Abstract
Portions of the posterior parietal cortex (PPC) play a role in working memory (WM) yet the precise mechanistic function of this region remains poorly understood. The pure storage hypothesis proposes that this region functions as a short-lived modality-specific memory store. Alternatively, the internal attention hypothesis proposes that the PPC functions as an attention-based storage and refreshing mechanism deployable as an alternative to material-specific rehearsal. These models were tested in patients with bilateral PPC lesions. Our findings discount the pure storage hypothesis because variables indexing storage capacity and longevity were not disproportionately affected by PPC damage. Instead, our data support the internal attention account by showing that (a) normal participants tend to use a rehearsal-based WM maintenance strategy for recall tasks but not for recognition tasks; (b) patients with PPC lesions performed normally on WM tasks that relied on material-specific rehearsal strategies but poorly on WM tasks that relied on attention-based maintenance strategies and patient strategy usage could be shifted by task or instructions; (c) patients’ memory deficits extended into the long-term domain. These findings suggest that the PPC maintains or shifts internal attention among the representations of items in WM.
Keywords: visual working memory, Balint’s syndrome, parietal lobe, strategy, recall, recognition
1. Introduction
Converging evidence from neuroimaging, neuropsychology, and brain stimulation studies indicates that portions of the posterior parietal cortex (PPC) are functionally involved in working memory (WM). The superior parietal lobe (SPL; BA 5 and 7) is known to be involved in spatial WM (Olson & Berryhill, 2009; Wager & Smith, 2003) while regions around the intraparietal sulcus and more inferior portions of the PPC, especially in the right hemisphere, appear to play an important role in object WM. For instance, BOLD activity in the intraparietal sulcus parametrically varies with the number of items held in WM, and with individual differences in WM capacity (Todd & Marois, 2004, 2005). Likewise, patients with right PPC damage struggle to remember a small number of sequentially presented objects or locations over brief delays (Berryhill & Olson, 2008b). The left PPC, around BA 39–40, has been associated with verbal WM in fMRI and lesion studies (e.g. Chein, Ravizza, & Fiez, 2003; Ravizza, Delgado, Chein, Becker, & Fiez, 2004).
These findings indicate that portions of the PPC play some role in WM, yet the precise mechanistic function(s) of these PPC regions in memory is poorly understood. One explanation, the pure storage account, suggests that areas of the PPC act as capacity-limited repositories for memory traces that are sustained over brief delays. A well-known example of this type of model is Baddeley’s multicomponent model, which emphasizes the use of material-specific (verbal, visuospatial) rehearsal and storage mechanisms (e.g. subvocal rehearsal). Specifically, Baddeley’s model proposes that WM involves storage of information in separate maintenance sub-systems, each devoted to short-term memory for distinct types of information. The phonological loop subsystem specializes in maintaining verbal information. The visuospatial sketchpad subsystem maintains visuospatial information. The episodic buffer maintains integrated multimodal information (Baddeley, 1986; Baddeley et al., 2000; Baddeley & Hitch, 1974; Baddeley & Logie, 1999; Repovs & Baddeley, 2006). Attempts to map these modules onto cortical regions has met with mixed success (Baddeley, 2003). Of interest here, based on neuropsychological and neuroimaging data, the left supramarginal gyrus (BA 40) is the proposed correlate of the phonological loop (Baddeley, 2003; but see Buchsbaum & D'Esposito, 2008). Contrasts between verbal and visuospatial WM suggest the right inferior parietal cortex (BA 40) as the location of the visuospatial sketchpad (Baddeley, 2003). The angular gyrus (BA 39) has been proposed as a putative site for the episodic buffer based on fMRI and EEG data (Vilberg & Rugg, 2008). Thus, pure storage accounts predict that PPC damage, particularly to the inferior parietal lobe (IPL), will cause material-specific WM deficits that become disproportionately large as maintenance demands increase. Predictions of the pure storage account are tested in Experiments 1–3.
An alternative account of PPC involvement in WM, the internal attention (IA) account, holds that the implicated areas of the PPC are not involved in storage per se, but rather, are the locus of a domain-general attentional mechanism that can be deployed to support WM. Of course, attention may play many different roles in a WM task. Attention could simply be engaged to select for the objects of information processing at the time of item encoding or retrieval. Alternatively, attention could play a more active role in revivifying representations as they are maintained in WM, a process that has been referred to as attentional refreshing (Chein & Fiez, in press; Chein et al., 2003; Lewandowsky, Oberauer, & Brown, 2009). According to the latter view, representations held in WM can be “boosted” by reentry into the focus of attention, thus preventing decay. The notion that attention may play an active role in covert maintenance is consistent with several attention-based models of WM (e.g. the embedded-processes model, Cowan, 1999; the time-based resource sharing model, (Barrouillet & Camos, 2009; Unsworth & Engle, 2007). These models are consistent with the notion that, although certain task conditions may also encourage the recruitment of material-specific maintenance strategies, we often alternatively rely on a default, or back-up, process in which general attentional mechanisms are used to reactivate information stored in WM.
As with the embedded-processes and time-based resource sharing models of WM, we assume the contribution of two maintenance mechanisms: a material-specific articulatory rehearsal mechanism that requires little attention, as well as an attentionally mediated refreshing mechanism (Barrouillet & Camos, 2009; Cowan, 2001; Lewandowsky & Oberauer, 2008). The attentional refreshing mechanism and subvocal rehearsal mechanism are complementary, although task and stimulus demands may bias one to be deployed more intensively than the other.
A prediction of the IA account that follows from these assumptions is that bilateral regions in the PPC should exhibit activity reflecting the attentional demands of the WM task, with the laterality of parietal engagement biased according to the hemisphere in which task memoranda are more strongly represented; e.g., the left hemisphere is more strongly activated during verbal tasks, and the right hemisphere is more strongly activated during visuospatial tasks. The recruitment of parietally mediated attentional mechanisms is especially likely in tasks that prohibit subvocal rehearsal. In contrast, tasks in which participants sustain WM traces through subvocal rehearsal should not rely on the PPC. Retrieval demands may be one important factor in determining the engagement of subvocal rehearsal, with rehearsal being more likely when memory is probed by recall than when it is probed by recognition. Consistent with this prediction, different results are obtained in fMRI and PET studies of verbal WM depending on the retrieval conditions. PPC activity in the IPL during the maintenance and/or retrieval stages is observed only when memory is probed by old/new recognition, but not when WM is probed by recall (Becker et al., 1994; Chein & Fiez, 2001; Chein, Moore, & Conway, in press; Fiez et al., 1996; Grasby et al., 1993; Jonides et al., 1998). These results are also consistent with our prior findings that patients with focal lesions to the IPL were impaired at object WM, but again, only when memory was probed by old/new recognition, and not when WM was probed by recall (Berryhill & Olson, 2008a, 2008b). Importantly, this dissociation is not limited to patients with brain damage. We recently observed that when cathodal transcranial direct current stimulation (tDCS) was applied to the right IPL of normal young adults before they performed a visual WM task, performance on recognition trials was impaired whereas performance on recall trials was normal (Berryhill, Wencil, Coslett, & Olson, 2010).
Our goal in this study was to understand the mechanistic function of the PPC in WM. Two influential views, the pure storage and the internal attention accounts, posit different roles for this region. The pure storage account proposes material-specific storage modules. The internal attention account proposes an attentional refreshing mechanism. In this paper we tested predictions of the pure storage account in Experiments 1–3 and the internal attention account in Experiments 4–6 in two rare patients with bilateral PPC damage.
