number of lysosomal glycosidases (Table 1). All of these glycosidases are known to acquire the Man-6-P recognition marker (Sleat et al., 2008), with the exception of β-glucocerebrosidase, which is targeted to the lysosomes by a Man-6-P–independent mechanism (Reczek et al., 2007). The activities of α-mannosidase, β-galactosidase, β-mannosidase, and β-glucuronidase were modestly decreased in the pancreas of the Gnptab −/− mice (39–61% of wild-type levels) and in the submandibular salivary gland (28–89% of wild-type levels). The activity of α-galactosidase, however, was slightly elevated in both of these tissues of the mutant mice, while the activity of β-hexosaminidase was greatly increased in the pancreas (13.2-fold) but modestly decreased in the submandibular salivary gland. β-Hexosaminidase, in contrast to the other five glycosidases, has been shown to be associated with the lysosomal membrane (Yadao et al., 1997; Magini et al., 2008), as is β-glucocerebrosidase, whose activity was increased in both −/− pancreas and salivary gland (2.7- and 2.1-fold, respectively). However, it is not clear at this point that the membrane association of β-hexosaminidase is related to the increased activity in the −/− pancreas, nor is it clear how this association would affect the targeting of this hydrolase.
TABLE 1:
Activity of acid hydrolases in wild-type (WT) and Gnptab –/– tissues and fibroblasts.a
| WT | –/– | Ratio –/–/WT | |
|---|---|---|---|
| Pancreas | |||
| α-Mannosidase | 6.8 ± 2.0 | 3.9 ± 0.9* | 0.57 |
| β-Galactosidase | 1.1 ± 0.2 | 0.7 ± 0.3* | 0.61 |
| α-Galactosidase | 4.0 ± 0.7 | 6.0 ± 4.6 | 1.50 |
| β-Mannosidase | 66.6 ± 39.4 | 25.7 ± 9.6* | 0.39 |
| β-Glucuronidase | 3.9 ± 0.6 | 2.2 ± 1.2* | 0.57 |
| β-Hexosaminidase | 1.7 ± 0.5 | 22.8 ± 18.9* | 13.2 |
| β-Glucocerebrosidase | 2.4 ± 0.7 | 6.5 ± 2.9* | 2.70 |
| Submandibular salivary gland | |||
| α-Mannosidase | 2 124.5 ± 1500.9 | 589.6 ± 604.1* | 0.28 |
| β-Galactosidase | 48.8 ± 15.2 | 20.5 ± 13.7* | 0.42 |
| α-Galactosidase | 21.7 ± 4.5 | 27.9 ± 13.8 | 1.28 |
| β-Mannosidase | 34.5 ± 13.6 | 24.6 ± 12.6 | 0.71 |
| β-Glucuronidase | 79.5 ± 22.2 | 70.5 ± 24.9 | 0.89 |
| β-Hexosaminidase | 117.6 ± 44.8 | 86.5 ± 36.6 | 0.74 |
| β-Glucocerebrosidase | 6.7 ± 1.3 | 14.0 ± 6.6* | 2.08 |
| Fibroblasts | |||
| α-Mannosidase | 8.6 ± 2.8 | 1.3 ± 0.3* | 0.15 |
| β-Galactosidase | 23.7 ± 9.0 | 2.0 ± 0.7* | 0.08 |
| α-Galactosidase | ND | ND | ND |
| β-Mannosidase | 6.8 ± 2.0 | 1.1 ± 0.5* | 0.16 |
| β-Glucuronidase | 31.0 ± 10.5 | 7.8 ± 2.8* | 0.25 |
| β-Hexosaminidase | 34.9 ± 9.2 | 4.1 ± 1.7* | 0.12 |
| β-Glucocerebrosidase | 24.1 ± 8.1 | 97.2 ± 29.8* | 4.04 |
| Brain | |||
| α-Mannosidase | 5.0 ± 0.6 | 7.6 ± 1.3* | 1.52 |
| β-Galactosidase | 13.9 ± 4.4 | 8.5 ± 0.8* | 0.61 |
| α-Galactosidase | 8.2 ± 1.0 | 5.8 ± 0.4* | 0.71 |
| β-Mannosidase | 3.5 ± 0.5 | 3.7 ± 0.6 | 1.05 |
| β-Glucuronidase | 19.4 ± 2.4 | 26.4 ± 5.5* | 1.36 |
| β-Hexosaminidase | 39.2 ± 5.2 | 55.6 ± 15.1* | 1.42 |
| β-Glucocerebrosidase | 15.3 ± 6.4 | 26.4 ± 5.5* | 1.73 |
| Liver | |||
| α-Mannosidase | 14.4 ± 5.1 | 26.8 ± 7.3* | 1.86 |
| β-Galactosidase | 16.5 ± 6.9 | 25.3 ± 6.4* | 1.54 |
| α-Galactosidase | 12.6 ± 2.0 | 18.1 ± 3.8* | 1.44 |
| β-Mannosidase | 12.5 ± 2.5 | 23.1 ± 5.2* | 1.85 |
| β-Glucuronidase | 83.6 ± 20.4 | 102.4 ± 3.5 | 1.22 |
| β-Hexosaminidase | 10.7 ± 3.3 | 33.7 ± 13.6* | 3.16 |
| β-Glucocerebrosidase | 9.6 ± 3.4 | 14.2 ± 3.5* | 1.47 |
| Muscle | |||
| α-Mannosidase | 1.6 ± 0.5 | 2.9 ± 0.7* | 1.79 |
| β-Galactosidase | 1.4 ± 0.5 | 1.1 ± 0.6 | 0.79 |
| α-Galactosidase | 1.3 ± 0.3 | 1.1 ± 0.6 | 0.84 |
| β-Mannosidase | 1.2 ± 0.4 | 1.8 ± 0.5 | 1.47 |
| β-Glucuronidase | 10.2 ± 3.1 | 10.9 ± 2.8 | 1.07 |
| β-Hexosaminidase | 2.0 ± 0.7 | 2.1 ± 0.6 | 1.02 |
| β-Glucocerebrosidase | 1.6 ± 0.3 | 2.1 ± 0.5* | 1.37 |
a Activities are expressed as nmol of methylumbellyferone released per mg protein per hour.
The values are the average ± SD, with n ≥ 6.
*p < 0.01; ND, not determined.
We next determined the steady-state level of the protease cathepsin D in wild-type and Gnptab −/− submandibular salivary gland and pancreas by Western blotting. As shown in Figure 3B, the level of cathepsin D was similar to wild-type or even slightly increased in these Gnptab −/− tissues. In both tissues the major band was detected at ∼39 kDa, which corresponds to the mature form of the enzyme. Some of the mature cathepsin D was processed into the heavy and light chains, as shown by the presence of the ∼27-kDa heavy chain band. Cathepsin D had a slightly higher molecular weight in Gnptab −/− tissues, reflecting the differential processing of its N-linked glycans, which occurs in the absence of phosphorylation of high-mannose–type sugar chains (Gelfman et al., 2007).
