Abstract
Osteomyelitis is a debilitating infectious disease of the bone. It is predominantly caused by S. aureus and is associated with significant morbidity and mortality. It is characterised by weakened bones associated with progressive bone loss. Currently the mechanism through which either bone loss or bone destruction occurs in osteomyelitis patients is poorly understood. We describe here for the first time that the major virulence factor of S. aureus, protein A (SpA) binds directly to osteoblasts. This interaction prevents proliferation, induces apoptosis and inhibits mineralisation of cultured osteoblasts. Infected osteoblasts also increase the expression of RANKL, a key protein involved in initiating bone resorption. None of these effects was seen in a mutant of S. aureus lacking SpA. Complementing the SpA-defective mutant with a plasmid expressing spa or using purified protein A resulted in attachment to osteoblasts, inhibited proliferation and induced apoptosis to a similar extent as wildtype S. aureus. These events demonstrate mechanisms through which loss of bone formation and bone weakening may occur in osteomyelitis patients. This new information may pave the way for the development of new and improved therapeutic agents to treat this disease.
Introduction
The skeleton is a dynamic organ system which is constantly being rejuvenated and remodelled [1]. Bone remodelling involves the coordinated effort of osteoclasts and osteoblasts. Osteoclasts drive resorption of bone by acidification and release of lysosomal enzymes. Osteoblasts are responsible for the deposition of bone matrix and are thought to facilitate the calcification and mineralisation of the bone matrix. Together these cells function to ensure healthy bone, giving strength and rigidity in the skeletal system [2]. When pathogenic bacteria gain entry to healthy bone this leads to a condition called osteomyelitis.
Osteomyelitis is a debilitating infectious disease of the bone which is associated with significant morbidity and mortality [3]. It typically develops in the distal femur and proximal tibia in children and in adults, but any bone can be infected [4]. It is characterised by severe inflammation and progressive bone destruction [5]. Infection can occur as a result of haematogeneous spread or from a contiguous source secondary to an open injury to bone caused by trauma, bone surgery or joint replacement [6]. As part of the infectious process dead or dying bone can detach from healthy bone forming an island surrounded by a ring of sclerosis, which in turn leads to the development of an independent focus of infection [7]. In acute and chronic disease there is some degree of uncontrolled bone remodelling which often leads to an associated deformity [8]. Treatment of osteomyelitis involves aggressive antibiotic therapy which is often coupled with surgical debridement of infected tissue [4]. Antibiotic therapy is often unsuccessful because developing sequestra are typically avascular [3]. Thus antibiotics and inflammatory cells cannot reach the infected area and treatment often fails. Treatment is also compromised by the emergence of antibiotic resistance [9].
S. aureus permanently colonizes the anterior nares of the nostrils of about 20% of the population and is transiently associated with the rest [10]. Colonisation is a risk factor for developing infection. Until recently S. aureus was regarded as an extracellular pathogen. However it is clear that the organism can adhere to and become internalized by a variety of host cells [6], including osteoblasts [11], and that this is likely to be important in disease pathogenesis. Uptake is promoted by fibronectin binding proteins that capture fibronectin and use it as a bridge between bacteria and the α5β1 integrin [12]. Integrin clustering results in signalling that lead to bacterial uptake into phagocytic vesicles. Once internalized bacteria can escape the phagosome and cause necrosis [13]. Slow growing variants (called small colony variants) often emerge allowing the bacteria and the infection to persist [14].
Protein A (SpA) is an important virulence factor of S. aureus. It binds to a variety of ligands including the Fc region of IgG [15], von Willebrand factor [16], tumour necrosis factor receptor-1 (TNFR-1) [17], the Fab-heavy chains of the Vh3 subclass [18] and the epidermal growth factor receptor (EGFR)[19]. Five ligand binding domains lie at the N-terminus followed by regions Xc and Xr that allow the protein to span the cell wall. It is covalently attached to peptidoglycan following sortase cleavage of the LPXTG motif at the C-terminus [20]. Each binding domain comprises a triple helical bundle [21]. The IgG Fc region binds to residues exposed on the face formed by helices I and II. TNFR-1 also binds to this face but there are some differences in the residues of SpA that are involved. In particular, leucine 17 is crucial for binding to IgG but not for TNFR-1 binding [17].
Although current epidemiological data suggest that S. aureus is responsible for greater than 80% of osteomyelitis cases there is a distinct paucity of information surrounding the mechanisms that S. aureus uses to weaken and trigger bone destruction. While previous studies have clearly identified mechanisms through which S. aureus can become internalised into osteoblasts, thus evading the immune system and avoiding antibiotic attack, these studies failed to identify mechanisms that lead to bone destruction. This study was undertaken to investigate the mechanism though which S. aureus binds to and triggers localised bone destruction. Understanding these mechanisms may leads to the development of new and improved therapeutic agents to treat this disease.
Results
S. aureus binding to osteoblasts is mediated by SpA
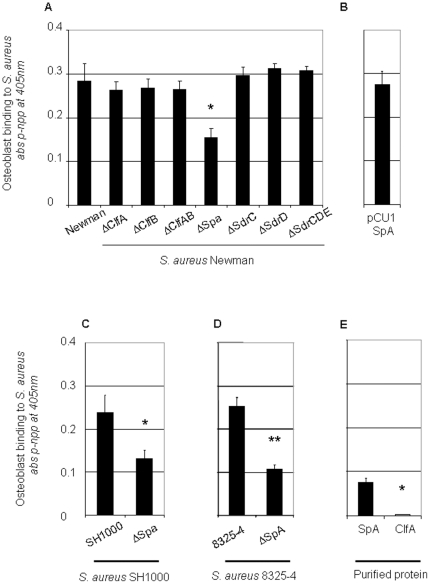
The contribution of cell surface molecules to the ability of S.aureus to support adherence to osteoblasts was investigated using isogenic mutants. It should be noted that strain Newman does not express fibronectin binding proteins so these strains are unlikely to be internalized rapidly. Mutants defective in several surface proteins previously shown to be involved in host recognition were immobilized and the ability of osteoblasts to adhere to the bacteria was measured. Mutants defective in expression of clumping factors A (ClfA) and B (ClfB), and serine asparate repeat proteins SdrC, SdrD and SdrE expression had no effect on osteoblast binding (Fig 1A, P = NS) whereas a mutant defective in SpA bound significantly less than the wildtype (Fig 1A, P<0.05). In order to confirm that SpA was involved in binding osteoblasts, a mutant of S. aureus Newman was complemented with a multicopy plasmid carrying the spa gene, pCU1spa. After growth, the expression of protein A was detected by western immunoblotting (data not shown). Complementation of the S. aureus SpA mutant with pCU1spa resulted in attachment to osteoblasts to a similar extent of that of the wildtype S. aureus Newman (Fig 1B, P = NS to the wildtype Newman control).