Part 1: Testing the Pure Storage Account
2. Experiment 1: Test of the Modality Specificity of Observed WM Deficits
In Berryhill & Olson (2008a), we reported a surprising dissociation between preserved WM recall and impaired WM recognition performance in patients with bilateral PPC damage. We first revealed this pattern of results in an order WM task in which participants observed four sequentially presented items (colors, shapes, objects), and after a brief delay, the task was either a recall judgment or an old/new recognition judgment regarding the temporal order of the stimuli. In the recall version, a single probe item appeared after the delay and participants reported the ordinal position of the probe (1st-4th). In the recognition task, either the same or a different order of items was shown, and the task was to make a same/different judgment regarding item order. The patients exhibited preserved recall and impaired recognition performance (Berryhill & Olson, 2008a). We then replicated this same pattern of data in an object WM task. The task was to remember four objects over a brief delay, and then make either a recall response (verbally name the objects that had been shown) or perform a recognition task. Again, the same pattern of preserved recall and impaired recognition was observed.
One explanation for this pattern is offered by Baddeley’s multicomponent model of WM (e.g. Baddeley, 2000; Baddeley & Hitch, 1974; Baddeley & Logie, 1999). According to the recent mappings between the multicomponent model and brain function (Smith & Jonides, 1998; Henson, 2001; Chein, et al., 2003), our patients’ PPC lesions may have selectively damaged the visuospatial sketchpad while leaving other modules intact. If this is true, the lesions may have prevented the comparison of memory representations to perceived images on recognition trials. In contrast, for recall trials, the PPC patients may have relied on a presumably intact phonological loop to rehearse each item up to the limits of WM capacity for each module. It then follows that bilateral PPC patients should perform normally on verbal WM tasks regardless of retrieval task because they would be able to rely on an intact phonological loop; Experiment 1 tested this prediction.
2.1 Method
Participants.In Experiments 1–5, the two bilateral PPC patients and 15 control participants were tested. In each experiment, patient and control participants were matched for age and education and there were no statistically significant differences in age (Exp. 1 mean = 46.2, Exp. 2 = 50.5, Exp. 3 = 49.2, Exp. 5 = 45.3, Exp. 6 = 47.3) or education (Exp. 1 mean = 14.0, Exp. 2 = 14.1, Exp. 3 = 13.2, Exp. 5 = 13.9, Exp. 6 = 14.0) between patients and controls (all nonparametric permutation tests: p > 0.2). The PPC patients have been described previously (Berryhill & Olson, 2008a; Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007) and their neuropsychological characteristics are summarized below. Participants signed informed consent documents and were reimbursed for participating. All experimental protocols were approved by the University of Pennsylvania Internal Review Board.
2.1.1 Patient EE555
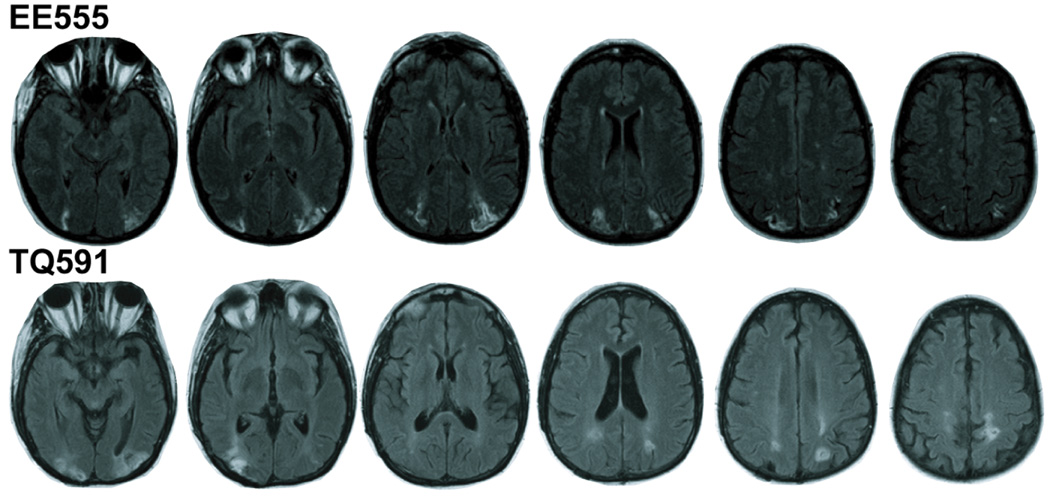
EE555 is a 42-year-old former teacher with 16 years of education. She had three sequential infarcts in 2004 affecting the watershed between the posterior and middle cerebral arteries. She experienced temporary acute symptoms including intense headaches and blindness. Following her third stroke, she was admitted to the Hospital of the University of Pennsylvania and treated for hypertension. The initial neurological evaluation revealed simultanagnosia, the inability to attend to more than one object at one time. Anatomical MRI scans revealed bilaterally symmetrical lesions extending from superior aspects of the occipital lobe through the angular gyrus (BA 39) in and around inferior and middle portions of the IPS; see Figure 1. Damage did not encroach into the precuneus or deep structures such as retrosplenial cortex.
Figure 1.
Lesion tracings. Lighter hypodensities represent the lesioned regions in patients EE555 (top) and TQ591 (bottom).
EE555’s primary deficit is simultanagnosia. She cannot ascertain the global meaning of pictures. For example, she sequentially describes isolated components of complex scenes: ‘there is a woman’, and ‘I see water’. In line cancellation tasks, she crosses off central items, ignoring peripheral items. She reports local elements when shown Navon letters. She suffers from optic ataxia, misreaching and mispointing to foveated and peripheral objects. EE555 does not have optic apraxia (inability to change location of fixation) nor does she suffer from left-right confusion. She speaks and understands language normally and performs at ceiling on the auditory tests of the Western Aphasia Battery. Visual acuity, object perception, and color perception are normal.
EE555’s attention was tested by the three auditory subtests (Elevator Counting, Elevator Counting with Distraction, and Lottery) of the Test of Everyday Attention (TEA) (Robertson, Ward, Ridgeway, & Nimmo-Smith, 1994). EE555 can maintain attention normally unless distracters are present. Her performance was at ceiling on the Elevator Counting Test, in which periodic tone pips are counted. She was in the low-normal range (scaled score 9, percentile 30.9–43.4) when she had to tally some tones while ignoring others. EE555 was also significantly impaired on the Lottery task, which required sustained attention to listen for a number cue and WM updating to retrieve the letters preceding the number cue. She performed in the significantly impaired range (scaled score 3, percentile .6–1.5).
2.1.2 Patient TQ591
TQ591 is a 51-year-old former preschool assistant teacher with 15 years of education. She suffered bilateral parieto-occipital damage due to CNS cerebral vasculitis in March 2006. She was treated at the Hospital of the University of Pennsylvania. TQ591’s MRI revealed signs of previous subacute posterior cerebral artery infarctions. The primary lesions are in bilateral PPC; see Figure 1. The left parietal lesion extends into IPS (BA 39) and slightly into the precuneus (BA 7). There are two right lesion sites: the inferior lesion is in superior aspects of the occipital lobe (BA 18 and 19), and the superior lesion is in the superior parietal lobe (BA 7). Bilaterally, the lesions extend into temporo-occipital (BA 19) regions and parietal white matter.
TQ591’s primary deficit is simultanagnosia. She is slow to describe scenes and complains that parts of scenes ‘disappear’ when she looks away and cannot be relocated. In line cancellation tasks, she identifies a few lines within a narrow visual field. She has a local bias with Navon letters. She suffers from mild optic ataxia, misreaching to objects in the periphery but not in the fovea. She also suffers from optic apraxia, making it effortful for her to move her eyes. She deliberately blinks to release attention. She has mild left-right confusion. Language comprehension and speech fluency are normal. She performs at ceiling on the auditory tests of the Western Aphasia Battery. Visual acuity, object perception, and color perception are normal.
TQ591 can maintain attention normally unless distracters are present. Her performance was at ceiling on the Elevator Counting Test of the TEA. She was in the significantly impaired range (scaled score 5, percentile 1.5–3.3) on the Elevator Counting with Distraction Test. TQ591’s performance was within the low-normal range (scaled score 8, percentile 20.2–30.9) on the Lottery task.
2.1.3 Stimuli
36 1-syllable words with a Francis-Kucera frequency of 10–12 per million were recorded in a female voice using GarageBand software.
