Abstract
Previous studies showed that A. gambiae L35 females, which are refractory (R) to Plasmodium infection, express higher levels of genes involved in redox-metabolism and mitochondrial respiration than susceptible (S) G3 females. Our studies revealed that R females have reduced longevity, faster utilization of lipid reserves, impaired mitochondrial State-3 respiration, increased rate of mitochondrial electron leak and higher expression levels of several glycolytic enzyme genes. Furthermore, when State-3 respiration was reduced in S females by silencing expression of the adenine nucleotide translocator (ANT), hydrogen peroxide generation was higher and the mRNA levels of lactate dehydrogenase increased in the midgut, while the prevalence and intensity of P. berghei infection were significantly reduced. We conclude that there are broad metabolic differences between R and S An. gambiae mosquitoes that influence their susceptibility to Plasmodium infection.
1. INTRODUCTION
Malaria is a deadly disease that causes more than a million human deaths every year, mostly in tropical areas of Africa, Americas and Asia (WHO, 2009). Anopheles gambiae is the main vector of Plasmodium falciparum malaria in Sub-Saharan Africa. The Plasmodium life-cycle in the mosquito begins when a female ingests blood containing Plasmodium gametocytes from a vertebrate host. The female gametocytes are fertilized in the midgut lumen forming zygotes that gives rise to motile forms, the ookinete. Ookinetes transverse the midgut epithelial layer and differentiate into oocysts upon reaching the basal lamina. Ookinetes face a range of mosquito anti-Plasmodium responses, such as generation of reactive oxygen (ROS) and nitrogen species (RNS) (Kumar et al., 2003; Luckhart et al., 1998; Molina-Cruz et al., 2008) activation of immune signaling pathways (Frolet et al., 2006; Garver et al., 2009) and of components of the mosquito complement-like system such as the Thioester-conatining Protein 1 (TEP1) (Blandin et al., 2004; Fraiture et al., 2009).
Despite great advances in our understanding of the mosquito immune system, the multiple interactions between anopheline mosquitoes and Plasmodium parasites that determine the efficiency of disease transmission are still poorly defined. Twenty four years ago, a strain of Anopheles gambiae (L3–5) was genetically selected to be refractory (R) to Plasmodium cynomolgy (monkey malaria) infection (Collins et al., 1986). In this strain, ookinete formation and midgut invasion take place, but ookinetes are killed by the TEP1 system and covered with melanin (an insoluble black pigment) as soon as they come in contact with the mosquito hemolymph (Blandin et al., 2004). Genetic mapping defined a major Plasmodium encapsulation 1 (Pen1) region, located in the right arm of chromosome 2 (2R), that is highly associated with P. cynomolgy melanization (Zheng et al., 1997). The R strain also exhibits refractoriness to many different species of Plasmodium, including P. berghei (a murine malaria) (Collins et al., 1986). The R strain of An. gambiae has a different TEP1 allele that is known to be a major determinant of P. berghei melanization (Blandin et al., 2004; Blandin et al., 2009). Additionally, microarray analysis and functional studies revealed broad physiological differences between R and the susceptible (S) G3 strains. R females are in a chronic state of oxidative stress that is exacerbated by blood feeding. Also, resistant mosquitoes promptly promote Plasmodium melanization and display remarkable differences in expression levels of genes related to mitochondrial energy metabolism (Kumar et al., 2003). However, the molecular mechanism(s) mediating refractoriness in the R strain is still not clear. Our studies compared the energy metabolism of the S (G3) and R (L3–5) strains of Anopheles gambiae and identified several differences in mitochondrial function between these strains that result in increased ROS generation in R females and are likely to contribute to the refractory phenotype.
2. MATERIALS AND METHODS
2.1. Insects
The experiments were carried out with female Anopheles gambiae from two different strains that differ from each other with respect to their susceptibility (G3) or refractoriness (L3–5) to malaria parasites (Collins et al., 1986). Mosquitoes were reared at 27°C and 80% humidity on a 12 hr light-dark cycle under standard laboratory conditions.