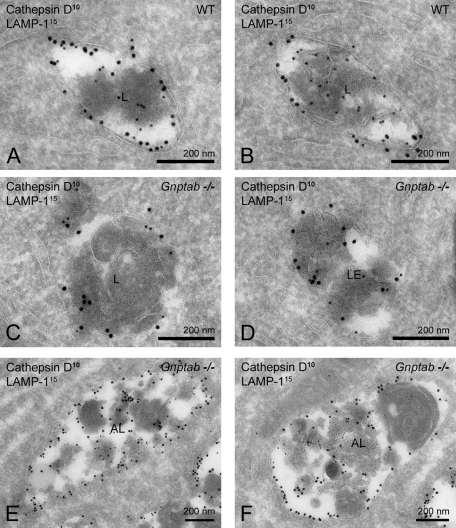
The intracellular localization of cathepsin D in wild-type and Gnptab −/− pancreatic acinar cells was then evaluated by immuno-EM. Cathepsin D was readily detected in LAMP-1 positive late endosomes and lysosomes of cells of both types of mice (Figure 5, A–D) and in the autolysosomes of the Gnptab −/− acinar cells (Figure 5, E and F). By quantification, the endolysosomes of wild-type cells contained 11.3 ± 11.6 gold particles per compartment, whereas lower values (5.3 ± 4.6 gold particles per compartment) were measured in the Gnptab −/− endolysosomes (p = 0.012). Moreover, we observed that the average surface area of the individual late-endosomal and lysosomal compartments in Gnptab −/− cells was 1.4× larger compared with wild-type levels. Consequently, the density of cathepsin D labeling in the late endosomes and lysosomes of the Gnptab −/− pancreatic acinar cells was reduced to 37% of the labeling density in wild-type cells (53.3 ± 50.1 vs. 144.5 ± 103.8 gold particles per μm2, respectively, p = 7.35 × 10−5), indicating decreased lysosomal targeting of cathepsin D in these cells.
FIGURE 5:
Lysosomes of Gnptab −/− pancreatic acinar cells contain reduced levels of cathepsin D. (A–F) Ultrathin cryosections of (A, B) wild-type and (C–F) Gnptab −/− pancreatic acinar cells immunogold labeled for cathepsin D (10-nm gold particles) and LAMP-1 (15 nm). (C, D) The endolysosomes in Gnptab −/− cells contain less cathepsin D compared with (A, B) wild-type cells. (E, F) Autolysosomes in Gnptab −/− pancreatic acinar cells contain low levels of cathepsin D. AL, autolysosome; L, lysosome; LE, late endosome.
The autolysosomes of the Gnptab −/− acinar cells contained low levels of cathepsin D labeling (Figure 5, E and F), consistent with dilution of the protease following fusion of the lysosomes with the autophagosomes. Because the total volume of autolysosomes in the Gnptab −/− acinar cells is so much larger than that of the lysosomes, a significant proportion of the total intracellular level of cathepsin D likely localizes to these compartments.
Loss of the Man-6-P signal does not affect tissues equally
Interestingly, in contrast to the exocrine glands of the Gnptab −/− mice, the liver, brain, and muscle of these mice contained normal levels of LAMP-2 and LC3-II (Figure 3A) and did not exhibit generalized vacuolization when examined by light or electron microscopy. In addition, the level of ubiquitinated proteins and GAPDH was unchanged in the muscle and brain of the Gnptab −/− mice compared with wild-type levels. Thus, at this level of analysis, the loss of the Man-6-P recognition system does not appear to impair the functioning of the autophagy or lysosomal system in these tissues. The liver of the mutant mice did exhibit a modest increase in the level of ubiquitinated proteins, although there was no accumulation of autolysosomes detected in this organ by EM (Supplemental Figure 1, A–D), and the levels of LC3-II and LAMP-2 were normal.
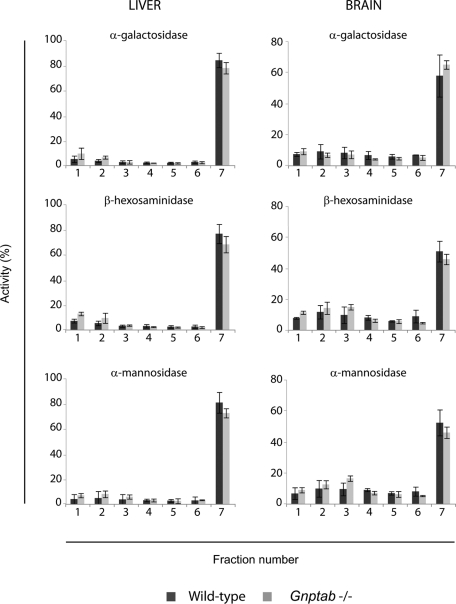
Direct enzyme assays revealed that the levels of the acid hydrolases in the brain and muscle of the Gnptab −/− mice were normal or even somewhat elevated compared with wild-type levels (1.0–1.8-fold) with the exception of β-galactosidase and α-galactosidase, whose activities were modestly decreased in the −/− brain by 39 and 29%, respectively (Table 1). The liver of the Gnptab −/− mice exhibited an increase in the activity of all the acid hydrolases measured (1.2–3.1-fold). To determine whether the acid hydrolases were actually in lysosomes, membrane fractions of brain and liver (from wild-type or Gnptab −/− mice) were fractionated on 18% self-forming Percoll density gradients as described previously (Boonen et al., 2009), a technique that allows concentration of lysosomes in the densest fraction of the gradient (Figure 6), while the other organelles remain in the lighter fractions (Supplemental Figure 2). The analysis of the distribution of three different acid hydrolases (β-hexosaminidase, α-galactosidase, and α-mannosidase) revealed that a similar proportion of acid hydrolases localized to the dense-lysosome fraction in both organs, indicating that the lysosomes of Gnptab −/− brain and liver receive normal levels of acid hydrolases (Figure 6).