2.1.3 Apparatus
All experiments were run on Dell computers using ePrime software (PST, PA).
2.1.4 Design
Trials began with the auditory presentation of 6 words. A set size of 6 was chosen after pilot testing showed that this set size approximated visual WM performance at a set size of 4 (Berryhill & Olson, 2008a). After a 1 s delay, participants performed a retrieval task. During the recall block, the task was to verbally report as many remembered words as possible, disregarding presentation order. During the recognition block, a probe word was presented aurally and the task was to decide whether the word was old or new (50% each). Participants were not instructed in any particular WM strategy. There were 15 recall trials and 60 recognition trials. Patient TQ591 completed all of the recall trials but tired after 40 recognition trials so testing was halted. Block order was counterbalanced and a break occurred between blocks. Breaks helped reduce interference and helped participants remain alert.
2.1.5 Analysis
Across all experiments recall performance is presented as raw accuracy. Recognition performance is presented as corrected recognition (hits minus false alarms). Chance performance for these measures was 0. Recall and recognition data were analyzed separately because they use different accuracy measures. The recall and recognition data were subjected to individual one-tailed non-parametric permutation tests analogous to parametric t-tests. One-tailed tests were used because we predicted that patients would be impaired relative to controls. This permutation test randomly reassigns group membership and performs a t-test 1000 times to create a distribution. The p-value represents the proportion of reassignments that were more different than the actual patient-group difference. Thus, there is no t-statistic. For other neuropsychological studies using this method see (Berryhill & Olson, 2008a; Konkel, Warren, Duff, Tranel, & Cohen, 2008; Olson, Moore, Stark, & Chatterjee, 2006).
2.2 Results and Discussion
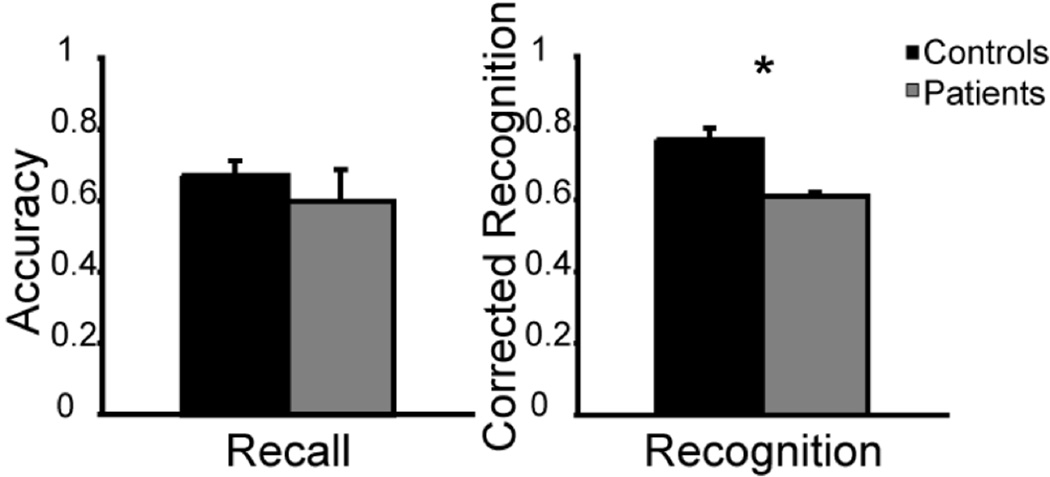
The data showed that patients had normal WM when tested by recall (patients M = .60; controls M = .67; p = .29) but impaired WM when tested by recognition (patients M = .61; controls M = .77; p = .04); see Figure 2. To ensure that patient TQ591’s fatigue did not drive this main effect, we conducted a second analysis using only the first half of the patients’ data and found the same pattern of results (p = .04).
Figure 2.
Experiment 1, verbal WM task. In all graphs recall and recognition performance is plotted as a function of accuracy/corrected accuracy for the control (black) and patient groups (gray). Error bars represent the standard error of the mean. Asterisks symbolize a significant difference between patients and controls.
These data counter the hypothesis that select damage to the putative visuospatial sketchpad module (Baddeley, 1986; Baddeley et al., 2000; Baddeley & Hitch, 1974; Baddeley & Logie, 1999; Repovs & Baddeley, 2006) produced the recall/recognition dissociation observed in our prior studies of WM in these patients. Moreover, these data extend our earlier visual WM findings (Berryhill & Olson, 2008a) by showing that the recall/recognition dissociation observed in a visual WM task after PPC damage generalizes to an auditory-verbal WM task. Furthermore, the pure storage model does not predict that there should be an effect of retrieval task on performance. In other words, impairment in recognition WM should be paralleled by impairment in recall WM since maintenance demands should remain constant across recall and recognition WM retrieval tasks. Thus, the results do not appear to be consistent with pure storage accounts of WM.
3. Experiment 2: Effects of Memory Load on Patient WM
A second prediction made by the pure storage account is that PPC damage should disproportionately affect WM performance when maintenance demands are high. This is because the visuospatial sketchpad and phonological loop have a time-based and quantity-based capacity that when damaged, should be dramatically limited. Thus, the pure storage account predicts that patients’ performance should be severely impaired when maintenance demands increase, regardless of response task. In contrast, the IA account is presumed to be less sensitive to high maintenance demands, because it predicts only a diminished capacity for attentional refreshing, which can be supplemented by verbal rehearsal. Thus, the IA account predicts that performance should drop off less acutely as maintenance demands increase. Two factors that increase maintenance demands are WM load and delay. These factors were tested in Experiments 2 (memory load) and 3 (delay).
3.1 Method
3.1.1 Stimuli
The stimuli were 20 grayscale tool stimuli used previously in visual WM studies (Berryhill & Olson, 2008a, 2008b).
3.1.2 Design
Trials began with a fixation cross (1000 ms), followed by the sequential presentation of 1, 4, or 6 stimuli (1000 ms/stimulus) at central fixation. After the last stimulus, a checkerboard mask appeared (1000 ms). During the block of recall trials, a text probe appeared which prompted participants to verbally report what they had seen; responses were recorded. During the block of recognition trials, a probe image appeared in the center of the screen and the participants made an old/new decision (50% chance). There were 15 recall trials and 20 recognition trials per set size for a total of 45 recall and 60 recognition trials.
3.1.3 Analysis
The data were subjected to a non-parametric permutation test analogous to repeated measures ANOVA with 2 levels of group (controls, patients) × 3 levels of set size (1, 4, 6). To ensure that results were not obscured by strong performance at a set size of 1, secondary analyses eliminating the set size 1 data were also conducted.
3.2 Results and Discussion
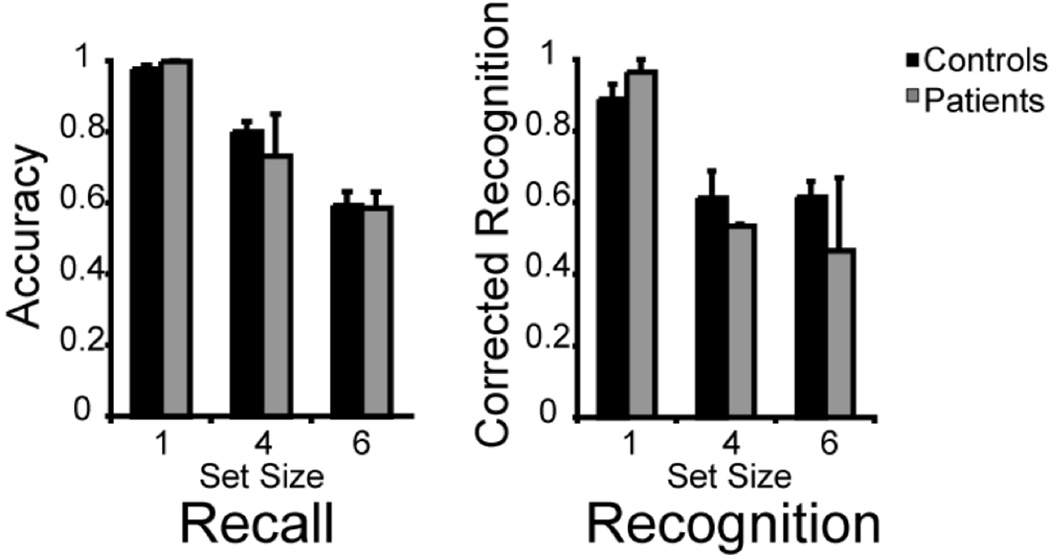
The patients and controls performed no differently at visual WM recall trials (patients M (1, 4, 6) = 1.00, .73, .59; controls M (1, 4, 6) = .98, .82, .59; F1, 15 = 4.35, p = .61); see Figure 3. As expected, both groups recalled fewer words as set size increased (F2, 30 = 116.26, p < .001). The interaction between group and set size was not significant (F < 1) showing that PPC patients were not disproportionately affected by large set sizes on recall trials. The secondary analysis in which the set size 1 data were eliminated showed the same pattern of results (group: F1, 15 = 8.52, p = .49, set size: F1, 15 = 104.94, p < .001, interaction: F1, 15 = 1.47, p = .27).
