Abstract
Life-threatening gastrointestinal (GI) diseases of prematurity are highly associated with systemic candidiasis. This implicates the premature GI tract as an important site for invasion by Candida. Invasive interactions of Candida spp. with immature enterocytes have heretofore not been analyzed. Using a primary immature human enterocyte line, we compared the ability of multiple isolates of different Candida spp. to penetrate, injure, and induce a cytokine response from host cells. Of all the Candida spp. analyzed, C. albicans had the greatest ability to penetrate and injure immature enterocytes and to elicit interleukin-8 (IL-8) release (p < 0.01). In addition, C. albicans was the only Candida spp. to form filamentous hyphae when in contact with immature enterocytes. Similarly, a C. albicans mutant with defective hyphal morphogenesis and invasiveness had attenuated cytotoxicity for immature enterocytes (p < 0.003). Thus, hyphal morphogenesis correlates with immature enterocyte penetration, injury and inflammatory responses. Furthermore, variability in enterocyte injury was observed among hyphal-producing C. albicans strains suggesting that individual organism genotypes also influence host-pathogen interactions. Overall, the finding that Candida spp. differed in their interactions with immature enterocytes implicates that individual spp. may employ different pathogenesis mechanisms.
INTRODUCTION
Invasive candidiasis is a significant cause of morbidity and mortality in premature neonates. Extremely low birthweight (< 1000 g) infants are most vulnerable, with invasive candidiasis rates reported to range from 3–23% of infants (1) and mortality rates of 20–60% (2). Invasive candidiasis also increases the risk of morbidities associated with prematurity including periventricular leukomalacia, bronchopulmonary dysplasia, retinopathy of prematurity and severe neurodevelopmental delay, even with appropriate anti-fungal treatment (3, 4).
In neonates, the gastrointestinal (GI) tract is the primary reservoir for Candida colonization (5). Several risk factors in the preterm infant that are associated with the development of invasive candidiasis also impact the integrity and/or microbiome of the gastrointestinal (GI) tract. For example, the use of H2 blockers and broad-spectrum antibiotics (especially third generation cephalosporins), lack of enteral feedings, and GI disease are reported to increase the risk of disseminated candidiasis (2). In particular, in a large retrospective cross-sectional study, 15% of infants diagnosed with necrotizing enterocolitis had concurrent invasive infections with Candida (6). Furthermore, in neonates with focal intestinal perforation, an entity distinct from necrotizing enterocolitis, the rate of invasive candidiasis was even higher, ranging from 44–50% (6, 7). This has led to the idea that Candida is either directly involved in damaging the GI epithelial barrier or takes advantage of injured GI epithelium in order to penetrate the host. Indeed, invasion of the bowel wall by Candida at the site of perforation has been observed in neonates with spontaneous intestinal perforation (8) and oral inoculation of C. albicans resulted in intestinal ulceration and necrosis and was associated with systemic dissemination of the fungus in a gnotobiotic piglet model (9).
The pathogenesis of invasive candidiasis is thought to involve colonization/adhesion of the fungus to host cells, penetration and invasion of host cell barriers and finally dissemination via the blood stream. Adhesion to adult and neonatal enterocytes in vitro differs among the Candida spp and this correlates with their incidence as colonizing organisms and as causes of sepsis. For example, C. albicans adheres to neonatal enterocytes to a greater extent as compared to other Candida spp, is the leading cause of invasive candidiasis in neonates and is isolated most frequently from the neonatal GI tract (10). C. parapsilosis is the second most frequent Candida spp. associated with these processes in neonates (10). C. albicans is also the most adherent to adult enterocytes relative to other Candida spp and the most frequent GI tract colonizer and cause of sepsis. In contrast to neonates, C. glabrata and C. tropicalis adhere to adult enterocytes better than C. parapsilosis and are also frequent colonizers of the adult GI tract and causes of sepsis in adults (10). Thus, colonization and adhesion to GI epithelia differ among the Candida spp. and between enterocytes derived from different human developmental stages.