Figure 1. Protein A is involved in binding of Staphylococcus aureus to osteoblasts.
Osteoblasts were allowed adhere to immobilised wildtype S. aureus and isogenic mutants (1×109 cells/ml), (A+B) strain Newman, (C) strain SH1000, (D) strain 8325-4 or (E) purified protein A or recombinant ClfA(50 µg/ml) for 45 mins at 37°C. Adhesion was determined by measuring the intracellular enzyme alkaline phosphatase content at 405 nm in a microplate reader. *P<0.05, **P<0.01, n = 7.
In order to determine if other strains of S. aureus, and in particular those expressing FnBPs, could bind osteoblasts and to examine the role of SpA, strains SH1000 and 8325-4 along with their isogenic spa-deficient mutants were tested. In both cases a significant reduction in osteoblast binding was observed (Fig 1C, P<0.01 and Fig 1D, P<0.005, respectively). Finally, to confirm the ability of SpA to interact with osteoblasts, the purified protein was immobilized and was shown to support binding of the osteoblasts (Fig 1E, P<0.01). As a control the recombinant A domain of clumping factor A did not promote attachment.
S. aureus binds to osteoblast Tumour Necrosis Factor Receptor 1
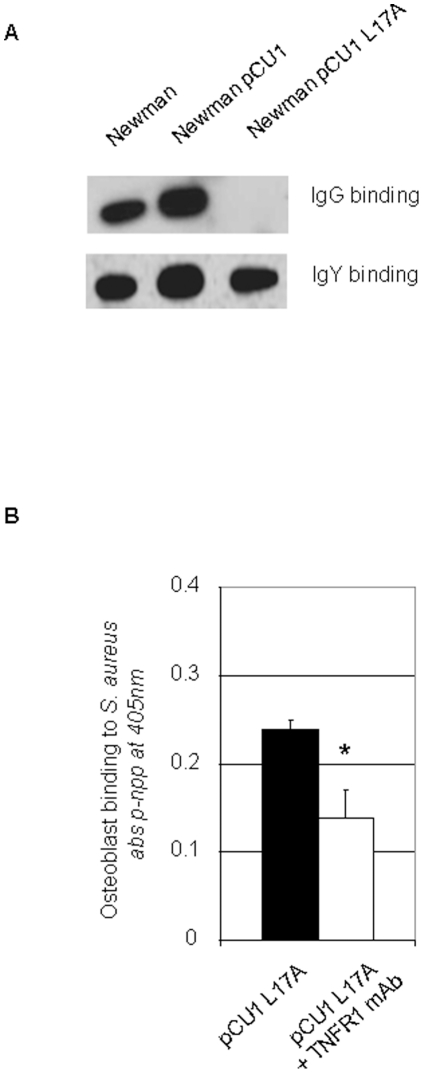
SpA is a multifunctional protein that has been shown to bind a number of ligands including IgG and Tumour Necrosis Factor Receptor1 (TNFR-1). Osteoblasts express high levels of TNFR-1. We investigated if SpA expressed by S. aureus could support binding to osteoblasts via TNFR-1. We used a strain of S. aureus Newman that expressed a variant of SpA where leucine 17 was substituted with an alanine in each of the five ligand binding domains. This substitution completely abolished IgG binding, but did not affect binding to TNFR-1 (Gomez et al., 2006). Immunoblotting was performed to show that the SpA variant L17A did not bind IgG. In contrast, Newman pCU1spa reacted with IgG in the blot. In control blots anti-SpA IgY bound to SpA from wildtype Newman, Newman pCU1spa and Newman pCU1spa L17A (Fig 2A). S. aureus Newman pCU1spa promoted adherence of osteoblasts equally as well as the Newman expressing wildtype SpA (99±1% of wildtype control, P = NS, n = 5). Furthermore, osteoblast-binding to Newman pCU1spa was significantly reduced by pretreatment of osteoblasts with a monoclonal anti-TNFR-1 IgG antibody (Fig 2B, P<0.05). These results indicate that immobilized S. aureus cells can support binding of cultured osteoblasts mediated by the interaction between SpA and TNFR-1.
Figure 2. Staphylococcus aureus SpA binds to osteoblast tumour necrosis factor receptor 1.
(A) S. aureus Newman, Newman pCU1spa and Newman pCU1spa L17A were lysed, separated on 7.5% SDS-PAGE gels, electroblotted onto PDVF membranes. Membranes were probed with either a nonspecific IgG or anti-SpA IgY antibody. Protein bands were detected using species specific horseradish peroxidase-conjugated secondary antibody and chemiluminescence. (B) Osteoblasts (5×105 cells/ml) were preincubated with either isotype control antibody or inhibitory monoclonal antibody against TNFR-1 for 15 mins prior to addition to S. aureus Newman pCU1spa L17A. Adhesion was determined by measuring the intracellular enzyme alkaline phosphatase content at 405 nm in a microplate reader. *P<0.05, n = 7.