FIGURE 6:
The subcellular distribution of acid hydrolases in the brain and liver of wild-type and Gnptab −/− mice is similar in a self-forming Percoll density gradient. Wild-type or Gnptab −/− liver and brain were homogenized in isotonic sucrose with a Potter-Elvehjem homogenizer and centrifuged at 1000 g to prepare a postnuclear supernatant (PNS). The PNS was subjected to centrifugation at 35,000 rpm in a 50Ti rotor for 40 min at 4ºC to obtain a membrane fraction which was resuspended in isotonic sucrose and loaded on top of an 18% Percoll solution. Self-forming gradients were generated by centrifugation at 25,000 rpm in a SW55Ti rotor for 40 min at 4ºC. Seven fractions were collected from the top (low density) to the bottom (densest fraction) and acid hydrolase content was determined by enzyme assays. Graphs represent the distribution of α-galactosidase, β-hexosaminidase, and α-mannosidase in wild-type (n = 3) or Gnptab −/− (n = 3) liver and brain, expressed as a percentage of the total activity recovered in each fraction.
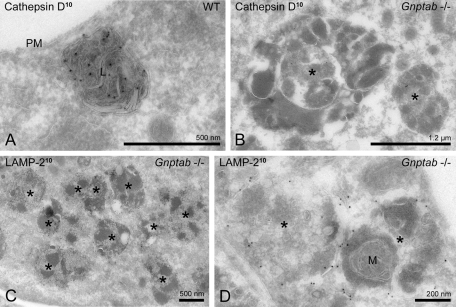
Skin fibroblasts of Gnptab −/− mice accumulate lysosomes and/or autolysosomes deficient in acid hydrolases
Because the presence of “inclusions” in fibroblasts of mucolipidosis type II patients is the hallmark of the disorder (also called I-cell disease for inclusion-cell disease), we were surprised that we did not detect morphological alterations in the fibrocytes and fibroblasts examined during the histologic survey of the Gnptab −/− mouse tissues (Vogel et al., 2009). Subsequently, we became aware of a 1975 publication by Aula et al. reporting that fibroblasts in tissues from a human mucolipidosis II fetus had detectable inclusions when the samples were embedded in epoxy, but not in paraffin, the method used for our prior histologic analysis (Aula et al., 1975). Therefore we isolated skin fibroblasts from the Gnptab −/− mice and examined them by immuno-EM. Figure 7A shows strong labeling of the lysosomal protease cathepsin D in skin fibroblasts of wild-type mice. Lysosomes with a diameter of 400–600 nm were detected, containing lamellar material as well as small internal membrane vesicles. By contrast, the fibroblasts derived from the Gnptab −/− mice had increased numbers of lysosomes that contained very low levels of cathepsin D (Figure 7B) but were positive for the lysosomal membrane protein LAMP-2 (Figure 7, C and D). This deficiency of cathepsin D reflects the known hypersecretion of this protease by this cell type (Lee et al., 2007). The lysosomes were enlarged (up to ∼1.5 μm in diameter), filled with dense amorphous material, and occupied much of the cytoplasm (Figure 7C). Mitochondrial remnants were occasionally detected within these compartments, indicating that they had received material via the autophagic pathway and had become autolysosomes (Figure 7D). The difference in size and composition of these enlarged lysosomes and autolysosomes (∼1.5 μm, mainly amorphous content) compared with their counterparts in the exocrine tissues (up to 4 μm with numerous organelle remnants) might explain why the latter gave rise to vacuolization that is readily detectable in paraffin sections while the other did not.
FIGURE 7:
Gnptab −/− mouse skin fibroblasts accumulate enlarged lysosomes and/or autolysosomes. (A) Electron micrograph of a typical lysosome in wild-type mouse skin fibroblasts immunogold labeled (10-nm gold particles) for cathepsin D. (B–D) Electron micrographs showing the accumulation of enlarged lysosomes and/or autolysosomes (asterisks) in Gnptab −/− mouse skin fibroblasts, recognized by their dense heterogeneous content, the occasional presence of (D) mitochondrial remnants, and (C, D) the detection of LAMP-2 in their membrane. (B) The enlarged lysosomes and autolysosomes contain low levels of cathepsin D. M, mitochondrion; PM, plasma membrane; *, swollen lysosome or autolysosome.
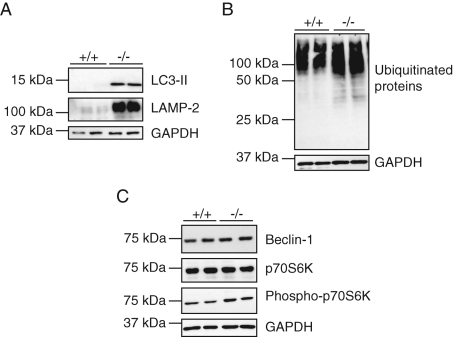
Measurements of the activity of five lysosomal hydrolases revealed extremely low levels in the Gnptab −/− fibroblasts (8–25% of wild-type levels), whereas the activity of β-glucocerebrosidase was increased fourfold over wild-type levels (Table 1). The Gnptab −/− fibroblasts also showed increased levels of LAMP-2 and LC3-II, as well as a slight increase in ubiquitinated proteins compared with wild-type fibroblasts (Figure 8, A and B). No difference in the level of GAPDH was detected, suggesting that the process of chaperone-mediated autophagy was not impaired in those cells. As in the exocrine glands, the level of phosphorylation of the mTOR target p70S6K was elevated in the Gnptab −/− fibroblasts compared with wild-type levels, indicating inhibition rather than activation of the macroautophagy pathway (Figure 8C). The cellular level of Beclin-1 was unchanged (Figure 8C).
FIGURE 8:
The level of LC3-II is elevated in Gnptab −/− mouse skin fibroblasts, while stimulation of macroautophagy via the Beclin-1 or mTOR pathway is not detected. (A, B) Immunoblots showing the increased levels of (A) LC3-II and LAMP-2 and (B) ubiquitinated proteins in Gnptab −/− mouse skin fibroblasts over wild-type levels. (Two independent cell lysates are shown for each genotype, indicated as +/+ and −/− for wild-type and Gnptab −/− mice, respectively.) (C) Detection by Western blotting of Beclin-1, whose levels are unchanged between +/+ and −/− cells, and of the mTOR target p70S6K, which is slightly more phosphorylated in the −/− fibroblasts compared with wild-type cells. (A–C) The level of GAPDH is similar in +/+ and −/− cells.
These findings show that the Gnptab −/− fibroblasts accumulate enlarged lysosomes and/or autolysosomes, most likely secondary to the severe depletion of acid hydrolases that impairs the degradative function of the lysosomes and autolysosomes. A similar decrease in acid hydrolase content was reported in fibroblasts isolated from mucolipidosis type II patients and from a Gnptab knock-in mouse model of mucolipidosis type II (Kornfeld and Sly, 2000; Kollmann et al., 2010). Moreover, Otomo et al. recently showed that fibroblasts of patients with mucolipidosis II accumulate autolysosomes that can account for the inclusions initially observed by light microscopy (Leroy and Demars, 1967; Otomo et al., 2009).