Figure 3.
Experiment 2, effects of variable set size on WM performance. Error bars represent the standard error of the mean.
Unexpectedly, patients and controls performed similarly when tested by recognition (patients M (1, 4, 6) = .97, .54, .47; controls M (1, 4, 6) = .90, .68, .63; F1, 15 = 20.50, p = .39); see Figure 3. Both groups generally performed worse as set size increased (F2, 30 = 24.65, p < .001). The interaction between group and set size was not significant (F2, 30 = 1.63, p = .21) indicating that the PPC patients were not disproportionately affected by large set sizes. The secondary analysis showed that when the set size 1 data were excluded, there were no significant differences between groups (F1, 15 = 34.75, p = .27) or set sizes (F1, 15 = 1.18, p = .29). The interaction remained nonsignificant (F1, 15 = .01, p = .91).
These findings do not support the prediction of the pure storage account, that PPC patients should have greater WM impairments when maintenance demands are higher. These results partially replicate our prior findings by showing that PPC damage does not affect WM performance as tested by recall. We were surprised to see that the patients also performed well on recognition trials whereas previously they were significantly impaired at set size 4. The high levels of patient performance across retrieval conditions may have been caused by the novel implementation of a covert rehearsal strategy on recognition trials where none was used previously. The interleaved set sizes imposed fluctuating WM demands that may have encouraged this type of strategy. This topic is further addressed in Experiment 4.
4. Experiment 3: Effects of Memory Delay on Patient WM
4.1 Stimuli, Design, and Analysis
The design was similar to the set size 4 condition used in Experiment 2. Three delay durations were included in separate blocks: 1, 10, or 20 s. Recall and recognition tests were blocked separately and breaks were taken between blocks. There were 10 recall and 20 recognition trials per delay duration for a total of 30 recall and 60 recognition trials. Data analysis was similar to that used in Experiment 2.
4.2 Results and Discussion
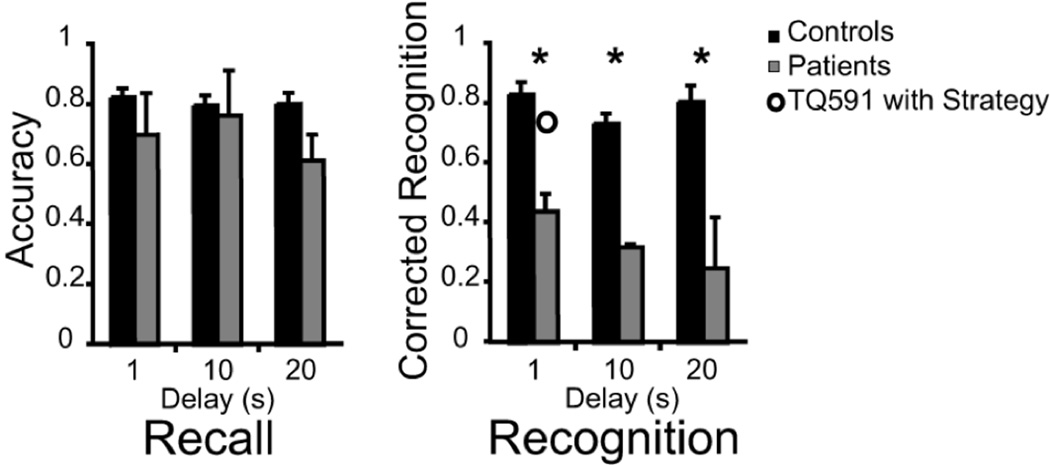
Patients and controls performed similarly when WM was probed by recall (patients M (1, 10, 20) = .70, .76, .61; controls M (1, 10, 20) = .84, .81, .81; F1, 15 = 18.72, p = .20); see Figure 4. There was no main effect of delay (F2, 30 = 1.34, p = .28) and the interaction of group × delay did not reach significance (F2, 30 = 2.36, p = .11) indicating that the PPC patients were not disproportionately affected by delay length.
Figure 4.
Experiment 3, the bars show the effects of variable delay interval on WM performance. The open circle shows patient TQ591’s performance when she was provided with a rehearsal strategy. She was instructed to rehearse the object names during the course of an old/new recognition trial.
In contrast, the patients were impaired when WM was probed by old/new recognition (patients M (1, 10, 20) = .44, .32, .25; controls M (1, 10, 20) = .83, .73, .75; F1, 15 = 94.76, p < .001); see Figure 4. Although there was a significant main effect of group, the main effect of delay duration did not reach significance (F2, 30 = 2.45, p = .10). The interaction of group x delay also failed to reach significance (F < 1). In summary, the PPC patients were impaired across delay durations on recognition WM trials. The degree of impairment did not increase as delay duration lengthened which provides a second indication that the PPC patients were not disproportionately affected by greater maintenance demands.
The data from Experiment 3 does not strongly support the pure storage account because PPC patients did not show disproportionate WM deficits as maintenance demands increased with longer delay intervals. However we cannot rule out the possibility that the absence of an interaction effect was due to low power since numerically, patient performance suffered more with extended delay periods. In concordance with our prior findings, the patients performed normally on WM trials that were probed by recall. On recognition trials, patients were impaired equally across different delay durations. These data show that the recall/recognition dissociation exists across a range of short delay intervals.
5. Part 2: Testing the Internal Attention Account
The results from Part 1 were not consistent with predictions of pure storage accounts. In Part 2 we tested an alternative WM model, the IA account, to see if it provided greater explanatory power regarding the role of the PPC in WM. At the center of the IA model is the idea that participants modulate their maintenance strategies to optimize performance and minimize effort, and this modulation is associated with the particular task demands. Thus, we began in Experiment 4 by asking whether normal participants show any evidence of strategic differences in the way they maintain information on recall versus recognition tasks. To foreshadow our results, we find evidence for a rehearsal-based maintenance process on recall trials that is not apparent during recognition trials. Linking this to our prior patient findings, we must assume that the non-rehearsal based maintenance strategy that is used on old/new recognition trials is disrupted by PPC damage. To gain further evidence for this, in Experiment 5 we interleaved recall and recognition WM trials to compel participants to use a rehearsal maintenance strategy for both trial types. In Experiment 6, we induced patients to drop a rehearsal-based strategy by exceeding WM capacity and lengthening the delay interval.