2.2. Total lipid content
Sugar-fed females were homogenized 1 or 5 days after emergence in ice-cold PBS buffer with complete protease inhibitor cocktail (Roche Diagnostics, Manheim, Germany) on ice. The homogenates were centrifuged at 3500 rpm at 10°C for 30 minutes and the supernatants were subjected to lipid extraction. Protein concentration was estimated using BCA Protein Assay Kit (Thermo Scientific-Pierce, Rockford, IL, USA) using bovine serum albumin (BSA) as standard. Lipid extraction was performed according to Bligh and Dyer (Bligh and Dyer, 1959) with the following modifications. Samples were mixed for 2h in a stoppered tube using methanol-chloroform-water solution (2:1:0.8, v/v/v) with intermittent agitation. The mixture was centrifuged and the supernatant was collected. The pellet was subjected to a second lipid extraction (1h). To the pooled supernatant, 1 ml of water and 1 ml of chloroform were added. The mixture was vigorously shaken. After centrifugation, the organic phase was removed and dried under a stream of nitrogen. Total lipid content was determined gravimetrically.
2.3. Oxygen consumption in flight muscle mitochondria
Oxygen consumption was determined using a Clark-type electrode (Oxytherm oxygraph – Hansatech Instruments) at 27.5 °C. Mitochondria from flight muscle were isolated as previously described (Gonçalves et al., 2009). Briefly, approximately 100 thoraces were dissected and mitochondrial were enriched by differential centrifugation in ice-cold isolation buffer (250 mM sucrose, 5 mM Tris-HCl, 2 mM EGTA, 1% (w/v) fatty acid-free bovine serum albumin (FAF-BSA), pH 7.4). The final pellet was resuspended in ice-cold respiration media (120 mM KCl, 5 mM KH2PO4, 3 mM Hepes, 1 mM EGTA, 1 mM MgCl2, and 2 mg/ml fatty FAF-BSA, pH 7.2) (Gonçalves et al., 2009). A 5μL sub-sample of this preparation was diluted in 10 volumes of water to lyze the mitochondria. Protein concentration was determined using the BCA Protein Assay Kit (Thermo Scientific-Pierce, Rockford, IL, USA). To determine the protein from the mitochondrial preparation, the protein added to the respiration buffer (2 mg/ml of FAF-BSA) was subtracted from the total protein concentration present in the homogenate. Respiratory substrates, inhibitors and uncouplers were sequentially added and the maximal rates of oxygen consumption in each condition were plotted. All the mosquitoes used were 4–7 days old and the values presented in figures represent the average of at least 6 runs analyzed in three independent batches of mosquitoes.
2.4. Electron leak from thorax mitochondria
The electron leak was measured as the ratio of hydrogen peroxide (H2O2) released divided by the oxygen (O2) consumed in the presence of pyruvate, proline and ADP as previously described (Herrero and Barja, 1997). Oxygen consumption and H2O2 generation in isolated mitochondria were determined using methodology previously described (Goncalves et al., 2009).
2.5. Permeabilized midgut preparations
Groups of 30 midguts were dissected in isolation buffer consisting of 250 mM sucrose, 5 mM Tris-HCl, 2 mM EGTA, 1% (w/v) fatty acid-free bovine serum albumin (FAF-BSA), pH 7.4 and placed on an oxygraph chamber with respiration buffer (120 mM KCl, 5 mM KH2PO4, 3 mM Hepes, 1 mM EGTA, 1 mM MgCl2, and 2 mg/ml FAF-BSA, pH 7.2) supplemented with 0.0025 % digitonin to permeabilize the cells as previously described (Kuznetsov et al., 2008). After 1 min. of equilibration, both NAD+-linked substrate (10 mM pyruvate + 10 mM proline) and FAD+- linked substrate (10 mM succinate) were added to the chamber. Subsequently, 1 mM ADP was added to induce the state-3 respiratory rate. State-4-like (oligomycin-stimulated) and uncoupled respiratory rates (carbonyl cyanide p-trifluoromethoxy-phenylhydrazone; FCCP-stimulated) were measured upon addition of 7 μg of oligomycin and up to 7 μM FCCP, respectively. Oxygen (O2) consumption was recorded in an oxygraph fitted with a Clark-type electrode in a water-jacketed chamber (Oxytherm). For all experiments, temperature was maintained at 27.5°C and the total reaction volume was 1.0 ml. The respiratory inhibitors rotenone, antimycin a, and cyanide completely inhibited midgut O2 consumption. All mosquitoes used for the respiration assay were 4–7 days old. All mitochondrial respiration results represent the average oxygen consumption of at least six different midgut preparations analyzed in three independent experiments.