DISCUSSION
We previously reported that acinar cells of the exocrine glands of Gnptab −/− (MLII) mice exhibit extensive vacuolization when examined by light microscopy (Gelfman et al., 2007; Vogel et al., 2009). These morphological abnormalities are similar to those described in the exocrine pancreas, salivary glands, and gastric chief cells of several MLII patients (Elleder and Martin, 1998). On the basis of our analysis of the acinar cells of the salivary and pancreatic glands of Gnptab −/− mice, we now demonstrate that the vacuolization represents the accumulation of autolysosomes in the mucous and serous cells of these organs. Our data show that these autolysosomes are defective in degradative capacity, accounting for their striking accumulation. Yet the involved exocrine glands retain substantial amounts of acid hydrolases despite the loss of the Man-6-P signal. This finding is in contrast to fibroblasts which have similar morphological alterations but extremely low levels of acid hydrolases due to massive hypersecretion of these enzymes.
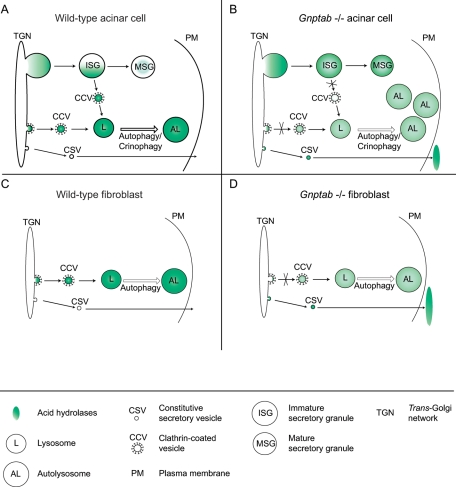
These observations can be explained by considering the unique role of the Man-6-P sorting pathway in regulated secretory cells compared with other cell types (i.e., the fibroblasts). In fibroblasts, the newly synthesized acid hydrolases are packaged at the TGN into clathrin-coated carriers by a Man-6-P–dependent mechanism and transported to the lysosomes (Figure 9C). When the synthesis of the Man-6-P signal is defective, as occurs in Gnptab −/− mice, the acid hydrolases are hypersecreted via the constitutive secretory pathway, resulting in very low cellular levels of the hydrolases (Figure 9D) (Lee et al., 2007). In the regulated secretory cells (Figure 9A), a portion of the newly synthesized acid hydrolases are similarly packaged into clathrin-coated carriers at the TGN and transported to lysosomes. However, studies of pancreatic beta-cells have shown that the majority of the hydrolases escape entry into the clathrin-coated carriers and instead enter immature secretory granules (ISGs) that bud from the TGN (Kuliawat and Arvan, 1994). During granule maturation, the Man-6-P–containing acid hydrolases are then removed by a mechanism involving binding to Man-6-P receptors (MPRs) and incorporation into AP-1 and syntaxin-6-positive clathrin-coated vesicles (CCVs) that bud from the ISGs and transport their cargo to endolysosomes (Tooze and Tooze, 1986; Kuliawat et al., 1997; Klumperman et al., 1998; Dittié et al., 1999). This process provides an “exit site” for acid hydrolases that enter the regulated secretory pathway, allowing retrieval to lysosomes. The inability to synthesize the Man-6-P marker in the Gnptab −/− mice would have several consequences in these cells (Figure 9B). At the TGN, the newly synthesized acid hydrolases would be poorly sorted into CCVs due to their inability to bind MPRs, which would result in increased secretion via the constitutive secretory pathway and even greater packaging of the hydrolases into ISGs. Most important, once the hydrolases enter the ISGs, they would not be retrieved by the Man-6-P–dependent system. This hypothesis is supported by the observations of Kuliawat et al., who reported that the loss of one of the two MPRs in mice, the cation-dependent MPR, resulted in a nearly fourfold increase in the presence of the precursor form of cathepsin B in the regulated secretory pathway of pancreatic beta-cells (Kuliawat et al., 1997). The lack of binding of acid hydrolases to both of the MPRs in Gnptab −/− mice exocrine cells would be expected to impair the sorting and retrieval of the acid hydrolases to lysosomes even more. As a result, their lysosomes would be deficient in acid hydrolases and unable to efficiently degrade material derived from the endocytic and autophagic pathways. The finding that the cathepsin D content of the pancreatic acinar cell lysosomes of Gnptab −/− mice is decreased to 37% of the wild-type levels whereas the total cellular content of this protease is normal or even increased is consistent with this model.
FIGURE 9:
Model for the consequences of the lack of the Man-6-P signal on the trafficking of acid hydrolases in fibroblasts and secretory cell types (salivary and pancreatic gland acinar cells). (A) In secretory cell types, acid hydrolases can reach the lysosomes by two pathways: direct sorting from the TGN, or retrieval from the ISGs that had incorporated a portion of the newly synthesized hydrolases while budding off the TGN. Both processes involve packaging of the hydrolases in CCVs by a Man-6-P–dependent mechanism. (B) In the absence of the Man-6-P signal (Gnptab −/− cells), the hydrolases either undergo constitutive secretion or are trapped within mature secretory granules due to the lack of retrieval from the ISGs. As a result, lysosomes (L) have low levels of acid hydrolases. The accumulation of autolysosomes (AL) in the salivary glands and pancreas of the Gnptab −/− mice is the consequence of the resulting defective lysosomal degradative capacity. (C) In normal fibroblasts, acid hydrolases are packaged into clathrin-coated transport carriers at the TGN and targeted to lysosomes (L) in a Man-6-P–dependent manner. (D) The loss of the Man-6-P signal in Gnptab −/− fibroblasts, and the subsequent lack of recognition of the hydrolases at the TGN, results in their hypersecretion via the constitutive secretory pathway. Consequently, lysosomes (L) and autolysosomes (AL) with low acid hydrolase content and poor degradative capacity accumulate.
In addition, the modest decrease in the intracellular levels of several other acid hydrolases suggests their increased secretion by the Gnptab −/− acinar cells, either via the constitutive secretory pathway and/or as the result of secretory granule fusion with the plasma membrane followed by release of their contents. It should be noted that the extent of this secretion could be underestimated in our assay. The steady-state level of the acid hydrolases in the acinar cells is determined by the rate of synthesis, the extent of secretion, and their half-life. Sardiello et al. reported that acid hydrolase synthesis is up-regulated in fibroblasts from patients with several forms of lysosomal storage diseases (Sardiello et al., 2009). If acid hydrolase synthesis is increased in the acinar cells of the Gnptab −/− mice, this could help maintain the intracellular levels of some acid hydrolases in the face of significant hypersecretion of these enzymes. Nevertheless the issue of entrapment in secretory granules would remain. Finally, because we only assayed for a limited number of acid hydrolases, we cannot exclude the possibility, although unlikely, that one particular acid hydrolase (out of the total 60 enzymes that reside in lysosomes) might be dramatically reduced in the lysosomes, leading to the impaired degradative capacity of these compartments.