S. aureus SpA inhibits proliferation of osteoblasts
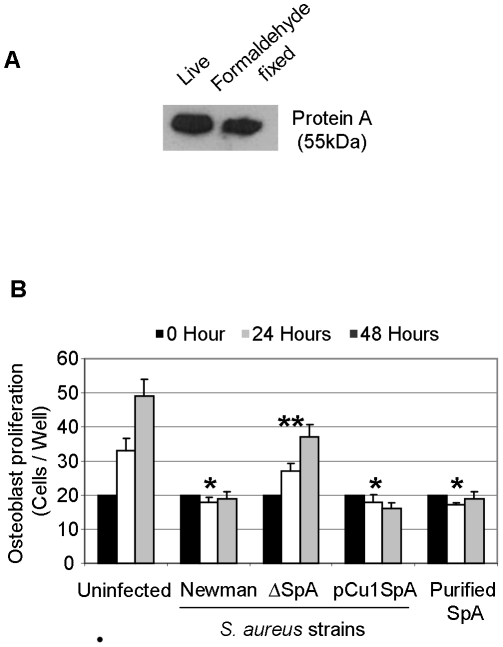
Given the observation that S. aureus SpA binds to osteoblasts we next investigated if SpA had any effect on osteoblast survival or proliferation. Live S. aureus can use nutrients in tissue culture media to divide. This process will starve osteoblasts of essential nutrients necessary for their growth. To address this we fixed S. aureus in a mild formaldehyde solution aimed to maintain bacterial cell integrity yet stunt their growth. This fixation step failed to have any effect on the SpA protein as determined by western blot (Fig 3A). Formaldehyde-fixed S. aureus Newman was added to osteoblasts and proliferation determined after 24 and 48 hrs. In the absence of S. aureus, osteoblasts proliferated as expected. Addition of S. aureus Newman Spa+ ablated proliferation (Fig 3B, P<0.01). A mutant defective in SpA did not prevent proliferation (P = NS to uninfected osteoblasts). Complementation of the S. aureus mutant with pCU1spa restored the inhibitory effect to the same level as wild type (P<0,01). Finally, addition of purified protein A to osteoblasts also prevented proliferation (P<0.01). These results suggest that S. aureus SpA binds to osteoblasts and triggers a signal transduction pathway that inhibits osteoblast proliferation.
Figure 3. Staphylococcus aureus lacking SpA does not inhibit osteoblast proliferation.
(A) Live or formaldehyde fixed S. aureus Newman was lysed in RIPA buffer containing 1 X protease inhibitor cocktail on ice for 10 minutes and probed with anti-protein A IgY antibodies. Protein bands were detected using species specific horseradish peroxidase-conjugated secondary antibody and chemiluminescence. (B) Osteoblasts (5×105 cells/ml) were preincubated with either control buffer or formaldehyde fixed S. aureus Newman, Newman SpA deficient or Newman pCU1spa (1×109 cells/ml) or purified protein A (50 µg/ml) for a total period of 21 days. After days 7, 14 and 21 osteoblasts were removed by trypsinization and proliferation was determined by counting cells on a haemocytometer. *P<0.01, **P = NS, n = 5.
S. aureus induces osteoblast apoptosis
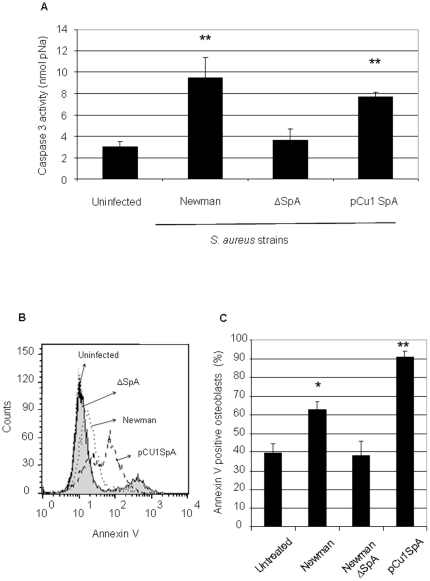
It has been reported in the literature that S. aureus can induce apoptosis in osteoblasts however the mechanism was not elucidated [22], [23]. We next investigated if S. aureus SpA binding to osteoblasts is responsible for inducing apoptosis. Apoptosis was first determined by measuring the amount of caspase 3 cleavage following incubation of cultured osteoblasts with formalin-treated S. aureus cells after a 24 hour period. Caspase 3 is a downstream effector caspase important in death receptor apoptotic mechanisms. In the absence of S. aureus, caspase 3 cleavage was minimal. However, after incubation with wildtype S. aureus Newman, caspase 3 activity was significantly increased, indicating that the osteoblasts were undergoing apoptosis (Fig 4A, P<0.01). No increase in caspase-3 cleavage occurred after addition of the S. aureus mutant defective in SpA (P = NS compared to the uninfected osteoblasts). Complementation with pCU1spa induced caspase 3 activity (P<0.01). Finally, addition of purified SpA to osteoblasts also increased caspase 3 activity (data not shown, P<0.05, n = 5).
Figure 4. Staphylococcus aureus lacking SpA does not trigger osteoblast apoptosis.
(A) Osteoblasts (5×105 cells/ml) were preincubated with either control buffer or formaldehyde fixed S. aureus Newman (1×109 cells/ml) for 24 hrs. Osteoblasts are removed, lysed and incubated with caspase 3 substrate (DEVD-pNA) for 1 hr at 37°C. Caspase 3 cleavage was measured at 405 nm in a microplate reader. (B+C) To measure Annexin V binding pelleted osteoblasts were re-suspended in 100 µl of FITC-Annexin V antibody. Suspensions were incubated in the dark for 15 min at RT and analysed by flow cytometry. *P<0.01, **P<0.05, n = 5.
Apoptosis was also detected by measuring the amount of FITC-Annexin V binding following incubation of cultured osteoblasts with formalin-treated S. aureus cells after a 24 hour period. In the absence of S. aureus 40% of osteoblasts appeared to bind Annexin V. Addition of S. aureus Newman to the osteoblasts led to a significant increase in Annexin V binding (Fig 4B+C, P<0.05). In contrast, addition of the S. aureus mutant defective in SpA failed to induce apoptosis above the uninfected levels (P = NS). Addition of S. aureus pCU1spa to the osteoblasts induced significant apoptosis (P<0.001). S. aureus pCU1spa induced significantly more apoptosis than the wildtype S. aureus strain (P<0.005).