2.6. Midgut ROS production
Midguts were dissected in PBS and incubated at room temperature in the dark in PBS with Amplex Red (Invitrogen, Carlsbad, CA) and horseradish peroxidase (HRP). Fluorescence intensity was measured in the supernatant in a spectrofluorometer plate reader (SpectraMax gemini XPS, Molecular Devices, USA) at Ex/Em – 530/590, respectively. After each experiment, a standard curve of reagent grade H2O2 (Merck KGaA, Darmstadt, Germany) was performed.
2.7. Gene expression analysis
RNA was extracted with TRIzol (Invitrogen, USA) and cDNA was synthesized using QuantiTect Reverse Transcriptase (Qiagen, USA). Quantitative real-time PCR (qPCR) was performed with SYBR green (DyNAmo HS; New England Biolabs, USA) in a Chromo4 system (Bio-Rad, USA). Anopheles gambiae S7 gene was used as an internal control. Gene expression analysis was performed using the 2-ΔΔCt method. Primer sequences used were: S7: FGGCGATCATCATCTACGTGC and R-GTAGCTGCTGCAAACTTCGG. Adenine nucleotide translocator (ANT) (Gene bank accession number AGAP006782-PA): FCAAGGGTATCGTCGATTGCT and R- ATCCGAGGTTACCCAGGAAG. Hexokinase (HK) (AGAP011208-PA): F-CACGAA GTACGTGTCGGAGA and R-CTCGGTTCGTCCATTTTGTT. Phosphofructokinase (PFK) (AGAP007642-PA): F-GACGGGTCGATCAATGCTAC and R-ATGCCCTCC TCCTCATAGGT. Pyruvate kinase (PK) (AGAP004596-PA): F-ATCGTGAAGGTG GTCAAACC and R-ACGCCAGATCAGACTTGTCC. Lactate dehydrogenase (LDH) (AGAP004880-PB): F-TCATGAAGAACGCTCACGTC and R-TGGAGACGACCA ACAGAATG.
2.8. dsRNA synthesis and RNAi experiments
A 906-bp fragment from ANT gene (AGAP006782-PA) was amplified using cDNA from Anopheles gambiae (G3) midgut using primers F-ATGACCAAGAAGGCTGATCC and R-CCAGCAGGGCCTTAACTT. An aliquot of this PCR reaction was used as a template for dsRNA synthesis in vitro using MEGAscript RNAi kit (Ambion INC, Austin, TX, USA) and the primers ANT-T7-F-TAATACGACTCACTATAGGGAACGGTCTGCTCGACTGTCT and ANT-T7-R-TAATACGACTCACTATAGGGAGCAGGGCCTTAACTTCGTC. The underlined sequences represent the T7 promoter sequence that were incorporated in both primers to allow the synthesis of a 431-bp dsRNA. This fragment was further purified according to the manufacture's protocol, eluted with water and concentrated to 3 μg/μL. For silencing experiments 2-day old Anopheles gambiae females (G3) were injected with 69 nL of dsRNA solution. RNA was extracted at different times after injection and gene silencing efficiency was validated using qPCR.
2.9. Plasmodium berghei infection
3–7 day old female Anopheles gambiae (G3) were fed with anesthetized Balb/C mice infected with P. berghei (GFP-CON transgenic 259cl2 strain) (Franke-Fayard et al., 2004). Infected mice were considered ideal for experiments with 3–5% parasitemia and 1–2 exflagellations/field. Fully engorged mosquitoes were kept at 21 °C and 80% relative humidity. Infection levels were determined 7 days after feeding by counting the number of oocysts present in individual midguts.