Unfortunately we were unable to formally demonstrate the missorting of the acid hydrolases into mature secretory granules of the mutant cells. By immuno-EM using cathepsin D antibodies, we only observed background labeling of the secretory granules of wild-type and mutant pancreatic acinar cells. In addition, homogenization and subcellular fractionation of the Gnptab −/− pancreas resulted in extensive autolysosome disruption, which made it impossible to determine whether the secretory granules in the mutant pancreas contained increased amounts of acid hydrolases. Meister et al. recently reported this same problem with pancreatic tissue of cation-independent MPR–deficient mice (Meister et al., 2010). Similar to our work, these authors described the formation of large cytoplasmic vacuoles in the pancreas of those mice, although much less severe than in the Gnptab −/− mice (Gelfman et al., 2007).
We did not detect convincing evidence for macroautophagy activation in the exocrine glands of the Gnptab −/− mice (3–4-mo-old mice), based on evaluation of Beclin-1 levels and activity of mTOR. However, we cannot rule out the possibility that stimulation of macroautophagy may have contributed to pathological development at an earlier stage of the process. In addition, the numerous profiles of ongoing crinophagy and microautophagy observed in the mutant salivary glands raise the possibility that these processes may be stimulated, perhaps representing an attempt to dispose of secretory granules that have been damaged by their content of acid hydrolases. This activation could contribute to the accumulation of autolysosomes. In the Gnptab −/− pancreas, the accumulation of autolysosomes was accompanied by a decrease in the number of lysosomes. These data are consistent with the recent report that inhibition of autolysosomal protein degradation with protease inhibitors abrogates lysosome reformation, leading to the accumulation of enlarged and long-lived autolysosomes (Yu et al., 2010). The accumulation of autolysosomes in our MLII mouse also points out an important difference compared with other lysosomal storage disorders with autophagy defects, including multiple sulfatase deficiency and mucopolysaccharidosis IIIA. Studies of mouse models of these disorders revealed that the affected cells exhibit an accumulation of autophagosomes, rather than autolysosomes, resulting from a defect in the fusion of the autophagosomes with lysosomes (Settembre et al., 2008; Fraldi et al., 2010). By contrast, the defect in the Gnptab −/− mice appears to be the failure to dispose of the engulfed material by lysosomes and autolysosomes deficient for critical lysosomal hydrolases.
Our data support previous reports that the requirement for the Man-6-P–dependent sorting pathway varies substantially among cell types. At one extreme, fibroblasts (cells of mesenchymal origin) are highly dependent on the Man-6-P pathway for efficient acid hydrolase sorting and lysosomal function. Our findings now demonstrate that the lysosomal function of the exocrine secretory cells is also highly dependent on the Man-6-P pathway. It is of note that exocrine secretory cells and organs, including the pancreas, appear to have a relatively high basal level of autophagy compared with other tissues (Mizushima et al., 2004). This might explain why the autolysosomes detected in the exocrine cells of the Gnptab −/− mice were generally more prevalent than observed in fibroblasts and often filled with easily recognizable remnants of material derived from macro-, micro-, or crinophagy. At the other extreme, the liver, brain, and muscle of the Gnptab −/− mice have normal or even elevated levels of acid hydrolases that are correctly localized within lysosomes, despite the loss of the Man-6-P signal, and do not exhibit any obvious morphological alterations. These findings are in agreement with those previously reported for postmortem samples obtained from patients with MLII (Kenyon et al., 1973; Martin et al., 1975, 1984; Nagashima et al., 1977; Owada and Neufeld, 1982; Waheed et al., 1982). The liver, which is known to have a high endocytosis capacity, had an increased level of all the measured acid hydrolases, which could reflect their capture from the plasma where the levels are extremely elevated in the mutant mice (Gelfman et al., 2007). However, while endocytosis might contribute to the normal or elevated levels of acid hydrolases in the brain and muscle, a more likely explanation is that these tissues utilize an intracellular Man-6-P–independent targeting pathway(s). This is consistent with previous studies demonstrating the existence of a Man-6-P–independent intracellular sorting pathway for cathepsin D in MLII lymphoblasts and thymocytes isolated from mice deficient in both Man-6-P receptors (Glickman and Kornfeld, 1993; Glickman et al., 1996; Dittmer et al., 1999). A major goal of future work will be to identify the molecular basis of the Man-6-P–independent pathway(s).
MATERIALS AND METHODS
Materials
Chemicals, including fluorogenic substrates for acid hydrolase enzyme assays, were obtained from Sigma Aldrich (St. Louis, MO) unless otherwise specified. Mouse anti-ubiquitin antibody and rabbit anti–Beclin-1, p70S6K, and phospho-p70S6K (Thr389) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Rabbit anti-LC3 and mouse anti-GAPDH antibodies were obtained from Novus Biologicals (Littleton, CO) and Sigma Aldrich, respectively, and both rat anti–LAMP-1 and rat anti–LAMP-2 antibodies from Developmental Studies Hybridoma Bank (Iowa City, IA). Goat anti-amylase antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and rabbit anti-amylase (AMY2B) from Proteintech Europe (Manchester, UK). The rabbit anti–cathepsin D used for Western blots was produced in our laboratory. Cathepsin D was detected in immuno-EM with a rabbit anti–cathepsin D antibody that was a generous gift from M. Koike (Juntendo University Graduate School of Medicine, Tokyo, Japan). Horseradish peroxidase (HRP)–coupled secondary antibodies were purchased from GE Healthcare (Piscataway, NJ). For immuno-EM, rabbit anti–rat immunoglobulin (Ig)G polyclonal antibody (DAKO, Heverlee, Belgium) was used as a bridging step between rat monoclonal antibody (mAb) and protein A-gold (Cell Microscopy Center, UMC Utrecht, the Netherlands). Cellgro Alpha-Minimum Essential Media and penicillin/streptomycin were obtained from Fisher Scientific (Pittsburgh, PA) and fetal calf serum (FCS) from ISC BioExpress (Kaysville, UT). The Gnptab −/− mice were described previously (Gelfman et al., 2007).