S. aureus inhibits mineralisation of osteoblasts
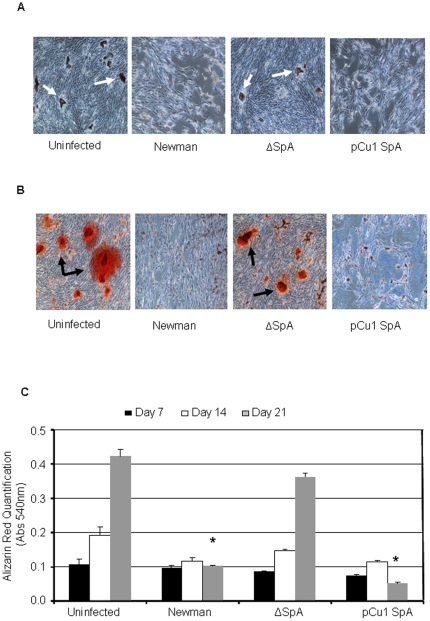
Following matrix deposition, osteoblasts facilitate the calcification and mineralisation of bone matrix thereby ensuring strength and rigidity to the skeletal system. We next investigated the effect of S. aureus on the mineralisation of osteoblasts. Mineralisation typically begins between day 7–10 and can be detected by staining the phosphates and calcium-rich deposits on the osteoblasts. Representative images obtained by brightfield microscopy showing staining for phosphates (von Kossa) from day 21 is shown in Fig 5A and for calcium deposition (Alizarin red) is shown in Fig 5B. Uninfected osteoblasts showed signs of both phosphate (Fig 5A) and calcium deposition (Fig 5B). Addition of either S. aureus Newman or S. aureus complemented with pCU1Spa prevented both phosphate (Fig 5A) and calcium deposition (Fig 5B). Critically, osteoblasts showed signs of both phosphate (Fig 5A) and calcium deposition (Fig 5B) when S. aureus SpA mutant was added. Quantification of the calcium deposition (alizarin red staining) supported these results demonstrating that over a 21 day period osteoblasts from both the uninfected and S. aureus SpA mutant stained for calcium deposition similarly (Fig 5C, P = NS), whereas S. aureus Newman and S. aureus complemented with pCU1Spa failed to do so (Fig 5C, P<0.01 and P<0.001, respectively).
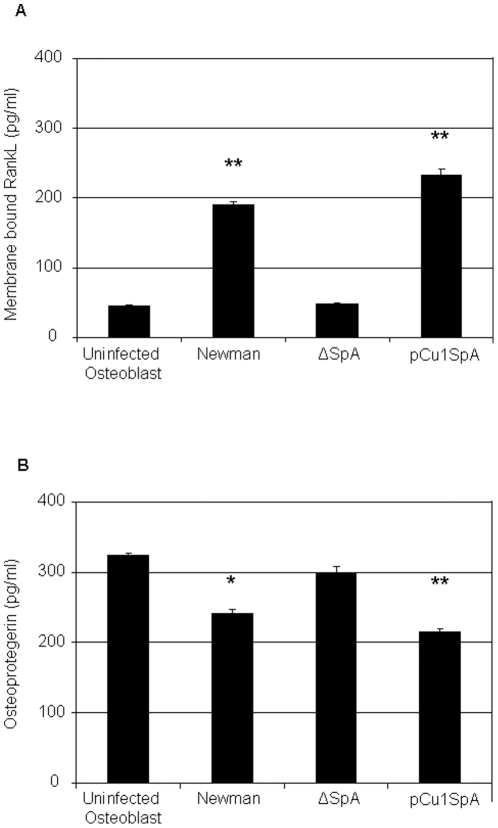
Figure 5. Staphylococcus aureus induces RANKL and inhibits OPG in osteblasts.
S. aureus (1×109 cells/ml) was added to the osteoblasts for 24 hrs. Two hundred micro litres of the RIPA buffer was added to each well resulting protein was removed and centrifuged at ×10,000 g for 2 minutes. (A) RANKL or (B) OPG was detected using an ELISA kit. *P<0.001, **P<0.0001, n = 3.
S. aureus induced RANKL expression in osteoblasts
RANKL is a key molecule involved in bone remodeling. Once expressed on osteoblasts it initiates bone resorption. Previous studies have shown that RANKL is upregulated in patients with staphylococcal osteomyelitis. We therefore investigated if S. aureus SpA leads to RANKL expression by osteoblasts. Quantitative analysis of membrane bound RANKL expression on osteoblasts after 24 hrs demonstrated that in uninfected osteoblasts a low level of RANKL was expressed. Addition of S. aureus Newman significantly increased RANKL expression following 24 hrs (Fig 6A. P<0.0001). Addition of the S. aureus SpA mutant failed to express significant levels of membrane bound RANKL compared to the uninfected sample (P = NS). However, complementation of the S. aureus mutant with pCU1spa led to significant expression of membrane bound RANKL (P<0.0001).
Figure 6. Staphylococcus aureus prevents osteoblast mineralisation.
Osteoblasts (5×105 cells/ml) were preincubated with either control buffer or formaldehyde fixed S. aureus Newman (1×109 cells/ml) for a total period of 21 days. (A) Following a 21 day infection von kossa stain was added to the osteoblasts to determine phosphate deposition. Phosphate nodules are indicated by white arrows. (B) Following a 21 day infection alizarin red stain (2%) was added to the osteoblasts. Calcium nodules are indicated by black arrows. (C) Staining was quantified by leeching the cells of the dye and reading absorbance at 540 nm. Representative images from day 21 for both stains were obtained using 20x bright field microscopy.. *P = NS, **P<0.05, ***P<0.01, n = 3.
Osteoprotegerin is a natural inhibitor of RANKL. By binding RANKL, osteoprotegrin prevents transcription via NF-κB and activation of osteoclasts, thereby preventing bone resorption. When RANKL expression is high osteoprotegrin levels are low and vice versa. Therefore we investigated this inverse relationship by measuring osteoprotegrin in infected and uninfected osteoblasts. Consistent with these findings osteoprotegrin was high in uninfected osteoblasts and significantly lower in S. aureus infected osteoblasts following 24 hrs (Fig 6B, P<0.001). Osteoprotegrin levels were high in cells exposed to the S. aureus SpA mutant and low in the S. aureus complemented with pCU1Spa (P<0.001). Collectively these results suggest that SpA binding to the osteoblasts is responsible for RANKL expression leading to activation of osteoclasts and bone breakdown.
Discussion
Bone is normally resistant to infection. However trauma, surgery to the skeleton or placement of a foreign body such as an orthopaedic implant may expose this otherwise sterile environment to infection. S. aureus is a common human pathogen which lives harmlessly on the skin and mucous membranes of healthy individuals. It is isolated from more than 80% of patients with bone infection or osteomyelitis [13]. Upon exposure to the bone, S. aureus induces a severe inflammatory response followed by progressive bone destruction and loss of the vasculature. As a result, sections of dead bone separate themselves from the healthy bone forming a sequestra [7]. This area of dead infected tissue is inaccessible to immune cells or to antibiotics, leading to persistent chronic infection. This is further complicated by the rapid emergence of strains of S. aureus that are resistant to multiple antibiotics which makes treatment of this disease very difficult [3]. A better understanding of the interactions between S. aureus and the bone may aid in the development of novel therapeutics to treat this disease.