Other than colonization and adhesion, there is relatively little known about how Candida spp. interact with the immature neonatal GI tract. Based on the observations that Candida spp differ in prevalence as colonizers and bloodstream isolates (10) and the severity of infections that they cause in infants (11), we hypothesized that Candida spp differ in their invasive interactions with the premature GI epithelium and that differences in these processes may underlie differences in pathogenesis mechanisms. The goal of this study was to compare the ability of Candida spp. to penetrate, invade, and induce a pro-inflammatory response from premature human enterocytes. To do this, we used the H4 cell line, a model of non-malignant immature small intestinal epithelial cells. In addition, we analyzed multiple Candida strains within an individual species to investigate the possibility of strain-to-strain differences in invasion and injury phenotypes.
METHODS
Enterocyte cell culture
Non-malignant primary immature human enterocytes (cell line H4, derived from the small intestine of 20 to 22 weeks gestation fetuses), their cultivation and maintenance are as previously described (12).
Candida strains and growth conditions
The Candida strains used in this study are described in Table 1 (13–17). Strains were propagated and maintained in Yeast Peptone Dextrose agar (18) and, for experiments, were grown in either Synthetic Dextrose Complete (SDC) (18) or Sabouraud’s (Difco Laboratories, Detroit, MI) liquid media at 30°C overnight. Cell concentrations were determined using a hemacytometer. In addition, growth of the Candida spp. was compared after incubation in H4 media for 8 h (the longest incubation time for the assays described below) by counting cells with a hemacytometer and was found to be similar among the strains.
Table 1.
Candida strains used in this study.
| Strain | Species | Source |
|---|---|---|
| SC5314 | C. albicans | (13) |
| A022b | C. albicans | Clinical isolate, neonate, pleural fluid* |
| A002 | C. albicans | Clinical isolate, neonate, peritoneal fluid* |
| A003 | C. albicans | Clinical isolate, neonate, blood* |
| A0038 | C. albicans | Clinical isolate, neonate, cerebrospinal fluid* |
| RSR1/RSR1 (9955) | C. albicans | (14) |
| rsr1/rsr1 (8880) | C. albicans | (14) |
| 4175 (A010) | C. parapsilosis | Clinical isolate, neonate, cardiac mass* |
| 4176 (A044) | C. parapsilosis | Clinical isolate, neonate, blood* |
| 4177 (A053) | C. parapsilosis | Clinical isolate, neonate, blood* |
| 4179 (4961) | C. parapsilosis | Clinical isolate, adult, blood* |
| 22019 | C. parapsilosis | ATCC† |
| 4173 (BG2) | C. glabrata | (15) |
| 10267 (ATCC2001) | C. glabrata | ATCC† |
| 66032 | C. glabrata | ATCC† |
| A037 | C. glabrata | Clinical isolate, neonate, urine* |
| YA059 | C. glabrata | Clinical isolate, neonate, blood* |
| 10265 (CD36) | C. dubliniensis | (16) |
| M2649 (R3b) | C. dubliniensis | (17) |
| M2650 (R1b) | C. dubliniensis | (17) |
| M2651 (16F) | C. dubliniensis | (16) |
| 3779 | C. dubliniensis | Clinical isolate, oropharynx* |
Clinical Microbiology Laboratories, University of Minnesota
American Type Culture Collection, (Manassas, VA)
Epithelial cell penetration assay
H4 cells, at a concentration of 2 × 105 cells were grown to ~80% confluence on circular glass microscope cover slips in 12-well tissue culture plates (BD Biosciences, San Jose, CA). H4 monolayers were inoculated with 1 × 105 yeast cells suspended in 1 ml H4 growth medium and incubated for 3h. Infected monolayers were analyzed by immunocytochemistry as previously described (20) using a polyclonal, biotin-conjugated rabbit anti-C. albicans immunoglobulin G (Biodesign International, Saco, Maine) as the primary antibody and streptavidin conjugated to the Alexa 568 fluorophore (Invitrogen, Carlsbad, CA) as the secondary antibody. In preliminary experiments lacking H4 cells, all Candida species had consistent, uniform staining of the cell wall using this strategy (≥ 90% of cells). After incubation, Candida cells were distinguished as either penetrating (unstained) or non-penetrating (stained) with respect to the epithelial cell layer. A total of ~15 fluorescent images along the z axis, in ~1 µm increments, were collected for each microscopic field to insure that any fluorescent signal was captured throughout the diameter of the fungal cell. Data are presented as averages of at least three independent experiments (n ≥ 100 cells for each) ± SEM.