Enzyme assays
Mouse skin fibroblasts were isolated from wild-type and Gnptab −/− mouse ears and maintained in α-MEM medium containing 20% FCS, 100 μg/ml penicillin, and 100 U/ml streptomycin. Organs were collected from 3–4-mo-old mice after perfusion through the heart with phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.5). Tissues or confluent cells in culture were suspended in PBS containing 1% Triton X-100 and a mixture of protease inhibitors (complete mini protease inhibitor tablets; Roche, Penzberg, Germany) for 15 min on ice and then sonicated and centrifuged for 15 min at 13,000 g. Lysosomal hydrolase activities were determined in the supernatants by fluorometric enzyme assays as described (Lee et al., 2007). Briefly, the samples were incubated with either 1 mM (tissue samples) or 5 mM (cultured fibroblasts lysates) of 4-methylumbelliferyl–coupled specific substrates in a 50-mM citrate buffer containing 0.5% Triton X-100 (pH 4.5) at 37ºC, except for the β-glucocerebrosidase assay that was performed in a buffer containing 0.1 M sodium citrate, 0.2 M Na2HPO4, 0.2% TX-100, and 0.25% sodium taurocholate, pH 5.2. Reactions were stopped by addition of 0.1 M glycine-NaOH solution (pH 10.3) and the fluorescence was read at 495 nm. Activities were expressed as nanomoles of hydrolyzed methylumbelliferone per milligram protein and per hour.
Percoll density gradients
Wild-type and Gnptab −/− mice that were 2–3 mo of age were anesthetized and subsequently perfused with PBS to remove blood from their organs. The brains and livers of these mice were removed and fractionated on an 18% self-forming Percoll density gradient as described previously (Boonen et al., 2009). Briefly, a membrane fraction was prepared, loaded on top of an 18% Percoll solution (18% [vol/vol] Percoll [Pharmacia, Uppsala, Sweden], 0.25 M sucrose, 2 mM EDTA, and 10 mM Tris/HCl [pH 7.4]) and centrifuged at 25,000 rpm in a Beckman SW55Ti rotor for 40 min at 4ºC. Seven fractions were collected from the top of the gradient. The distribution of three different lysosomal hydrolases was determined by enzyme assays as described earlier.
Western blotting
Identical amounts of wild-type or Gnptab −/− protein samples (lysed in PBS containing 1% Triton X-100 and protease inhibitors as described earlier) were resolved on polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA), which then were blocked in 10% fat-free milk in PBS/Tween 0.1% for 30 min at room temperature (RT) prior to incubation overnight at 4ºC with primary antibodies diluted in PBS/Tween 0.1% containing 0.2% fat-free milk. After washing with PBS/Tween 0.1%, blots were incubated for 45 min at RT with HRP-coupled secondary antibodies diluted in PBS/Tween 0.1% containing 0.2% fat-free milk and washed. Immunoreactive bands were revealed by chemiluminescence (ECL Amersham, Piscataway, NJ).
EM
Wild-type and Gnptab −/− mice at 1.5–2 mo of age were sedated and perfused via the heart with either 2% wt/vol paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) pH 7.4 or 2% wt/vol PFA, 0.2% wt/vol glutaraldehyde (GA, from Polysciences Europe GmbH, Eppelheim, Germany) in 0.1 M PB, pH 7.4. Following the perfusion fixation with 2% PFA, the tissues were removed and immediately immersed in 4% wt/vol PFA in 0.1 M PB and kept overnight at 4°C. In case of the perfusion fixation with 2% PFA, 0.2% GA, the tissues of interest were divided into two halves and either immersed in the same fixative, kept overnight at 4°C, and processed for cryosectioning or immersed in 2% wt/vol PFA, 2.5% wt/vol GA in Na-cacodylate buffer (Karnovsky fixative) for 2 h at RT. Subsequently, the Karnovsky fixative was replaced for 0.1 M Na-cacodylate buffer, pH 7.4. Prior to Epon embedding, the tissues were postfixed with 1% wt/vol OsO4, 1.5% wt/vol K3Fe(III)(CN)6 in 0.065 M Na-cacodylate for 2 h at 4°C. Subsequently the tissues were stained with 0.5% uranyl acetate for 1 h at 4°C, dehydrated with ethanol, and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate.
For cryosectioning and immuno-EM, the fixed tissues were further processed as described (Slot and Geuze, 2007). In short, the tissues were cut into small blocks, rinsed with PBS and PBS containing 0.02 M glycine, and embedded in 12% gelatin. The blocks were incubated overnight in 2.3 M sucrose at 4°C, mounted on metal pins, and frozen in liquid nitrogen. Ultrathin cryosectioning was performed at −120°C in a Leica UCT-FCS ultracryomicrotome (Leica Microsystems, Vienna, Austria). The sections were transferred to copper grids by pickup with a 1:1 mixture of 2.3 M sucrose in PBS and 2% wt/vol methyl cellulose. For immunogold labeling, the grids were incubated first on 2% gelatin/PBS at 37°C, blocked with 0.02 M glycine/PBS and 1% bovine serum albumin/PBS, and subsequently incubated with primary antibodies followed by protein A-gold. The sections were contrasted with 2% uranyl acetate/oxalate, pH 7, followed by 0.4% uranyl acetate pH 4 and 1.8% methyl cellulose (Slot and Geuze, 2007).
Mouse skin fibroblasts were fixed by addition of an equal volume of 4% wt/vol PFA in 0.1 M PB to the culture medium. After ∼10 min, the fixative was replaced for 4% wt/vol PFA in 0.1 M PB for at least 2 h at RT and the cells were processed for cryosectioning and immunogold labeling.
All sections were analyzed in a JEOL 1200 EX (Tokyo, Japan) electron microscope at 60 or 80 kV for Epon or cryosections, respectively. Quantification of the number of autophagosomes, autolysosomes, and lysosomes was performed in ultrathin cryosections of wild-type and Gnptab −/− pancreas labeled for LAMP-2. Values are average numbers per cell profile ± SE of the mean. Autophagosomes were defined as compartments containing cytoplasmic material but that were devoid of LAMP-2 labeling. Autolysosomes were distinguished by the presence of undegraded cytoplasmic material and the presence of LAMP-2 on their limiting membrane, whereas lysosomes were defined as LAMP-2–positive compartments with homogeneous content that occasionally contained visible internal membrane sheets. In total, 15 cell profiles from two grids of wild-type or Gnptab −/− pancreatic acinar cells were assessed for the presence of lysosomes and autolysosomes and 30 cell profiles for the presence of autophagosomes. Quantification of the cathepsin D labeling in late endosomes and lysosomes of wild-type and Gnptab −/− pancreatic acinar cells was performed by random screening of LAMP-1 and cathepsin D double-labeled sections. Late endosomes and lysosomes were defined as LAMP-1–positive compartments with a diameter size >200 nm that were devoid of undegraded autophagic material. The number of gold particles representing cathepsin D was scored per compartment. In total, 29 endolysosomal compartments were assessed in both wild-type and Gnptab −/− cells, out of which the average cathepsin D labeling level was determined; ± values are standard deviations. The surface of the late endosomes and lysosomes was measured with a square lattice overlaying the electron micrographs by counting the number of points within the compartment (point hit method, Griffiths, 1993). The mesh size of the square lattice was adjusted to the magnification of the electron micrographs: 7.5, 10.28, and 12.45 mm for magnifications of 30,000×, 40,000×, and 49,000×, respectively. The labeling density of cathepsin D was calculated by dividing the number of gold particles for cathepsin D by the product of the number of points and the area associated with one point (0.0088 μm2). In this way the numerical density of gold over an area was obtained in gold particles per μm2 (Griffiths, 1993). The size of the secretory granules in wild-type and Gnptab −/− pancreatic acinar cells was determined by using the same point hit method. In total, 165 and 211 secretory granules were evaluated in the wild-type and Gnptab −/−, respectively. P values were determined by performing a t test.