Previous reports demonstrated that S. aureus is capable of invading osteoblasts from human, murine and chicken sources in both culture and in vivo [24]; [25]; [26]. Invasion is dependent on the S. aureus fibronectin binding proteins FnbpA and FnbpB which bind to the extracellular matrix protein fibronectin and serves as a bridging molecule to integrin α5β1 [12]. In the absence fibronectin or using mutants deficient in FnbpA and FnbpB, S. aureus invades osteoblasts very poorly. Interestingly bacteria still adhere to the osteoblast surface [11] even in the absence of FnbpA and FnbpB. Thus, S. aureus is also capable of directly interacting with osteoblasts in the absence of extracellular matrix proteins such as fibronectin.
S. aureus expresses several components that are capable of interacting with osteoblasts. A major virulence factor known to promote evasion of/or interference with the host immune system is capsular polysaccharides expressed on the bacterial cell surface [27]; [28]. Deletion of capsule from S. aureus Newman or using the capsule negative strain SH1000 failed to have any effect on binding, suggesting that the polymeric carbohydrates that are found in the capsule do not mediate attachment to osteoblasts (data not shown). S. aureus owes much of its pathogenicity to an array of cell wall-anchored surface proteins. These adhesins facilitate binding to various matrix proteins or host cells [29].
SpA is one of the most prevalent cell wall proteins on S. aureus and appears to play an important role in the success of S. aureus as a human pathogen [10]; [16]. By binding the Fc portion of immunoglobulin, SpA assists S. aureus in evasion of phagocytosis by neutrophils [30]. SpA also binds to von Willebrand factor which may play a role in supporting platelet adhesion in the early stages of thrombosis[16]. In addition, Gomez and colleagues demonstrated that SpA binds to the EGFR to regulate TNFR1 availability[19]. Deletion of SpA in several different strains of S. aureus (Newman, SH1000, 8325-4) significantly reduced binding to osteoblasts. Complementing the SpA mutant with the pCU1spa plasmid restored binding.
Recently, S. aureus SpA was shown to bind directly to TNFR-1 in lung epithelial cells resulting in proinflammatory signalling in the pathogenesis of staphylococcal pneumonia[17]. Osteoblasts also express high levels of the TNFR-1[31] and engagement with its ligand TNFα has been implicated in a wide spectrum of bone diseases including osteoporosis and rheumatoid arthritis[32]. Here we demonstrate that inhibition of TNFR-1 on osteoblasts significantly reduced binding to S. aureus. These results suggest that SpA maybe binding to the osteoblast TNFR-1, however additional experiments using TNFR-1 null mice or siRNA to reduce the receptor are required to confirm this.
TNFR-1 is known as a death receptor because its engagement triggers a series of events that culminates in apoptosis [33]. Several reports have demonstrated that S. aureus can induce apoptosis in osteoblasts [22], [23]. However the mechanism by which this occurs has not yet been elucidated. Caspase 3 is a key component of the proteolytic cascade that leads to apoptosis which is typically followed by membrane blebbing. Active caspase 3 and extrusion of annexin V (as a measure of membrane blebbing) was detected in the uninfected control osteoblasts and is most likely due to apoptosis and cell turnover in the in vitro osteoblast tissue culture system. Consistent with previous observations, when osteoblasts were exposed to S. aureus, caspase 3 and annexin V were significantly increased [23]. The SpA mutant yielded similar results to the uninfected osteoblasts. The complemented mutant or addition of purified protein A increased caspase 3 and annexin V in osteoblasts to levels similar to S. aureus Newman. Previous results demonstrated that S. aureus α-toxin induced apoptosis in Jurkat cells via the Bcl-2-controlled mitochondrial death pathway which involves caspase 3 [34] However this mechanism is not playing a role in these experiments as the S. aureus cells are formalin-fixed and cannot produce α-toxin. These results therefore suggest that S. aureus SpA binds to osteoblasts, possibly through an interaction with the death receptor TNFR-1 which induces caspase 3 activation and membrane blebbing with an end point of apoptosis. S. aureus-induced apoptosis is not unique to osteoblasts as endothelial and epithelial cells undergo apoptosis following S. aureus infection [35]–[37].
Mineralisation is a process where phosphate and calcium becomes deposited in bone[1]. This gives the bones additional strength and rigidity. During S. aureus infection, mineralisation (phosphate and calcium deposition) is completely inhibited. Deletion of SpA from S. aureus allowed both phosphate and calcium deposition. Interestingly, when TNFα binds to its receptor it results in inhibition of bone mineralisation. These results further suggest that engagement of the TNFR leads to a signal that prevents mineralisation such as the one that S. aureus SpA provides.
RANKL is a homotrimeric molecule displayed on the membrane of osteoblasts that stimulates differentiation in osteoclasts and is a key induction molecule involved in bone resorption leading to bone destruction[38]. Osteoprotegerin (OPG) is also produced and released by osteoblasts and is an endogenous inhibitor of RANKL signalling. It typically acts as a decoy receptor that binds to RANKL and prevents its association with RANK with the net result of preventing excessive bone destruction through activation of osteoclasts[39]. Consistent with previous results we found that S. aureus infection of osteoblasts led to a significant increase in RANKL expression in their membrane[40]. The increase in RANKL is likely to trigger osteoclast-induced bone resorption and bone destruction and may help explain why patients with osteomyelitis have significant bone loss. These results are consistent with the observation that TNFα binding to TNFR-1 on osteoblasts triggers ostoclast differentiation and subsequent bone destruction[41]. Critically, deletion of SpA from S. aureus prevented RANKL expression, possibly because S. aureus is unable to bind to TNFR-1 to trigger an increase in RANKL expression.