6. Experiment 4: Strategic Differences Between Recall and Recognition Tasks in Normal Participants
The IA model presumes that individuals have two WM strategies at their disposal and that they preferentially adopt an attention based maintenance approach when the task is dominated by old/new recognition trials, and a rehearsal based approach with when the task is dominated by verbal recall trials. Here, we sought evidence for this distinction in healthy participants by testing explicit and implicit measures of WM strategy. First, we asked participants to describe what strategy they were using after completing a short WM task. We anticipated that participants would have poor insight into this so we included a second, more implicit measure: word-length. Word-length is inversely related to WM capacity (Baddeley, Thomson, Buchanan, 1975). The interpretation of the word-length effect is that longer items require longer rehearsal time, which means they may be lost from WM while awaiting rehearsal (but see Nairne, 2002 for a review of alternative interpretations). The IA model predicts that the word-length effect would be apparent on recall trials since performance on these trials is reliant on rehearsal, but not on old/new recognition trials, since an attention based maintenance strategy, not rehearsal, is used on these trials.
6.1 Method
6.1.1. Participants
We tested two groups of 22 healthy young adults (ages 18–25) from the Temple University psychology subject pool. Participants received course credit for their time.
6.1.2 Stimuli
Two types of colorized Snodgrass pictures were used as stimuli: those depicting items with one-syllable names (e.g., sun, eye) and those depicting items with 2–5 syllable names (e.g. accordion, refrigerator).
6.1.3 Design
The design was similar to the WM design used in Experiment 2 except it was a between-groups design with Recall and Recognition WM groups. There were two blocks: a short syllable block followed by an assessment of strategy, and a long syllable block followed by a second assessment of strategy. Block order was counterbalanced.
During each trial in a 10-trial block, participants viewed four sequentially presented colorized Snodgrass pictures (1 s/image). After a 1 s delay participants made their WM response. The Recall group typed in the names of all the items they remembered. Responses were considered correct if spelling was approximate. Multiple correct answers were considered correct if they were reasonable, for example for the refrigerator, a response of ‘fridge’ was accepted. The Recognition group viewed one probe image and made an unspeeded old/new decision by key press. They were then asked to describe in writing what their strategy had been and then to endorse rehearsal or attention-based strategies from a checklist of options.
6.1.4 Analysis
For the explicit strategy analysis, we tallied the participants’ explicitly stated primary strategy and calculated the proportion of participants endorsing a rehearsal based or attention based strategy. For the implicit strategy analysis, we examined the change in performance between the first and second halves of the study to identify a word-length effect. As above, for Recall raw accuracy was used and for Recognition we used corrected recognition.
6.2 Results and Discussion
The majority of participants in both groups stated that they relied on a verbal rehearsal strategy to perform WM trials (M Recognition = .86, M Recall = .85) suggesting that participants are not consciously aware of strategy differences between recall and recognition WM tasks. The assessment of word-length revealed a different story. The Recall group performed worse when the stimuli were pictures with longer names (M short words = .89, M long words = .84, t21 = 2.15, p = .04). In contrast, the length of the picture labels did not significantly affect performance in the Recognition group (M short words = .71, M long words = .80, t21 = 1.41, p = .17). Moreover, an ANOVA comparing word length effect and group revealed a significant interaction (F1, 42 = 4.29, p = .045) indicating that the effect of word length depended on the retrieval task.
These findings are consistent with the idea that participants adopt different strategies depending on how WM is probed. When they know that WM will be probed by recall, participants unknowingly rely more heavily on verbal rehearsal than during similar recognition WM tasks. An alternative explanation is that the output demands of typing the words during recall may have enhanced the word length effect by creating longer maintenance times, thus leading to greater decay (reviewed in Nairne, 2002). However, this interpretation is not supported by the data: participants were able to recall 3.56 items in the recall task, but only 3.01 items in the recognition task, as calculated by Cowan’s K (Cowan, 2001). A second concern is that the word frequencies differed between the two tasks, causing output differences. However the stimuli that we used were based on the Snodgrass and Vanderwort (1980) stimulus set that controlled for familiarity, which correlates with frequency.
7. Experiment 5: Encouraging Rehearsal with Interleaved Trials
The IA account makes the basic prediction that attentionally demanding WM tasks should rely more heavily on the PPC than WM tasks that require little internal attention. As such, patients with damage to the PPC should perform well on tasks that encourage rehearsal-based maintenance because little internal attention is required to maintain information. In contrast, tasks that cannot be supported by material-specific rehearsal should rely on the PPC for sustaining the memory trace and thus, PPC patients should be impaired.
This idea can explain the recall/recognition dissociation that we previously observed because recall and recognition trials were presented in separate blocks. Blocking makes it possible for participants to apply different retrieval strategies for each trial type. Thus, participants may have sustained WM traces with active rehearsal on recall trials whereas a default attention-scanning strategy may have been used on recognition trials. In Experiment 4 we reasoned that if recall and recognition trials were intermingled, making the retrieval demands unpredictable, participants would default to a covert rehearsal strategy since this would lead to optimum performance across both trial types. PPC patients would then adopt the effective strategy typically implemented only during recall trials, and show normal WM performance on recognition trials.
7.1 Method
7.1.1 Design
The design was the same as Experiment 2 with three exceptions (1) one set size of 4 was tested; (2) colorized Snodgrass pictures were used as stimuli instead of grayscale tools; and most importantly (3) recall and recognition trials were randomly interleaved. Retrieval condition was indicated at test. There were 64 trials, evenly divided between recall and recognition.
7.2 Results and Discussion
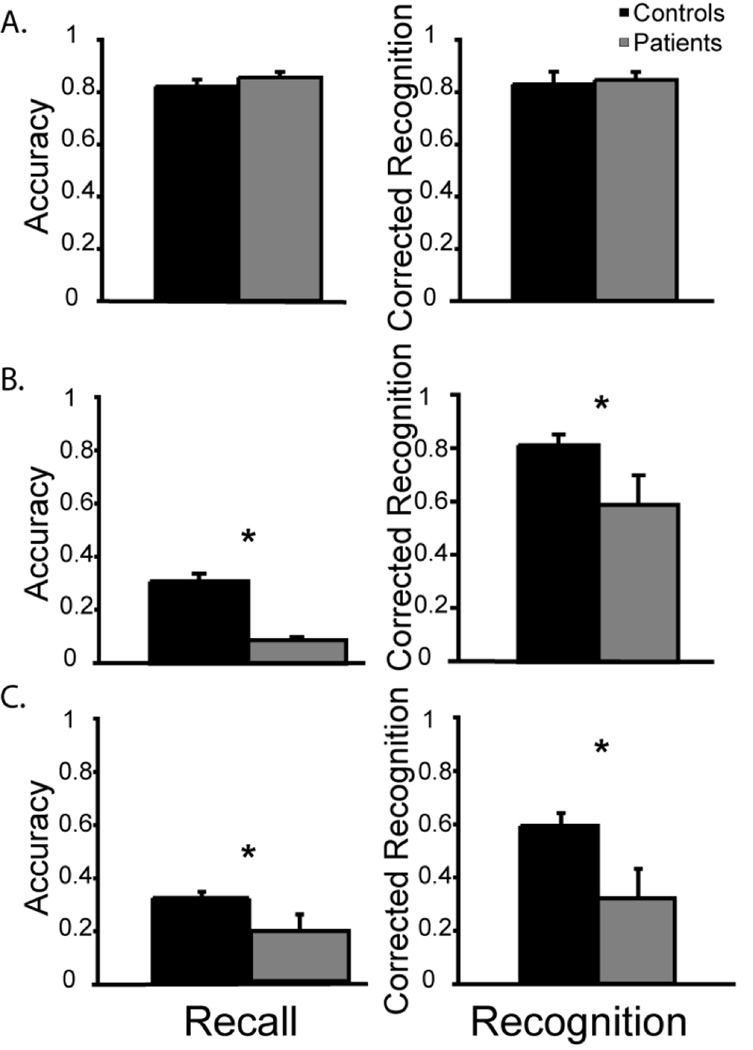
The results revealed no difference in performance between patients and controls on recall (patient M recall: .86; control M recall: .84; p > .60) or recognition trials (patient M recognition: .85; control M recognition: .86; p > .45); see Figure 5.
Figure 5.