3. RESULTS
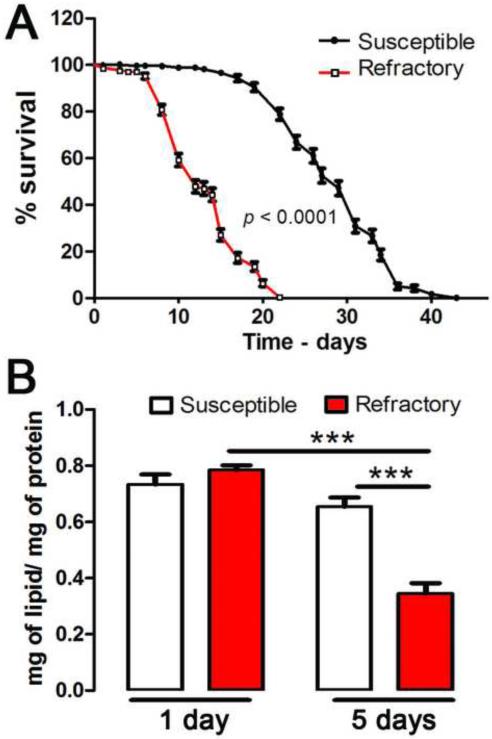
We analyzed the longevity of sugar-fed mosquitoes and observed that S females had a median life-span of 29 days while R females lived substantially less (median of 12 days) (p < 0.0001) (Figure 1A). To explore whether the decrease in longevity could be due to differences in energy metabolism, the total lipid content of whole body sugar-fed females was determined 1 and 5 days after emergence. The lipid content (normalized for protein content) was indistinguishable between 1 day-old R and S females (Figure 1B). Strikingly, the average size of adult R females was not significantly different from S (wing length R = 2.72 ± 0.02 mm, n=42 vs. S= 2.73 ± 0.02 mm, n=29). However, four days later, the lipid reserves remained unchanged in S females, but decreased to about half in the R strain (p < 0.001) (Figure 1B). The lipid content of 5 days-old R females was also significantly lower than that of S females (p < 0.001).
Figure 1. Comparison of the lifespan and lipid stores of Refractory (R) and Susceptible (S) adult sugar-fed mosquito females.
(A) Survival curves of S (n = 283) and R (n = 318) females. This experiment was repeated 5 times with independent batches of mosquitoes. All the data were included in the figure (Log-rank test analysis using GraphPad Prism 5). (B) Whole body lipid content of 1 and 5 day-old S and R females (*** indicates p < 0.001 using the ANOVA-Tukey's test).
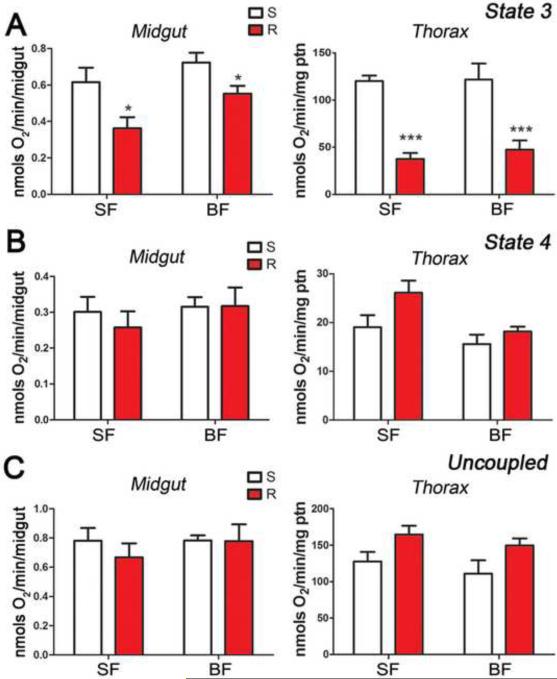
The hypothesis that the reduced lifespan and increased rate of lipid utilization in R females was due to differences in mitochondrial function was investigated. The ADP-stimulated respiratory rate (state-3) of S and R females was determined in permeabilized midguts and in preparations enriched for flight muscle mitochondria. The R strain exhibited reduced state-3 respiration in midguts of sugar-fed (p = 0.0248) and blood-fed mosquitoes (p = 0.0391) (Figure 2A, left panel) relative to the S strain and this difference was even more striking in mitochondria from flight muscle of sugar-fed (p < 0.0001) and blood-fed (p < 0.001) females (Figure 2A, right panel). Oligomycin, an inhibitor of F1FoATP synthase, was added to the preparations to determine the phosphorylation-independent oxygen consumption (state-4). There was no difference in state-4 respiration between mosquito strains in the midgut or mitochondria of sugar-fed or blood-fed females (Figure 2B).
Figure 2. Mitochondrial respiration in midgut and thorax preparations from sugar-fed (SF) and blood-fed (BF) females.