Epithelial cell injury assay
The ability of Candida to damage H4 cells was assessed using the Cyto-Tox-96® assay (Promega, Madison, WI), which measures the amount of lactate dehydrogenase (LDH) released from injured epithelial cells. H4 cells were cultured at a concentration of 2 × 104 cells/well and grown to ~80% confluence in 96-well flat-bottomed tissue culture plates (BD Biosciences, San Jose, CA). H4 monolayers were infected with yeast-form Candida resuspended in H4 media in a 10:1 ratio to the number of epithelial cells seeded (ratio determined in preliminary experiments to give results in the linear range of the spectrophotometer (data not shown)) for 8 h and the assay was then carried out according to the manufacturer’s instructions and as previously described (20). Cell damage (% cytotoxicity) is expressed as the average of three independent experiments, each performed in triplicate, ± SEM.
Determination of IL-8 secretion by H4 enterocytes
H4 cells were cultured at a concentration of 1 × 105 cells/ml and grown to ~80% confluence in twenty-four well tissue culture plates (BD Biosciences, San Jose, CA). H4 monolayers were inoculated with yeast-form Candida suspended in H4 growth media at a concentration of 1×106 cells/ml. Supernatant samples of uninfected and infected monolayers were collected at 12 h after inoculation. Human IL-8 was measured using a commercially-available ELISA kit according to the manufacturer’s instructions (Quantikine, R&D Systems, Minneapolis, MN). Data are expressed as the average of three independent experiments, each performed in triplicate, ± SEM.
Statistical analyses
Statistical results were obtained using SPSS 15.0 for Windows (SPSS, Chicago, IL) and were analyzed using one-way ANOVA, followed by post-hoc separation of means using Tukey’s Honestly Significant Differences Test. A p value < 0.05 was considered statistically significant.