Supplementary Material
Acknowledgments
We thank all our colleagues for their advice, as well as Marc van Peski and René Scriwanek for their photographical expertise and Wang-Sik Lee for his help with the cathepsin D Western blotting. E.v.M. is supported by VICI grant 918.56.611 of the Netherlands Organization for Scientific Research (NWO), awarded to J.K. M.B. and S.K. are supported by NIH grant CA-008759–44.
Abbreviations used:
- CCV
clathrin-coated vesicle
- EM
electron microscopy
- FCS
fetal calf serum
- GA
glutaraldehyde
- GlcNAc-1-phosphotransferase
UDP-GlcNAc:lysosomal enzyme GlcNAc-1-phosphotransferase
- Gnptab
gene encoding the alpha/beta subunits of GlcNAc-1-phosphotransferase
- HRP
horseradish peroxidase
- ISG
immature secretory granule
- Man-6-P
mannose 6-phosphate
- MLII
mucolipidosis type II
- MPR
mannose 6-phosphate receptor
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PNS
postnuclear supernatant
- RT
room temperature
- TGN
trans-Golgi network
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-07-0584) on February 16, 2011.
REFERENCES
- Aniento F, Roche E, Cuervo AM, Knecht E. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J Biol Chem. 1993;268:10463–10470. [PubMed] [Google Scholar]
- Aula P, Rapola J, Autio S, Raivio K, Karjalainen O. Prenatal diagnosis and fetal pathology of I-cell disease (mucolipidosis type II) J Pediatr. 1975;87:221–226. doi: 10.1016/s0022-3476(75)80583-x. [DOI] [PubMed] [Google Scholar]
- Boonen M, Vogel P, Platt KA, Dahms N, Kornfeld S. Mice lacking mannose 6-phosphate uncovering enzyme activity have a milder phenotype than mice deficient for N-acetylglucosamine-1-phosphotransferase activity. Mol Biol Cell. 2009;20:4381–4389. doi: 10.1091/mbc.E09-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- Cathey SS, Leroy JG, Wood T, Eaves K, Simensen RJ, Kudo M, Stevenson RE, Friez MJ. Phenotype and genotype in mucolipidosis II and III alpha/beta: a study of 61 probands. J Med Genet. 2010;47:38–48. doi: 10.1136/jmg.2009.067736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy: many paths to the same end. Mol Cell Biochem. 2004;263:55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- Dittié AS, Klumperman J, Tooze SA. Differential distribution of mannose 6-phosphate receptors and furin in immature secretory granules. J Cell Sci. 1999;112:3955–3966. doi: 10.1242/jcs.112.22.3955. [DOI] [PubMed] [Google Scholar]
- Dittmer F, Ulbrich EJ, Hafner A, Schmahl W, Meister T, Pohlmann R, von Figura K. Alternative mechanisms for trafficking of lysosomal enzymes in mannose 6-phosphate receptor-deficient mice are cell type-specific. J Cell Sci. 1999;112:1591–1597. doi: 10.1242/jcs.112.10.1591. [DOI] [PubMed] [Google Scholar]
- Elleder M, Martin JJ. Mucolipidosis type II with evidence of a novel storage site. Virchows Arch. 1998;433:575–578. doi: 10.1007/s004280050292. [DOI] [PubMed] [Google Scholar]
- Fraldi A, et al. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 2010;29:3607–3620. doi: 10.1038/emboj.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gelfman C, Vogel P, Issa TM, Turner A, Lee WS, Kornfeld S, Rice DS. Mice lacking α/β subunits of GlcNAc-1-phosphotransferase exhibit growth retardation, retinal degeneration, and secretory cell lesions. Invest Ophthalmol Vis Sci. 2007;48:5221–5228. doi: 10.1167/iovs.07-0452. [DOI] [PubMed] [Google Scholar]
- Glickman JN, Kornfeld S. Mannose 6-phosphate-independent targeting of lysosomal enzymes in I-cell disease B lymphoblasts. J Cell Biol. 1993;123:99–108. doi: 10.1083/jcb.123.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman JN, Morton PA, Slot JW, Kornfeld S, Geuze HJ. The biogenesis of the MHC class II compartment in human I-cell disease B lymphoblasts. J Cell Biol. 1996;132:769–785. doi: 10.1083/jcb.132.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. Fine Structure Immunocytochemistry. Berlin, Heidelberg: Springer-Verlag; 1993. Quantitative aspects of immunocytochemistry; pp. 371–445. [Google Scholar]
- Hickman S, Neufeld EF. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972;49:992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon KR, Sensenbrenner JA. Mucolipidosis II (I-cell disease): ultrastructural observations of conjunctiva and skin. Invest Ophthalmol. 1971;10:555–567. [PubMed] [Google Scholar]
- Kenyon KR, Sensenbrenner JA, Wyllie RG. Hepatic ultrastructure and and histochemistry in mucolipidosis II (I-cell disease) Pediatr Res. 1973;7:560–568. doi: 10.1203/00006450-197306000-00003. [DOI] [PubMed] [Google Scholar]
- Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann K, et al. Mannose phosphorylation in health and disease. Eur J Cell Biol. 2010;89:117–123. doi: 10.1016/j.ejcb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Kornfeld S, Sly WS. I-cell disease and pseudo-Hurler polydystrophy: disorders of lysosomal enzyme phosphorylation and localization. In: Scriver CR, et al., editors. In: The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2000. pp. 3469–3483. [Google Scholar]
- Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell. 2009;33:517–527. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic beta-cells. J Cell Biol. 1994;126:77–86. doi: 10.1083/jcb.126.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R, Klumperman J, Ludwig T, Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J Cell Biol. 1997;137:595–608. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Payne BJ, Gelfman CM, Vogel P, Kornfeld S. Murine UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase lacking the gamma-subunit retains substantial activity toward acid hydrolases. J Biol Chem. 2007;282:27198–27203. doi: 10.1074/jbc.M704067200. [DOI] [PubMed] [Google Scholar]