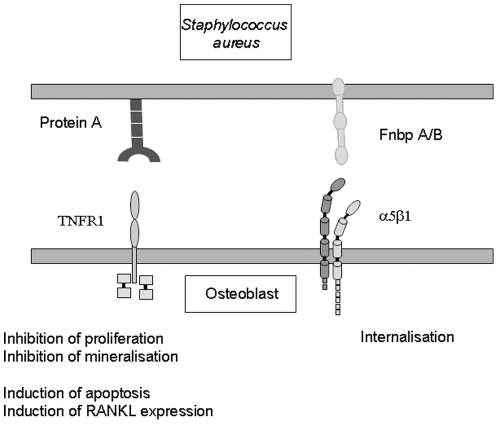
To date the mechanism by which S. aureus causes weakening of the bones in osteomyelitis is not fully understood. Previous results demonstrate that osteoblasts internalise the S. aureus via an indirect interaction between the fibronectin binding proteins that bind fibronectin and form a bridge to osteoblast α5β1 as a result S. aureus can evade immune responses and antibiotics. Here we describe for the first time that the major S. aureus virulence protein, SpA also binds directly to osteoblasts. This interaction results in the generation of multiple signals leading to inhibition of osteoblast proliferation, induction of osteoblast apoptosis, inhibition of mineralisation and release of mediators capable of inducing bone resorption via osteoclast activation (Fig 7). Given the finding that inhibition of the TNFR1 significantly reduces binding to osteoblasts it is tempting to suggest that this receptor plays a role in the pathogenesis of the disease process however, additional studies are required to confirm the role of the TNFR-1 in mediating these responses. Regardless of this, SpA binding to osteoblasts is an important step as it occurs in the absence of matrix proteins and is most likely the initial event in the development of bone infection. Results presented in this study provides evidence for the first time that SpA is likely to play a critical role in the success of S. aureus as a human pathogen in osteomyelitis and is a potential novel drug target for the treatment of this debilitating disease.
Figure 7. Proposed model of Staphylococcus aureus – osteoblast interaction.
Methods
Bacterial strains and growth conditions
The Staphylococcus aureus strains used in this study are listed in Table 1. They were grown to stationary phase at 37°C in Brain Heart Infusion broth (Oxoid, Basingstoke, United Kingdom). Bacteria were harvested and washed by centrifugation at 15,000 g for 5 min and finally re-suspended in phosphate buffered saline (PBS) pH 7.4. For all experiments, S. aureus suspensions were adjusted to 1×109 cells/ml. In some studies S. aureus were treated with 100 µg/ml trypsin-EDTA for 30 minutes with constant rotation, in order to remove cell wall proteins. Following this, samples were centrifuged at 15,000 g for 5 min, then the supernatant was removed and the pellet was re-suspended in 1 ml PBS. Trypsin-treated S. aureus were adjusted to 1×109 cells/ml for adhesion studies.
Table 1. List of strains of Staphylococcus aureus used in this study.
| Strain or plasmid | Relevant Characteristics | Reference |
| S. aureus | ||
| Newman | NCTC 8178 wildtype | Duthie and Lorenz, 1952 |
| Newman clfA | clfA:: Emr defective in clumping factor A | McDevitt et al 1994. |
| Newman clfB | Newman clfB:: lacZ Emr defective in clumping factor B | McAleese et al, 2001 |
| Newman spa | Newman spa:: Kar defective in protein A | O'Brien et al, 2002 |
| Newman sdrC | sdrC:: pG+Host Emr defective in Serine aspartate repeat protein C | O'Brien et al, 2002 |
| Newman sdrD | sdrD:: pG+Host Emr defective in Serine aspartate repeat protein D | O'Brien et al, 2002 |
| Newman ΔsdrCDE | ΔsdrCDE:: Tcr. Deletion mutant lacking SdrC SdrD and SdrE | O'Brien et al, 2002 |
| Newman cap | cap:: Tcr defective in capsular polysacccharide | Wann et al, 1999 |
| SH1000 | 8325-4 with repaired defect in rbsU | Horsburgh et al 2002 |
| SH1000 spa | spa:: Tcr transduced from 8325-4 spa | This study |
| 8325-4 | NCTC8325 cured of prophages | Novick 1967 |
| 8325-4 spa | spa:: Tcr. Protein a defective mutant | Hartlieb et al, 2000 |
| Newman clfA clfB | clfA:: Emr clfB: Tcr Defective in clumping factors A and B | Ni Eidhin et al, 1998 |
| Plasmids | ||
| pCU1spa | Shuttle vector capable of replicating in E. coli and S. aureus. Cmr Apr spa gene cloned into pCU1 | This study |
| pCU1 spaL17A | Expresses spa with an L17A substitution in each IgG binding domain | This study |
Bacterial strains and plasmids
S. aureus wildtype strains and mutants are listed in Table 1. pCU1spa was constructed by amplifying DNA encoding the spa gene including the promoter region and cloning into the multiple cloning site. The region encoding the SpA IgG binding domains EDABC was deleted by inverse PCR and an AccI site introduced forming pCU1ΔspaΔEDABC. The DNA region encoding the SpA domains bearing an L17A substitution in each ligand binding domain and flanked by AccI sites was synthesized commercially. It was cut with AccI and cloned into the introduced AccI site in pCU1ΔspaΔEDABC. The resulting plasmid, pCU1spaL17A. was verified by DNA sequencing.
Cell culture conditions
The mouse clonal MC3T3-E1 pre-osteoblastic cell line (ATCC, Middlesex, UK) was used for these experiments. This is a standard osteoblast cell line and is used routinely for the assessment of osteoblasts in different culture conditions [42], [43], [44] These cells were cultured in standard tissue culture flasks containing α-MEM supplemented with 10% FBS, 2% penicillin-streptomycin solution and 1% L-glutamine (Biosera Ltd., East Sussex, United Kingdom). The media was replaced every 3–4 days and after confluency, cells were harvested using trypsin and re-suspended in medium.
Osteoblast proliferation assay
Overnight cultures of S. auerus were harvested, washed and fixed in 4.8% formaldehyde under rotation and washed to eliminate any residual formaldehyde. S. aureus were centrifuged at 15,000 g for 5 minutes were re-suspended in α-MEM. A 6-well plate was coated with 1 ml formaldehyde fixed S. aureus (1×109 cell/ml) or 50 µg/ml of purified protein A. The plate was incubated at 37°C with 5% CO2 for 2hrs. Following this, the unbound bacteria or purified protein A was removed by gentle aspiration. MC3T3-E1 osteoblasts (5×105 cells/ml) were added into each well. Un-infected osteoblasts were seeded and cultured in the absence of bacteria as a control. Osteoblast proliferation was determined by counting cells on a haemocytometer in a 1∶1 dilution with Trypan Blue.