(A) State-3 respiratory rates (phosphorylating state) in permeabilized midguts and isolated flight muscle mitochondria from SF and BF Susceptible (S = white bars) and Refractory (R = red bars) females. (B) State-4-like respiratory rate (phosphorylation-independent oxygen consumption). (C) Uncoupled respiration or maximal respiratory capacity. (* p < 0.05), ** p < 0.01), *** p < 0.0001, T-test).
Because the electrochemical gradient in the intermembrane space can limit the rate of mitochondrial oxygen consumption, addition of the proton ionophore FCCP disrupts the gradient and makes it possible to measure the maximal respiratory capacity (or uncoupled oxygen consumption). The maximum respiratory capacity was not different between S and R females (Figure 2C), indicating that the amount of functional mitochondria was similar in the two preparations and that the observed differences in mitochondrial function were due to a specific impairment of state-3 respiration in R females.
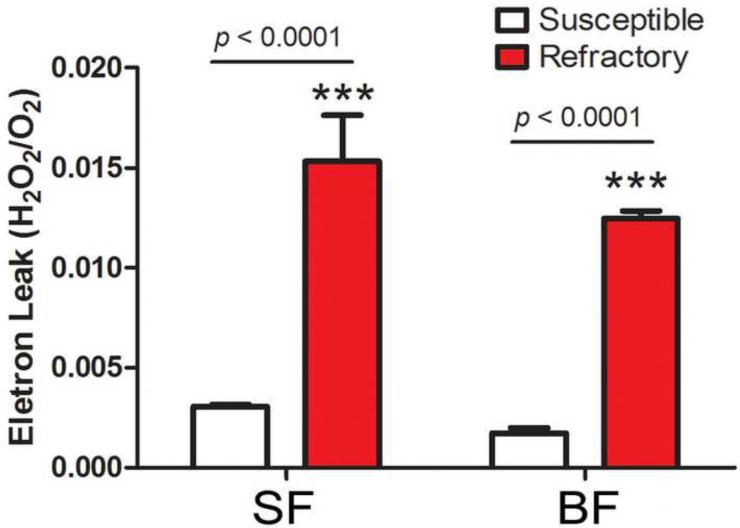
We investigated whether the decrease in state-3 respiration was associated with increased ROS generation by measuring the mitochondrial electron leak, which represents the ratio of hydrogen peroxide (H2O2) generated by the respiratory chain per O2 consumed under a specific mitocondrial metabolic state (Barja, 2002). The electron leak in state-3 respiration was four fold higher (p<0.0001) in sugar-fed and blood-fed R females (Figure 3), incriminating mitochondria as one of the sources responsible for increased ROS levels in R mosquitoes.
Figure 3. Mitochondria electron leak in permeabilized midguts from sugar-fed (SF) and blood-fed (BF) females.
The rate of electron leak was determined in midguts from Susceptible (white bars) or Refractory (red bars) adult females (*** = p < 0.0001, T-test).
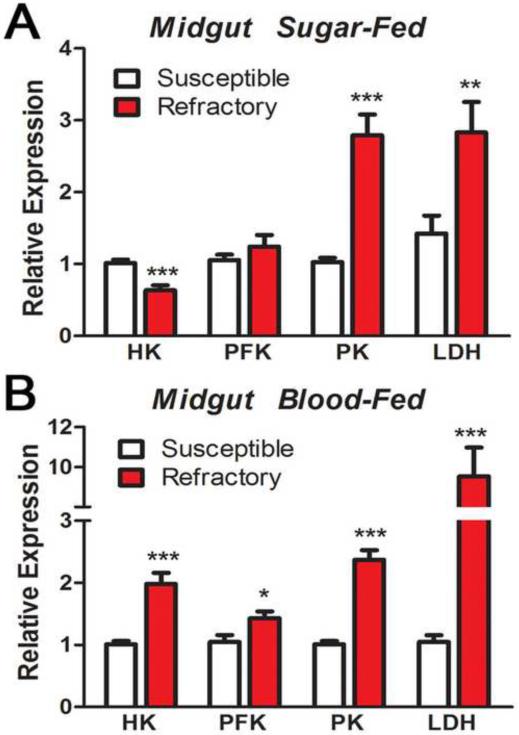
To explore whether the R strain compensates for the reduced efficiency in oxidative phosphorylation by increasing the rate of anaerobic glycolysis, the relative expression of four key glycolytic enzymes, hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK) and lactate dehydrogenase (LDH) was compared between S and R females. Sugar-fed R females indeed exhibited increased mRNA levels of PK and LDH (Figure 4A). The transcripts of all enzymes were significantly elevated 24 h after blood-feeding in the R strain (Figure 4B), with LDH reaching levels 10 fold higher than in the S strain.