A) Experiment 5, the unpredictable retrieval task equates performance across patient and control groups. B–C) Experiment 6, the prevention of rehearsal for (B) visual or (C) verbal stimuli reveals impaired performance by the patients when responding probed by either recall or recognition. Error bars reflect the standard error of the mean.
This finding supports the IA model by showing that when patients used a ‘recall’ rehearsal strategy, PPC damage no longer affected WM performance on recognition trials. Further support for this view was found when in a separate testing session, in which we instructed patient TQ591 to use a verbal rehearsal strategy when performing the recognition WM trials tested in Experiment 3 (delay of 1 s). Her performance on this task improved to normal levels (CR = .75); see open circular symbol in Figure 4.
8. Experiment 6: Preventing Rehearsal with Long Lists and Long Delays
A prediction of the IA model is that whenever material-specific rehearsal is prohibited by task design, or if it is difficult to implement, participants will use an attentionally demanding maintenance strategy, regardless of retrieval demands. Because the locus of the attention-based mechanism is thought to lie in the PPC, it is predicted that patients with PPC lesions will be impaired on both WM and long-term memory tasks when they cannot use rehearsal. We tested this prediction by extending the task into the long-term memory domain by extending the set size to exceed WM span and by lengthening the delay period to make rehearsal onerous.
8.1 Method
8.1.1 Stimuli
Stimuli consisted of two matched sets of words (text) and images (colorized drawings). The items were objects (e.g. a picture of a football or the word football), animals (e.g. a picture of a dog or the word dog), and fruits (e.g. a picture of a cherry or the word cherry). Verbal stimuli were spoken aloud to ensure that participants apprehended the stimuli.
8.1.2 Design
The procedure for verbal and visual stimuli were identical. At encoding, 40 stimuli were sequentially presented for 3 s each. Next, a conversation cue appeared and the experimenter and participant discussed the topic for 5 minutes to limit information rehearsal. There were two retrieval tasks. First, participants were asked to freely recall as many of the encoded items as possible. Second, recognition was tested by presenting a stimulus on the computer screen, and requiring participants to make an old/new decision by key press. In both cases, chance performance = 0. Separate visual and verbal blocks were counterbalanced across participants.
8.1.3 Analysis
The recall and recognition data were compared with separate non-parametric permutation analyses with the factors of group (control, patient) and stimulus type (visual, verbal). Raw accuracy is presented for the recall data; corrected recognition (hits - false alarms) is presented for the recognition data, such that chance is equal to 0.
8.2 Results and Discussion
The patients’ recall performance of 9% for visual and 19% for verbal stimuli was significantly worse than that of the control participants (M pictures = .35; M words = .32, F1, 15 = 524.39, p < .037); see Figure 5BC. There was no main effect of stimulus type (F < 1) and no interaction of stimulus type and group (F < 1) indicating that the patients were similarly impaired for visual and verbal stimuli.
The recognition data were similar. The patients’ corrected recognition performance of 59% for visual and 32% for verbal stimuli was significantly worse than that of control participants (M pictures = .83; M words = .62, F1, 15 = 167.90, p < .0001); see Figure 5BC. There was a main effect of stimulus type (F1, 15 = 17.47, p = .0004) such that performance was generally superior for visual stimuli. The interaction of group and stimulus type did not reach significance (F < 1) indicating that the patients were similarly impaired for visual and verbal stimuli.
These findings support our prediction: when subvocal rehearsal strategies were onerous, as in this long-term memory paradigm, PPC patients were impaired across both recall and recognition trials. Similar results were obtained in a preliminary pilot study, with the only difference being that a smaller number of stimuli were tested and the experimental design was 2-alternative forced choice, rather than old/new recognition. In both this study and the pilot study, patient performance was above chance, indicating that the observed results were not due to catastrophic memory failure.
9. General Discussion
The goal of this paper was to specify the mechanistic function of the PPC in WM. To do this, we leveraged our previous finding showing a WM deficit limited to old/new recognition after inferior PPC damage. Strikingly, this damage did not appear to affect performance on WM trials probed by recall (Berryhill & Olson, 2008a).
The dissociation between performance on recall and recognition WM tasks has important theoretical implications. It was a first indication that our results failed to support pure storage models of WM. Pure storage models propose that portions of the PPC store a capacity limited number of verbal or visuospatial items in WM over short delays through automatic and explicit rehearsal (Baddeley, 1986; Baddeley et al., 2000; Baddeley & Hitch, 1974; Baddeley & Logie, 1999; Repovs & Baddeley, 2006). In the present study we tested this account more fully. We manipulated two variables that modulate the difficulty of WM maintenance - set size and delay. Contrary to the predictions of the pure storage account neither variable strongly affected patient performance (Experiments 2–3). However, the retrieval task (recognition, recall) affected patient performance in a way that the pure storage model does not predict.
Instead, our results confirm predictions of an IA account (Chein et al., 2003) derived from attentional models of WM such as the embedded-processes model (Cowan, 1988, 1993, 1995, 1999). This unitary model proposes that WM capacity is governed by the focus of internal attention on memoranda. In other words, WM performance is a function of IA. Representations of items are kept active in WM by focusing attention on them. When internal attention is redirected, decay is initiated. A portion of the PPC has been proposed as the seat of this attentional process (Chein et al., 2003). The present data support several predictions of this model. First, this model predicts that portions of the PPC, specifically regions in the IPL, are necessary for WM when an attentionally-demanding maintenance strategy is used, but not when a subvocal rehearsal-based maintenance strategy is used. This was observed in the dissociation between recall/recognition performance described in Experiments 1 and 3 (see also Berryhill & Olson, 2008a). However some caution must be exercised in completely rejecting pure storage models as we did not find the expected recall-recognition dissociation in Experiment 2. In Experiment 4, we verified that normal individuals are more likely to use a rehearsal-based strategy on WM trials tested by recall than on WM trials tested by recognition. Second, the model predicted that when PPC patients are forced to use a rehearsal-based covert maintenance strategy for recognition trials, their performance should improve to normal levels; this prediction was confirmed in Experiment 5. Third, the model predicted that the opposite should also be observed: when patients were unable to use a rehearsal-based maintenance strategy, their performance should drop to abnormal levels even on recall trials; this was observed in Experiment 6. These deficits are compounded by the fact that the patients have difficulty sustaining attention in the presence of distracters.
Our interpretation relates to WM maintenance, but it remains distinct from the pure storage view advocated by the multimodal model of WM and supported by some neuroimaging data (Todd & Marois, 2004). The primary difference is that the pure storage view affords no role for PPC involvement in internal attention. According to the present interpretation, neural activity in portions of the PPC during WM maintenance reflects access to or shifts in the focus of internal attention. Thus, fMRI studies showing parametric modulations of PPC activity around the IPS corresponding to WM capacity (e.g. Todd & Marois, 2004) may reflect the number of items currently active within the focus of internal attention, or the number of items being maintained through rapid attentional shifting. Several recent fMRI studies support this view. For instance, similar patterns of bilateral IPS activity during perceptual attention and WM tasks have been reported (Mitchell & Cusack, 2008; Magen, Emmanouil, McMains, Kastner, & Treisman, 2009).
9.1 Predictions of the IA Model and Available Evidence
The IA model predicts that disabling portions of the PPC will impair WM performance on tasks placing heavy demands on the attentional functions of the PPC. We have identified three categories of WM tasks that meet this criteria: (1) most old/new recognition tasks, because participants may adopt a less onerous ‘wait and see’ approach that relies on attentional refreshing rather than a taxing verbal rehearsal strategy (for discussion see (Chein & Fiez, in press; Chein et al., 2003)); (2) WM tasks requiring information manipulation or dual task performance (e.g. complex span tasks like operation span), because these tasks demand rapid shifts of attention regardless of retrieval task; and (3) WM tasks that require the maintenance of difficult-to-rehearse information, such as spatial location, regardless of probe task. In the next paragraphs, we marshal evidence that directly speaks to these predictions.