Osteoblast binding studies
Microtitre plates (96 well) were coated with 100 µl of bacteria (1×109 cells/ml) or purified protein A (50 µg/ml) (Sigma, Wicklow, Ireland). The plate was incubated at 37°C for 2 hours. Following this, the plate was washed with PBS and blocked with 1% bovine serum albumin (BSA) for a further 1 hour at 37°C. The plate was then washed once in PBS to remove any unbound bacteria or protein. MC3T3-E1 cells (1.5×106 cells/ml) were added to each well and allowed to adhere for 45 minutes at 37°C. In some studies, osteoblasts were pre-incubated with 50 µg/ml of anti-TNFR-1 antibody or isotype control antibody (Santa Cruz, Heidelberg, Germany) for 30 minutes at room temperature (RT) prior to their addition to the plate. Each well was gently washed with 100 µl PBS to remove any non-adhered osteoblasts. Adherent osteoblasts were then lysed with 100 µl lysis buffer containing a substrate for acid phosphatase (0.1 M Na acetate pH 5.5, 0.1% Triton X-100, 10 mM p-nitrophenol phosphate) and incubated for 20 minutes at 37°C and the A405nm read in a microplate reader (Wallac Victor2, Perkin Elmer, Cambridge, United Kingdom).
Osteoblast apoptosis
Caspase 3 is a downstream effector caspase important in death receptor apoptotic mechanisms. Caspase 3 activity was measured using a Caspase 3 colorimetric assay kit (Clontech Laboratories, CA (US) according to manufacturer's instructions. Briefly, cultured MC3T3-E1 osteoblasts were incubated with S. aureus or purified protein A (50 µg/ml). Untreated osteoblasts were used as a negative control and 100 µM dexamethasone- treated osteoblasts as a positive control. Cell lysates were incubated with the Caspase 3 substrate DEVD-pNA at 37°C for 1 hour. Samples were read at A 405nm in a microplate reader (Wallac Victor2, Perkin Elmer, Cambridge, United Kingdom).
Annexin V-FITC staining to infected osteoblasts was carried out according to the manufacturer guidelines (Trevigen). Cultured MC3T3-E1 osteoblasts were infected over 24 h with 4.8% formaldehyde fixed S. aureus Newman WT, NewmanΔSpa and Newman pCu1Spa (OD = 1.6), un-infected osteoblasts were used as negative control. Briefly un-infected and infected cells (1.5×105) were collected by centrifugation at 300×g for 5 min at RT. Pellets were washed once in cold (4°C) PBS and re-centrifuged at 300 ×g for 5 min at RT. Pellets were then re-suspended in 100 µl of Annexin V Incubation Reagent. Suspensions were incubated in the dark for 15 min at RT. Following addition of 400 µl of 1 X Binding Buffer, samples were processed by flow cytometry.
Immunoblotting
S. aureus Newman (formaldehyde fixed or unfixed) were lysed in RIPA buffer containing 1X protease inhibitor cocktail on ice for 10 minutes. Lysates were separated on a 10% sodium dodecylsulfate polyacrylamide (SDS-PAGE) gel. Proteins were electroblotted onto polyvinyldifluoride membranes (Roche, UK) for 1 hr. The membrane were probed with either a primary IgG antibody (clone 3E8) or primary chicken anti-protein A IgY antibody (clone SPA-27) overnight at 4°C with constant inversion. Unbound antibody was removed by 3×10 min washes with TS buffer. Protein bands were detected using species-specific horseradish peroxidase-conjugated secondary antibody and developed by chemiluminescence.
Quantification of RANKL and OPG
MC3T3-E1 cells (1×106 cells/well) were seeded on six well plates. S. aureus was added to the osteoblasts for 24 hours. Two hundred microlitres of the RIPA buffer was added to each well resulting protein was removed and centrifuged at ×10,000 g for 2 minutes. RANKL or OPG was detected using an ELISA kit (R&D Systems, Minneapolis, MN) according to manufacturer's instructions.
Statistical Analysis
Statistics were performed using SSC-Stat V2.12. Data shown are the means plus or minus standard error of the mean (SEM). Comparisons between mean values were performed using a 2-tailed student T-test.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a grant from the Science Foundation of Ireland Research Frontiers Programme awarded to SWK (no. BMT/2008/1709), a Health Research Board of Ireland project grant RP/2006/2 to TJF and the Royal College of Surgeons in Ireland Alumni Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hill PA. Bone remodelling. Br J Orthod. 1998;25:101–107. doi: 10.1093/ortho/25.2.101. [DOI] [PubMed] [Google Scholar]
- 2.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 3.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 4.Carek PJ, Dickerson LM, Sack JL. Diagnosis and management of osteomyelitis. Am Fam Physician. 2001;63:2413–2420. [PubMed] [Google Scholar]
- 5.Klosterhalfen B, Peters KM, Tons C, Hauptmann S, Klein CL, et al. Local and systemic inflammatory mediator release in patients with acute and chronic posttraumatic osteomyelitis. J Trauma. 1996;40:372–378. doi: 10.1097/00005373-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Garzoni C, Kelley WL. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 2009;17:59–65. doi: 10.1016/j.tim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am. 2004;86-A:2305–2318. doi: 10.2106/00004623-200410000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Marriott I, Gray DL, Tranguch SL, Fowler VG, Jr, Stryjewski M, et al. Osteoblasts express the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Am J Pathol. 2004;164:1399–1406. doi: 10.1016/S0002-9440(10)63226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vander Have KL, Karmazyn B, Verma M, Caird MS, Hensinger RN, et al. Community-associated methicillin-resistant Staphylococcus aureus in acute musculoskeletal infection in children: a game changer. J Pediatr Orthop. 2009;29:927–931. doi: 10.1097/BPO.0b013e3181bd1e0c. [DOI] [PubMed] [Google Scholar]