Figure 4. Relative mRNA expression of glycolytic enzymes in Susceptible and Refractory mosquitoes.
Gene expression analysis of key glycolytic enzymes: hexokinase (HK), phoshofructokinase (PFK), pyruvate kinase (PK) and lactate dehydrogenase (LDH) in the midgut of (A) sugar-fed and (B) blood-fed (24h) of Susceptible (white bars) or Refractory (red bars) adult females (* p < 0.05, ** p < 0.01, *** p < 0.0001, T-test).
Mitochondria require reduced substrates that donate electrons to the electron transport chain (ETC) and generate the proton-motive force to drive the activity of F1Fo ATP synthase. Adenosine diphosphate (ADP) and inorganic phosphate (Pi) must also be available in the mitochondrial matrix to allow adequate ATP synthesis. The adenine nucleotide translocator (ANT) mediates the exchange of ADP for ATP across the inner mitochodnrial membrane. Because ANT activity is required to maintain mitochondrial state-3 respiration, the hypothesis that ANT silencing in S females would mimic the R phenotype was investigated.
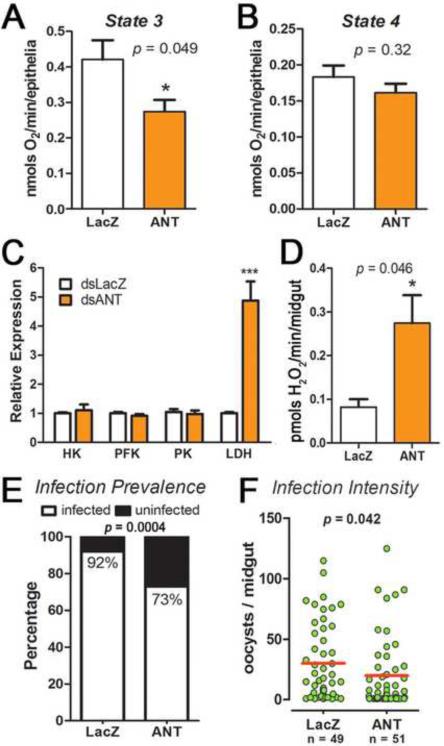
S females were injected with ANT double-stranded RNA (dsANT) or dsLacZ as a control. The efficacy of midgut silencing was 98 and 96%, at 2 and 4 days after dsRNA injection, respectively, (Figure S1). ANT silencing reduced State-3 respiration in the midgut (Figure 5A), but did not affect State-4 respiration (Figure 5B). Furthermore, ANT knock-down also increased LDH expression (Figure 5C), as was observed in R females (Figure 4A and 4B). However, the expression of other glycolytic enzymes such as HK and PK were not affected by ANT silencing.
Figure 5. Effect of silencing the adenine nucleotide translocator (ANT) in Susceptible females on mitochondrial respiration, expression of glycolytic enzymes, H2O2 production, and susceptibility to Plasmodium infection.
(A) State-3 (ADP-stimulated) respiration in control (dsLacZ-injected, white bars) and ANT-silenced (orange bars) midguts (B) State-4-like respiratory rate (phosphorylation-independent oxygen consumption). (C) Gene expression analysis of key glycolytic enzymes in the midgut: hexokinase (HK), phoshofructokinase (PFK), pyruvate kinase (PK) and lactate dehydrogenase (LDH) in midguts 2 days after dsRNA injection. (D) Hydrogen peroxide production in the midgut (*p < 0.05, *** p < 0.0001, T-test). Effect of ANT silencing on the (E) prevalence (p < 0.0004, Chi-square test) and (F) Intensity (p = 0.042, Kolmogorov-Smirnov Test) of infection with P. berghei. Each dot represents the number of oocysts present on an individual midgut and the median is indicated by the red line(p = 0.042, Kolmogorov-Smirnov Test)
We next investigated whether the phenotype produced by ANT silencing would impact ROS generation and the insect's susceptibility to Plasmodium infection. As expected, hydrogen peroxide production in the midgut was significantly higher (p<0.05) in ANT-silenced mosquitoes (Figure 5D). ANT knock-down also reduced the prevalence of infection from 92% in the dsLacZ control to 73% (p < 0.0004, χ2) (Figure 5E), as well as the intensity of infection from a median of 29 to 19 oocysts/midgut (p<0.05, Kolmogorov-Smirnov test) (Figure 5F).