First, our previous data, along with the data in Experiments 1–3 of this paper, showed that unilateral or bilateral PPC lesions impair performance on old/new recognition tasks but not recall WM performance (Berryhill & Olson, 2008a, 2008b). Functional neuroimaging studies testing verbal WM recall do not report PPC activations, whereas recognition WM performance does activate the PPC (Chein & Fiez, 2001, in press; Chein et al., in press; Fiez et al., 1996). Broad, bilateral superior parietal activations are reported when the WM task requires the active maintenance of verbal or visuospatial information, with greater left hemispheric activations for verbal information and greater right hemispheric activations for visuospatial information (Chein et al., in press). Furthermore, we recently found that transcranial direct current stimulation (tDCS) to the right IPL selectively reduces object WM in healthy young adults only when tested by old/new recognition but not when tested by recall (Berryhill, Drowos, & Olson, 2010). And finally, transcranial magnetic stimulation (TMS) designed to disrupt the superior parietal lobe can reduce WM for both passively maintaining letters and manipulated letters in an alphabetization task as tested by old/new recognition (Postle et al., 2006).
Second, WM tasks requiring information manipulation or dual task performance appear to rely on the PPC. For instance, it was reported that WM for letter order with a manipulation component was decreased by TMS applied to the superior parietal lobe (Postle et al., 2006). Another group reported that WM for auditory pitch in an N-back task was affected by right IPL TMS and performance in an auditory WM task was affected by left IPL TMS (Imm et al., 2008). Likewise, Koenigs and colleagues (2009) tested a large group of patients with unilateral left or right superior PPC lesions on an array of WM tasks. They found that the patients performed normally on WM tasks requiring maintenance alone, such as digit span. Note that these tasks all required a recall response, which, as we have shown, is typically accompanied by normal levels of performance after PPC damage. The IA model predicts that WM performance should fail on recall tasks when material-specific maintenance is difficult to implement or when the task puts heavy demands on the attentional processes of the parietal lobe – which is exactly what Koenigs and colleagues found in a different set of WM tasks requiring manipulation and rearrangement of information, such as digit-span backwards and N-back tasks (Koenigs, Barbey, Postle, & Grafman, 2009). This maintenance-manipulation dissociation is especially interesting because it directly supports the IA hypothesis. We suggest that manipulating information within WM puts a heavy load on the attentional functions of the PPC, by requiring many shifts of attention between the original stimuli and the updated stimuli.
Third, the IA model predicts that the PPC should be necessary for WM tasks that require the maintenance of difficult-to-rehearse information, such as locations or chromatic hue, regardless of probe task. Information that varies along a continuous dimension cannot be easily maintained using subvocal rehearsal. In support of this, several studies have shown that TMS stimulation applied to the right, and sometimes left PPC decreases spatial WM as tested by old/new recognition (Hamidi, Slagter, Tononi, & Postle, 2009; Hamidi, Tononi, & Postle, 2008; Koch et al., 2005; Yamanaka, Yamagata, Tomioka, Kawasaki, & Mimura, 2009). Predating brain stimulation findings, numerous studies of patients with right-lateralized PPC lesions reported that these patients had spatial WM deficits on both recall and recognition trials (Olson & Berryhill, 2009).
The IA model predicts that TMS to the same regions of the PPC should not disrupt performance on WM tasks in which a material-specific rehearsal mechanism, requiring little internal attention, can be used to maintain information. Tasks that meet these criteria include digit span, and other immediate recall tasks lacking a manipulation component. The only brain stimulation study testing this prediction is our recent tDCS study (Berryhill, Wencil, et al., 2010) in which we confirmed this prediction. Neuropsychological studies by our group and by others (Berryhill & Olson, 2008a, 2008b; Koenigs et al., 2009) also support this prediction.
9.2 Limitations and Open Questions
One limitation of the present study is that the patients’ lesions mostly spared the precuneus, supramarginal gyri, and anterior portions of the intraparietal sulcus. It is possible that we did not find support for the pure storage view because the regions necessary for pure maintenance were spared in our patients. Related to this issue, we were unable to precisely link PPC structure to function since our patients’ lesions were large and bilateral. While neuroimaging findings indicate a relatively dorsal parietal site (extending from the superior supramarginal gyrus, through the intraparietal sulcus, and into the superior parietal lobule) as the likely locus of attention-based maintenance in WM (e.g., Chein et al., in press), recent work suggests that more ventral regions of the parietal cortex (inferior supramarginal gyrus, temporo-parietal junction) might also subserve attentional processes engaged to support WM (Ravizza, Hazeltine, Ruiz, & Zhu, 2010). We are also unable to ascertain the hemispheric laterality of the effects in the present study. Our review of relevant brain stimulation studies revealed similar structure-function limitations within that literature, a problem which is now being remedied by using fMRI to guide the selection of stimulation sites (see Postle & Feredoes, 2010). Some prior findings indicate that there is a strong right parietal lateralization for attentional processes (reviewed in Corbetta & Shulman, 2002). However, we speculate that the lateralized engagement of parietally-mediated attention mechanisms may be a function of the hemisphere in which the mental objects of attention are represented. Other limitations of our study include small sample size and the inherent problems associated with studying a population that is in poor health.
One question that arises is why our patients did not use a successful material-specific rehearsal strategy during old/new recognition WM trials. We do not have a firm answer to this question. We do know that when instructed to apply a rehearsal strategy during a WM test with old/new recognition trials, patient TQ591’s performance improved to normal levels (see Figure 4). There are other recorded instances in which lesion patients unknowingly exhibit a suboptimal processing strategy. For instance, Adolphs and colleagues (Adolphs et al., 2005) reported that a patient with amygdala damage showed an impaired ability to recognize fear from facial expressions that was due to lack of spontaneous fixations towards the eyes of freely viewed faces. When instructed to look at the eyes of others’ faces, her ability to recognize fear returned to normal. In normal adults, performance on whole report (recall) and change detection (recognition) measures is more similar when the retrieval demands are unpredictable (Cusack, Lehmann, Veldsman, & Mitchell, 2009). This finding suggests that when there is task uncertainty, it is strategic to adopt the more rigorous recall strategy that will lead to success regardless of retrieval task demands. These findings support the view that WM strategy plays an important role in WM in the normal population. However, unlike individuals with PPC lesions, the default strategy applied by neurologically normal individuals during recognition WM trials is fairly successful.
9.3 Multistore View of Memory
The fact that damage to inferior portions of the PPC caused deficits on both WM and LTM tasks (also see Berryhill, et al., 2007; Berryhill, Drowos, & Olson, 2010; Berryhill & Olson, 2008b) calls into question the multistore dichotomy partitioning the neural correlates of short and long term forms of memory. Instead, brain regions associated with long-term memory (e.g. the hippocampus) or short-term forms of memory (e.g. the PPC) appear to have roles spanning this classic distinction (for recent reviews see Graham, Barense, & Lee, 2010; Jonides et al., 2008). Attentional processes may be recruited during WM maintenance and LTM retrieval, but in a complementary fashion. In WM maintenance, attention can serve to refresh information as a way to overcome decay or interference. In LTM, attention may contribute to the search for trial-relevant representations. However we have observed that PPC lesions affect only some types of LTM (Berryhill, et al., 2007; Drowos, et al., 2010); whether attentional demands are the critical factor denoting which types of LTM rely on PPC computations (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008) requires further investigation. Nevertheless, the convergence of these perspectives suggests a widespread attentional role for the PPC across perception, WM, and some forms of LTM.