- 10.Foster TJ. Colonization and infection of the human host by staphylococci: adhesion, survival and immune evasion. Vet Dermatol. 2009;20:456–470. doi: 10.1111/j.1365-3164.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S, Meghji S, Williams RJ, Henderson B, Brock JH, et al. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect Immun. 2001;69:2872–2877. doi: 10.1128/IAI.69.5.2872-2877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha B, Francois PP, Nusse O, Foti M, Hartford OM, et al. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha5beta1. Cell Microbiol. 1999;1:101–117. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 13.Wright JA, Nair SP. Interaction of staphylococci with bone. Int J Med Microbiol. 300:193–204. doi: 10.1016/j.ijmm.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Eiff C, Bettin D, Proctor RA, Rolauffs B, Lindner N, et al. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin Infect Dis. 1997;25:1250–1251. doi: 10.1086/516962. [DOI] [PubMed] [Google Scholar]
- 15.Cedergren L, Andersson R, Jansson B, Uhlen M, Nilsson B. Mutational analysis of the interaction between staphylococcal protein A and human IgG1. Protein Eng. 1993;6:441–448. doi: 10.1093/protein/6.4.441. [DOI] [PubMed] [Google Scholar]
- 16.O'Seaghdha M, van Schooten CJ, Kerrigan SW, Emsley J, Silverman GJ, et al. Staphylococcus aureus protein A binding to von Willebrand factor A1 domain is mediated by conserved IgG binding regions. FEBS J. 2006;273:4831–4841. doi: 10.1111/j.1742-4658.2006.05482.x. [DOI] [PubMed] [Google Scholar]
- 17.Gomez MI, O'Seaghdha M, Magargee M, Foster TJ, Prince AS. Staphylococcus aureus protein A activates TNFR1 signaling through conserved IgG binding domains. J Biol Chem. 2006;281:20190–20196. doi: 10.1074/jbc.M601956200. [DOI] [PubMed] [Google Scholar]
- 18.Viau M, Zouali M. Effect of the B cell superantigen protein A from S. aureus on the early lupus disease of (NZBxNZW) F1 mice. Mol Immunol. 2005;42:849–855. doi: 10.1016/j.molimm.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 19.Gomez MI, Seaghdha MO, Prince AS. Staphylococcus aureus protein A activates TACE through EGFR-dependent signaling. EMBO J. 2007;26:701–709. doi: 10.1038/sj.emboj.7601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uhlen M, Guss B, Nilsson B, Gotz F, Lindberg M. Expression of the gene encoding protein A in Staphylococcus aureus and coagulase-negative staphylococci. J Bacteriol. 1984;159:713–719. doi: 10.1128/jb.159.2.713-719.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouda H, Torigoe H, Saito A, Sato M, Arata Y, et al. Three-dimensional solution structure of the B domain of staphylococcal protein A: comparisons of the solution and crystal structures. Biochemistry. 1992;31:9665–9672. doi: 10.1021/bi00155a020. [DOI] [PubMed] [Google Scholar]
- 22.Tucker KA, Reilly SS, Leslie CS, Hudson MC. Intracellular Staphylococcus aureus induces apoptosis in mouse osteoblasts. FEMS Microbiol Lett. 2000;186:151–156. doi: 10.1111/j.1574-6968.2000.tb09096.x. [DOI] [PubMed] [Google Scholar]
- 23.Alexander EH, Rivera FA, Marriott I, Anguita J, Bost KL, et al. Staphylococcus aureus - induced tumor necrosis factor - related apoptosis - inducing ligand expression mediates apoptosis and caspase-8 activation in infected osteoblasts. BMC Microbiol. 2003;3:5. doi: 10.1186/1471-2180-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bost KL, Ramp WK, Nicholson NC, Bento JL, Marriott I, et al. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J Infect Dis. 1999;180:1912–1920. doi: 10.1086/315138. [DOI] [PubMed] [Google Scholar]
- 25.Hudson MC, Ramp WK, Nicholson NC, Williams AS, Nousiainen MT. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 26.Reilly SS, Hudson MC, Kellam JF, Ramp WK. In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone. 2000;26:63–70. doi: 10.1016/s8756-3282(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson IM, Lee JC, Bremell T, Ryden C, Tarkowski A. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun. 1997;65:4216–4221. doi: 10.1128/iai.65.10.4216-4221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luong TT, Lee CY. Overproduction of type 8 capsular polysaccharide augments Staphylococcus aureus virulence. Infect Immun. 2002;70:3389–3395. doi: 10.1128/IAI.70.7.3389-3395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 30.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 31.Bu R, Borysenko CW, Li Y, Cao L, Sabokbar A, et al. Expression and function of TNF-family proteins and receptors in human osteoblasts. Bone. 2003;33:760–770. doi: 10.1016/j.bone.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 33.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 34.Menzies BE, Kourteva I. Staphylococcus aureus alpha-toxin induces apoptosis in endothelial cells. FEMS Immunol Med Microbiol. 2000;29:39–45. doi: 10.1111/j.1574-695X.2000.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 35.Bayles KW, Wesson CA, Liou LE, Fox LK, Bohach GA, et al. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahl BC, Goulian M, van Wamel W, Herrmann M, Simon SM, et al. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect Immun. 2000;68:5385–5392. doi: 10.1128/iai.68.9.5385-5392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haslinger-Loffler B, Kahl BC, Grundmeier M, Strangfeld K, Wagner B, et al. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell Microbiol. 2005;7:1087–1097. doi: 10.1111/j.1462-5822.2005.00533.x. [DOI] [PubMed] [Google Scholar]
- 38.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Somayaji SN, Ritchie S, Sahraei M, Marriott I, Hudson MC. Staphylococcus aureus induces expression of receptor activator of NF-kappaB ligand and prostaglandin E2 in infected murine osteoblasts. Infect Immun. 2008;76:5120–5126. doi: 10.1128/IAI.00228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, et al. Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J Pathol. 2002;198:220–227. doi: 10.1002/path.1190. [DOI] [PubMed] [Google Scholar]
- 42.Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–466. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 43.Plunkett NA, Partap S, O'Brien FJ. Osteoblast response to rest periods during bioreactor culture of collagen-glycosaminoglycan scaffolds. Tissue Eng Part A. 2010;16:943–951. doi: 10.1089/ten.TEA.2009.0345. [DOI] [PubMed] [Google Scholar]
- 44.Tierney CM, Jaasma MJ, O'Brien FJ. Osteoblast activity on collagen-GAG scaffolds is affected by collagen and GAG concentrations. J Biomed Mater Res A. 2009;91:92–101. doi: 10.1002/jbm.a.32207. [DOI] [PubMed] [Google Scholar]