4. DISCUSSION
Mitochondrial metabolism plays a central role in the control of both energy and redox homeostasis and recent work has pointed out emerging new functions of this organelle in mosquitoes (Goncalves et al., 2009). Our current findings revealed important differences in mitochondrial function between An. gambiae strains with different susceptibilities to Plasmodium infection. The R strain had reduced mitochondrial oxygen consumption specifically associated to ATP synthesis. This mitochondrial impairment appears to be systemic, as it was observed in both midgut and thorax mitochondrial preparations (Figure 2A).
Previous studies showed that R females have higher systemic levels of H2O2 that increase further after ingestion of a blood-meal (Kumar et al., 2003). Oral administration of antioxidants decreased H2O2 levels and reduced melanization of Plasmodium and of implanted Sephadex beads (Kumar et al., 2003) in the R strain, suggesting that high ROS levels promote melanization. Ookinete invasion induces high levels of nitric oxide synthase expression in midgut cells that is followed by the induction of peroxidase activity, as cells undergo apoptosis (Kumar et al., 2004). Apoptotic cells exhibit extensive protein nitration that appears to be mediated by a two-step reaction in which an inducible epithelial heme peroxidase potentiates nitration by a mechanism similar to what has been described in human macrophages (Pfeiffer et al., 2001). Higher levels of H2O2 in the R strain are expected to accelerate epithelial nitration and reduce Plasmodium survival because H2O2 is a substrate required by heme-peroxidases and nitration responses would be deleterious to the parasite (Kumar et al., 2004).
The reduction in mitochondrial state-3 respiration in the R strain is associated with a dramatic increase in mitochondrial electron leak (Figure 3) and is likely to contribute to the elevated systemic levels that have been associated with the refractory phenotype (Kumar et al., 2003). It is interesting that ANT gene silencing in the S strain induces a similar phenotype in which state-3 respiration is affected, H2O2 production is increased, LDH expression is high and the prevalence and intensity of Plasmodium infection is reduced. It is known that the TEP1 allele present in the R strain (TEP1-R1) is a major determinant of refractoriness to P. berghei. This allele is not present in the Susceptible (G3) strain and could explain the milder phenotype that was observed when ANT expression was silenced S females. The metabolic changes also appear to be less drastic in ANT-silenced S females, as the expression of some glycolytic enzymes such as HK and PK were not affected. There could also be other sources of ROS generation in the R strain besides mitochondria. In Drosophila, the dual oxidase (Duox) enzyme has been shown to be activated in response to bacterial elicitors and to generate ROS within the gut lumen (Ha et al., 2005). In An. gambiae, Duox (Kumar et al., 2004; Kumar et al., 2010) and NADPH-oxidase (Nox) (G. A. Oliveira and C. Barillas-Mury, unpublished) expression is induced in midgut epithelial cells in response to Plasmodium invasion. These genes are expressed in many other tissues and, in vertebrates, are known to play a central role in generating ROS as final effectors of the immune system (reviewed by Rada and Leto, 2008). Deregulation of some immune pathway in the R strain could result in constitutive activation of oxidases such as Duox or Nox. The constant generation of ROS would lead to mitochondrial damage and cause the impairment in state-3 respiration and the increased rate of mitochondrial electron leak we have observed. This, in turn, would further increase ROS generation. The Pen1 region (Chr. 2R) is a major determinant of An. gambiae refractoriness to Plasmodium cynomolgy B (Zheng et al., 1997) and the same region (Pbmel1) is associated with P. berghei melanization (Blandin et al., 2009). The complex metabolic changes that are observe at a systemic level in the R strain suggest that the gene mediating refractoriness is probably a regulatory protein that affects the function of multiple genes. One can envision, for example, a constitutively active transcription factor or a non-functional suppressor of a signal transduction pathway.