- Leroy JG, Demars RI. Mutant enzymatic and cytological phenotypes in cultured human fibroblasts. Science. 1967;157:804–806. doi: 10.1126/science.157.3790.804. [DOI] [PubMed] [Google Scholar]
- Leroy JG, Ho MW, MacBrinn MC, Zielke K, Jacob J, O’Brien JS. I-cell disease: biochemical studies. Pediatr Res. 1972;6:752–757. doi: 10.1203/00006450-197210000-00002. [DOI] [PubMed] [Google Scholar]
- Lightbody J, Wiesmann U, Hadorn B, Herschkowitz N. I-cell disease: multiple lysosomal-enzyme defect. Lancet. 1971;1:451. doi: 10.1016/s0140-6736(71)92435-4. [DOI] [PubMed] [Google Scholar]
- Magini A, Mencarelli S, Tancini B, Ciccarone V, Urbanelli L, Hasilik A, Emiliani C. Identification and characterization of mature beta-hexosaminidases associated with human placenta lysosomal membrane. Biosci Rep. 2008;28:229–237. doi: 10.1042/BSR20080075. [DOI] [PubMed] [Google Scholar]
- Martin JJ, Leroy JG, Farriaux JP, Fontaine G, Desnick RJ, Cabello A. I-cell disease (mucolipidosis II). A report on its pathology. Acta Neuropathol (Berl) 1975;33:285–305. doi: 10.1007/BF00686161. [DOI] [PubMed] [Google Scholar]
- Martin JJ, Leroy JG, Van Eygen M, Ceuterick C. I-cell disease. A further report on its pathology. Acta Neuropathol (Berl) 1984;64:234–242. doi: 10.1007/BF00688114. [DOI] [PubMed] [Google Scholar]
- Meister T, Niehues R, Hahn D, Domschke W, Sendler M, Lerch MM, Schnekenburger J. Missorting of cathepsin B into the secretory compartment of CI-MPR/IGFII-deficient mice does not induce spontaneous trypsinogen activation but leads to enhanced trypsin activity during experimental pancreatitis—without affecting disease severity. J Physiol Pharmacol. 2010;61:565–575. [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Sakakibara K, Endo H, Konishi Y, Nakamura N, Suzuki Y, Abe T. I-cell disease (mucolipidosis II). Pathological and biochemical studies of an autopsy case. Acta Pathol Jpn. 1977;27:251–264. [PubMed] [Google Scholar]
- Otomo T, Higaki K, Nanba E, Ozono K, Sakai N. Inhibition of autophagosome formation restores mitochondrial function in mucolipidosis II and III skin fibroblasts. Mol Genet Metab. 2009;98:393–399. doi: 10.1016/j.ymgme.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Owada M, Neufeld EF. Is there a mechanism for introducing acid hydrolases into liver lysosomes that is independent of mannose 6-phosphate recognition? Evidence from I-cell disease. Biochem Biophys Res Commun. 1982;105:814–820. doi: 10.1016/0006-291x(82)91042-7. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–323. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Pohl S, Marschner K, Storch S, Braulke T. Glycosylation- and phosphorylation-dependent intracellular transport of lysosomal hydrolases. Biol Chem. 2009;390:521–527. doi: 10.1515/BC.2009.076. [DOI] [PubMed] [Google Scholar]
- Reczek D, Schwake M, Schröder J, Hughes H, Blanz J, Jin X, Brondyk W, Van Patten S, Edmunds T, Saftig P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Sardiello M, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A. A block of autophagy in lysosomal storage disorders. Hum Mol Genet. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- Sleat DE, Della Valle MC, Zheng H, Moore DF, Lobel P. The mannose 6-phosphate glycoprotein proteome. J Proteome Res. 2008;7:3010–3021. doi: 10.1021/pr800135v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. Cryosectioning and immunolabeling. Nat Protoc. 2007;2:2480–2491. doi: 10.1038/nprot.2007.365. [DOI] [PubMed] [Google Scholar]
- Smith RE, Farquhar MG. Lysosome function in the regulation of the secretory process in cells of the anterior pituitary gland. J Cell Biol. 1966;31:319–347. doi: 10.1083/jcb.31.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondeur M, Vamos-Hurwitz E, Mockel-Pohls S, Dereume JP, Cremer N, Loeb H. Clinical, biochemical, and ultrastructural studies in a case of chondrodystrophy presenting the I-cell phenotype in culture. J Pediatr. 1971;79:366–378. doi: 10.1016/s0022-3476(71)80143-9. [DOI] [PubMed] [Google Scholar]
- Tooze J, Tooze SA. Clathrin-coated vesicular transport of secretory proteins during the formation of ACTH-containing secretory granules in AtT20 cells. J Cell Biol. 1986;103:839–850. doi: 10.1083/jcb.103.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P, Payne BJ, Read R, Lee WS, Gelfman CM, Kornfeld S. Comparative pathology of murine mucolipidosis types II and IIIC. Vet Pathol. 2009;46:313–324. doi: 10.1354/vp.46-2-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A, Pohlman R, Hasilik A, von Figura K, Elsen AV, Leroy JG. Deficiency of UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase in organs of I-cell patients. Biochem Biophys Res Commun. 1982;105:1052–1058. doi: 10.1016/0006-291x(82)91076-2. [DOI] [PubMed] [Google Scholar]
- Wiesmann UN, Lightbody J, Vasella F, Herschkowitz N. Multiple enzyme deficiency due to enzyme leakage? New Engl J Med. 1971a;284:109–110. doi: 10.1056/NEJM197101142840221. [DOI] [PubMed] [Google Scholar]
- Wiesmann U, Vasella F, Herschkowitz N. I-cell disease: leakage of lysosomal enzymes into extracellular fluids. New Engl J Med. 1971b;285:1090–1091. doi: 10.1056/NEJM197111042851922. [DOI] [PubMed] [Google Scholar]
- Yadao F,, Hechtman P, Kaplan F. Formation of a ternary complex between GM2 activator protein, GM2 ganglioside and hexosaminidase A. Biochim Biophys Acta. 1997;1340:45–52. doi: 10.1016/s0167-4838(97)00027-7. [DOI] [PubMed] [Google Scholar]
- Yu L, et al. Autophagy termination and lysosome reformation regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.