Acknowledgments
We would like to thank David Drowos and David McCoy for their help testing control participants, Dr. Marianna Stark for scheduling patients, and Dr. Anjan Chatterjee for use of the Hospital of the University of Pennsylvania Patient Database. This work was supported by a (NRSA NS059093 to M.B.) and (NIMH MH071615 grant to I.O.). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. 1986 [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature Reviews Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Bueno O, Cahill L, Fuster JM, Izquierdo I, McGaugh JL, et al. The brain decade in debate: I. Neurobiology of learning and memory. Braz J Med Biol Res. 2000;33(9):993–1002. doi: 10.1590/s0100-879x2000000900002. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Hitch GJ, editors. Working memory. Vol. 8. New York: Academic press; 1974. [Google Scholar]
- Baddeley A, Logie RH. Working memory: The multiple-component model. In: Miyake A, Shah P, editors. Models of Working Memory. New York: Camberidge University Press; 1999. [Google Scholar]
- Baddeley AD, Thomson N, Buchanan M. Word length and the structure of short-term memory. Journal of Verbal Learning and Verbal Behavior. 1975;14(6):575–589. [Google Scholar]
- Barrouillet P, Camos V. Interference: unique source of forgetting in working memory? Trends Cogn Sci. 2009;13(4):145–146. doi: 10.1016/j.tics.2009.01.002. author reply 146–147. [DOI] [PubMed] [Google Scholar]
- Becker JT, Mintun MA, Diehl DJ, Dobkin J, Martidis A, Madoff DC, et al. Functional neuroanatomy of verbal free recall: A replication study. Hum Brain Mapp. 1994;1:284–292. doi: 10.1002/hbm.460010406. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Drowos DB, Olson IR. Bilateral parietal cortex damage does not impair associative memory for paired stimuli. Cogn Neuropsychol. 2010:1–14. doi: 10.1080/02643290903534150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. Is the posterior parietal lobe involved in working memory retrieval? Evidence from patients with bilateral parietal lobe damage. Neuropsychologia. 2008a;46(7):1775–1786. doi: 10.1016/j.neuropsychologia.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. The right parietal lobe is critical for visual working memory. Neuropsychologia. 2008b;46(7):1767–1774. doi: 10.1016/j.neuropsychologia.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience. 2007;(27):14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Wencil EB, Coslett HB, Olson IR. A Selective Working Memory Impairment after Transcranial Direct Current Stimulation to the Right Parietal Lobe. Neurosci Lett. 2010;479:312–316. doi: 10.1016/j.neulet.2010.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, D'Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20(5):762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Fiez JA. Dissociation of verbal working memory system components using a delayed serial recall task. Cereb Cortex. 2001;11(11):1003–1014. doi: 10.1093/cercor/11.11.1003. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fiez JA. Evaluating models of working memory through the effects of concurrent irrelevant information. J Exp Psychol Gen. 139(1):117–137. doi: 10.1037/a0018200. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Moore AB, Conway AR. Domain-general mechanisms of complex working memory span. Neuroimage. doi: 10.1016/j.neuroimage.2010.07.067. (in press) [DOI] [PubMed] [Google Scholar]
- Chein JM, Ravizza SM, Fiez JA. Using neuroimaging to evaluate models of working memory and their implications for language processing. Journal of Neurolinguistics. 2003;16:315–339. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cowan N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychol Bull. 1988;104(2):163–191. doi: 10.1037/0033-2909.104.2.163. [DOI] [PubMed] [Google Scholar]
- Cowan N. Activation, attention, and short-term memory. Mem Cognit. 1993;21(2):162–167. doi: 10.3758/bf03202728. [DOI] [PubMed] [Google Scholar]
- Cowan N. Attention and memory:An integrated framework. New York: Oxford University Press; 1995. [Google Scholar]
- Cowan N. An embedded-processes model of working memory. In: Miyake A, Shah P, editors. Models of working memory: mechanisms of active maintenance and executive control. New York: Cambridge University Press; 1999. pp. 62–101. [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral & Brain Sciences. 2001;24(1):87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cusack R, Lehmann M, Veldsman M, Mitchell DJ. Encoding strategy and not visual working memory capacity correlates with intelligence. Psychon Bull Rev. 2009;16(4):641–647. doi: 10.3758/PBR.16.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drowos D, Berryhill ME, Andre JM, Olson IR. True memory, false memory, and subjective recollection deficits after focal parietal lobe lesions. Neuropsychology. 2010;24(4):465–475. doi: 10.1037/a0018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci. 1996;16(2):808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010 doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Bench C, Frackowiak RS, Dolan RJ. Functional mapping of brain areas implicated in auditory--verbal memory function. Brain. 1993;116(Pt 1):1–20. doi: 10.1093/brain/116.1.1. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Repetitive Transcranial Magnetic Stimulation Affects behavior by Biasing Endogenous Cortical Oscillations. Front Integr Neurosci. 2009;3:14. doi: 10.3389/neuro.07.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Tononi G, Postle BR. Evaluating frontal and parietal contributions to spatial working memory with repetitive transcranial magnetic stimulation. Brain Res. 2008;1230:202–210. doi: 10.1016/j.brainres.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R. Neural working memory. In: Andrade J, editor. Working memory in perspective. Philadelphia, PA: Psychology Press; 2001. pp. 151–173. [Google Scholar]
- Imm JH, Kang E, Youn T, Park H, Kim JI, Kang JI, et al. Different hemispheric specializations for pitch and audioverbal working memory. Neuroreport. 2008;19(1):99–103. doi: 10.1097/WNR.0b013e3282f36f91. [DOI] [PubMed] [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, et al. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18(13):5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Carlesimo GA, Turriziani P, Caltagirone C. rTMS evidence of different delay and decision processes in a fronto-parietal neuronal network activated during spatial working memory. Neuroimage. 2005;24(1):34–39. doi: 10.1016/j.neuroimage.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;29(47):14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowsky S, Oberauer K. The word-length effect provides no evidence for decay in short-term memory. Psychon Bull Rev. 2008;15(5):875–888. doi: 10.3758/PBR.15.5.875. [DOI] [PubMed] [Google Scholar]
- Lewandowsky S, Oberauer K, Brown GD. No temporal decay in verbal short-term memory. Trends Cogn Sci. 2009;13(3):120–126. doi: 10.1016/j.tics.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Magen H, Emmanouil TA, McMains SA, Kastner S, Treisman A. Attentional demands predict short-term memory load response in posterior parietal cortex. Neuropsychologia. 2009;47(8–9):1790–1798. doi: 10.1016/j.neuropsychologia.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DJ, Cusack R. Flexible, capacity-limited activity of posterior parietal cortex in perceptual as well as visual short-term memory tasks. Cereb Cortex. 2008;18:1788–1798. doi: 10.1093/cercor/bhm205. [DOI] [PubMed] [Google Scholar]
- Nairne JS. Remembering over the short-term: the case against the standard model. Annu Rev Psychol. 2002;53:53–81. doi: 10.1146/annurev.psych.53.100901.135131. [DOI] [PubMed] [Google Scholar]
- Olson IR, Berryhill M. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiol Learn Mem. 2009;91:155–165. doi: 10.1016/j.nlm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. Journal of Cognitive Neuroscience. 2006;18(7):1087–1097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Postle BR, Feredoes E. Stronger inference with direct manipulation of brain function. Cortex. 2010;46(1):121–123. doi: 10.1016/j.cortex.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson M, et al. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. J Cogn Neurosci. 2006;18(10):1712–1722. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22(2):562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Hazeltine E, Ruiz S, Zhu DC. Left TPJ activity in verbal working memory: Implications for storage-and sensory-specific models of short term memory. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience. 2006;139(1):5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, Nimmo-Smith I. The Test of Everyday Attention. London: Harcourt Assessment; 1994. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95(20):12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428(6984):751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn Affect Behav Neurosci. 2005;5(2):144–155. doi: 10.3758/cabn.5.2.144. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: active maintenance in primary memory and controlled search from secondary memory. Psychol Rev. 2007;114(1):104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from event-related fMRI. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Yamagata B, Tomioka H, Kawasaki S, Mimura M. Transcranial Magnetic Stimulation of the Parietal Cortex Facilitates Spatial Working Memory: Near-Infrared Spectroscopy Study. Cereb Cortex. 2009;20(5):1037–1045. doi: 10.1093/cercor/bhp163. [DOI] [PubMed] [Google Scholar]