In conclusion, we found clear evidence that, besides mitochondrial respiration, there are other major metabolic differences between R and S females. The genes of several glycolytic enzymes are expressed at higher levels in R females (Figure 4) and lipid stores are consumed faster (Figure 1B), suggesting a major metabolic shift, probably to compensate for the reduced efficiency in oxidative phosphorylation. We have previously shown that enzymes involved in ROS detoxification, such as catalase, play a central role in modulating the balance between immunity and fecundity (DeJong et al., 2007; Molina-Cruz et al., 2008). Although the higher ROS levels in the R strain enhance their ability to kill Plasmodium and to survive a bacterial challenge (Molina-Cruz et al., 2008), this comes with a high fitness cost, as it also causes a rapid loss of fecundity with age and triggers a series of metabolic changes that reduce the lifespan of adult female mosquitoes.
Supplementary Material
Acknowledgments
We thank Lindsey Garver and Alvaro Molina-Cruz for their comments and insight and André Laughinghouse and Kevin Lee for insectary support. This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. R.L.S.G. was funded by a fellowship from the Brazilian Government through CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior); J.H.M.O. was funded by CAPES and CNPq Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico). M.F.O. and P.L.O. are research scholars from CNPq.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barja G. The quantitative measurement of H2O2 generation in isolated mitochondria. J Bioenerg Biomembr. 2002;34:227–233. doi: 10.1023/a:1016039604958. [DOI] [PubMed] [Google Scholar]
- Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Blandin SA, Wang-Sattler R, Lamacchia M, Gagneur J, Lycett G, Ning Y, Levashina EA, Steinmetz LM. Dissecting the genetic basis of resistance to malaria parasites in Anopheles gambiae. Science. 2009;326:147–150. doi: 10.1126/science.1175241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, Collins WE, Campbell CC, Gwadz RW. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- DeJong RJ, Miller LM, Molina-Cruz A, Gupta L, Kumar S, Barillas-Mury C. Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae. Proc Natl Acad Sci U S A. 2007;104:2121–2126. doi: 10.1073/pnas.0608407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, Hoffmann JA, Blandin SA, Levashina EA. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, Sinden RE, Waters AP, Janse CJ. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Boosting NF-kappaB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity. 2006;25:677–685. doi: 10.1016/j.immuni.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Garver LS, Dong Y, Dimopoulos G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves RL, Machado AC, Paiva-Silva GO, Sorgine MH, Momoli MM, Oliveira JH, Vannier-Santos MA, Galina A, Oliveira PL, Oliveira MF. Blood-feeding induces reversible functional changes in flight muscle mitochondria of Aedes aegypti mosquito. PLoS One. 2009;4:e7854. doi: 10.1371/journal.pone.0007854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–50. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Herrero A, Barja G. ADP-regulation of mitochondrial free radical production is different with complex I- or complex II-linked substrates: implications for the exercise paradox and brain hypermetabolism. J Bioenerg Biomembr. 1997;29:241–249. doi: 10.1023/a:1022458010266. [DOI] [PubMed] [Google Scholar]
- Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimopoulos G, Kafatos FC, Barillas-Mury C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci U S A. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Gupta L, Han YS, Barillas-Mury C. Inducible peroxidases mediate nitration of anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J Biol Chem. 2004;279:53475–53482. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–8. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci U S A. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, Jaramillo-Gutierrez G, Barillas-Mury C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. Bioenergetics. Vol. 3. Academic Press; 2002. [Google Scholar]
- Pfeiffer S, Lass A, Schmidt K, Mayer B. Protein tyrosine nitration in cytokine-activated murine macrophages. Involvement of a peroxidase/nitrite pathway rather than peroxynitrite. J Biol Chem. 2001;276:34051–34058. doi: 10.1074/jbc.M100585200. [DOI] [PubMed] [Google Scholar]
- Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–87. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Ten Facts on Malaria. 2009 2009 at: http://wwwwhoint/features/factfiles/malaria/en/
- Zheng L, Cornel AJ, Wang R, Erfle H, Voss H, Ansorge W, Kafatos FC, Collins FH. Quantitative trait loci for refractoriness of Anopheles gambiae to Plasmodium cynomolgi B. Science. 1997;276:425–428. doi: 10.1126/science.276.5311.425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.