RESULTS
Immature enterocyte penetration by Candida spp
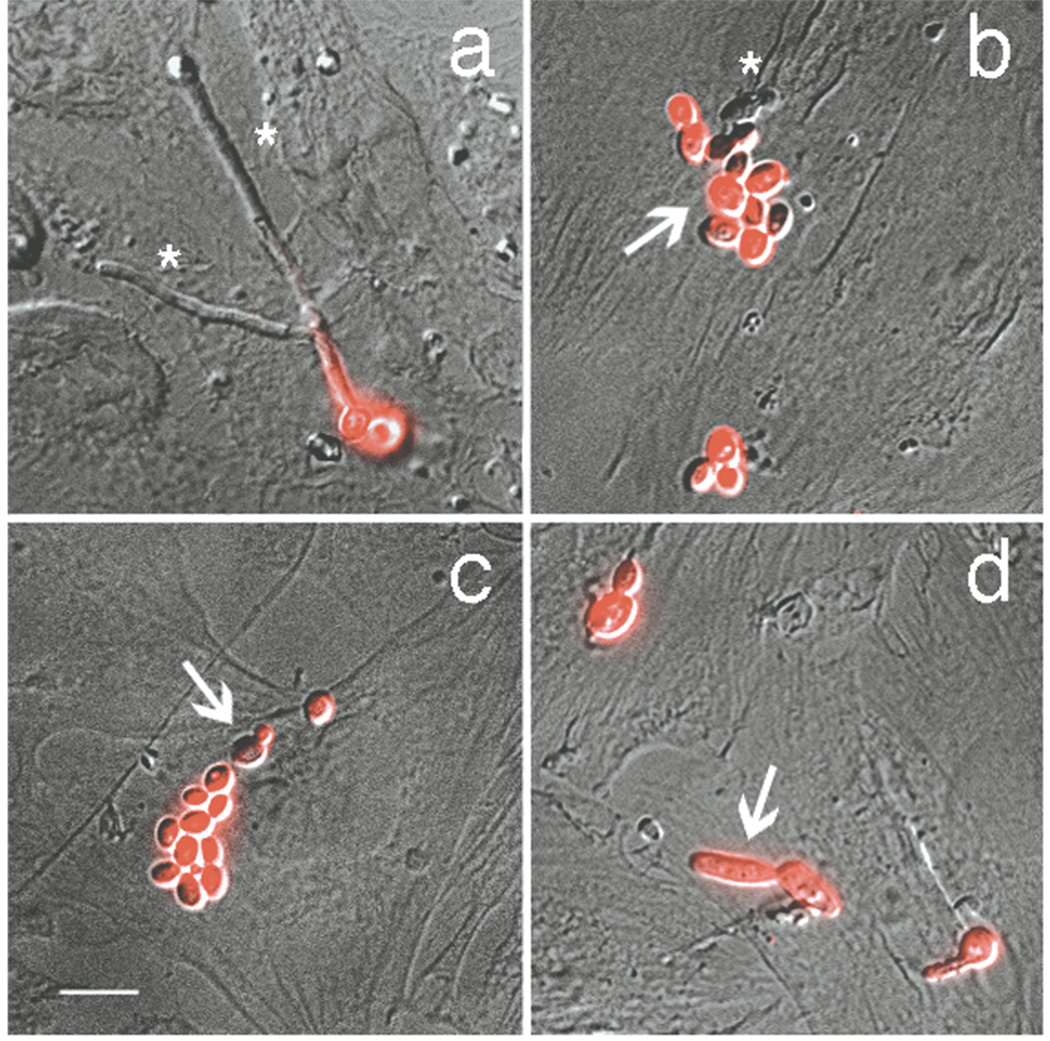
To understand how Candida spp. physically interact with immature human enterocytes, we used immunocytochemistry to visualize fungal penetration of H4 cells. C. albicans was located intraepithelially more frequently than C. parapsilosis, C. dubliniensis and C. glabrata (Figs. 1 and 2). In addition, C. albicans was the only Candida spp that formed abundant hyphal filaments after incubation with H4 cells. Furthermore, C. albicans hyphal tips were most often the portion of the cell that penetrated the enterocyte (Fig. 1a). Interestingly, C. dubliniensis, capable of forming hyphae in vitro albeit less efficiently than C. albicans (21), formed infrequent (~40% cells/high power field), short filaments (8–15 µm in length, example, Fig. 1d) that only rarely penetrated the H4 cells (Fig. 2A, E) as compared to C. albicans (~90% of cells/high power field; 35–40 µm in length). Similarly, C. parapsilosis and C. glabrata were only rarely observed within enterocytes (Fig. 1b and 2A, C, D) and existed primarily as budding yeast (Fig. 1b, c). Together, these data indicate an association between hyphal morphogenesis and enterocyte penetration.
Figure 1.
Representative photomicrographs of H4 enterocytes inoculated with the Candida spp. used in the quantitation depicted in Fig. 2A. Z-stacks of DIC and fluorescence images were merged to obtain the images shown. Penetrating Candida cells lack fluorescent signal (asterisks). Non-penetrating Candida cells exhibit fluorescent signal (arrows). a, C. albicans SC5314; b, C. parapsilosis 4175; c, C. glabrata 4173; d, C. dubliniensis 10265. Scale bar, 10 µm.
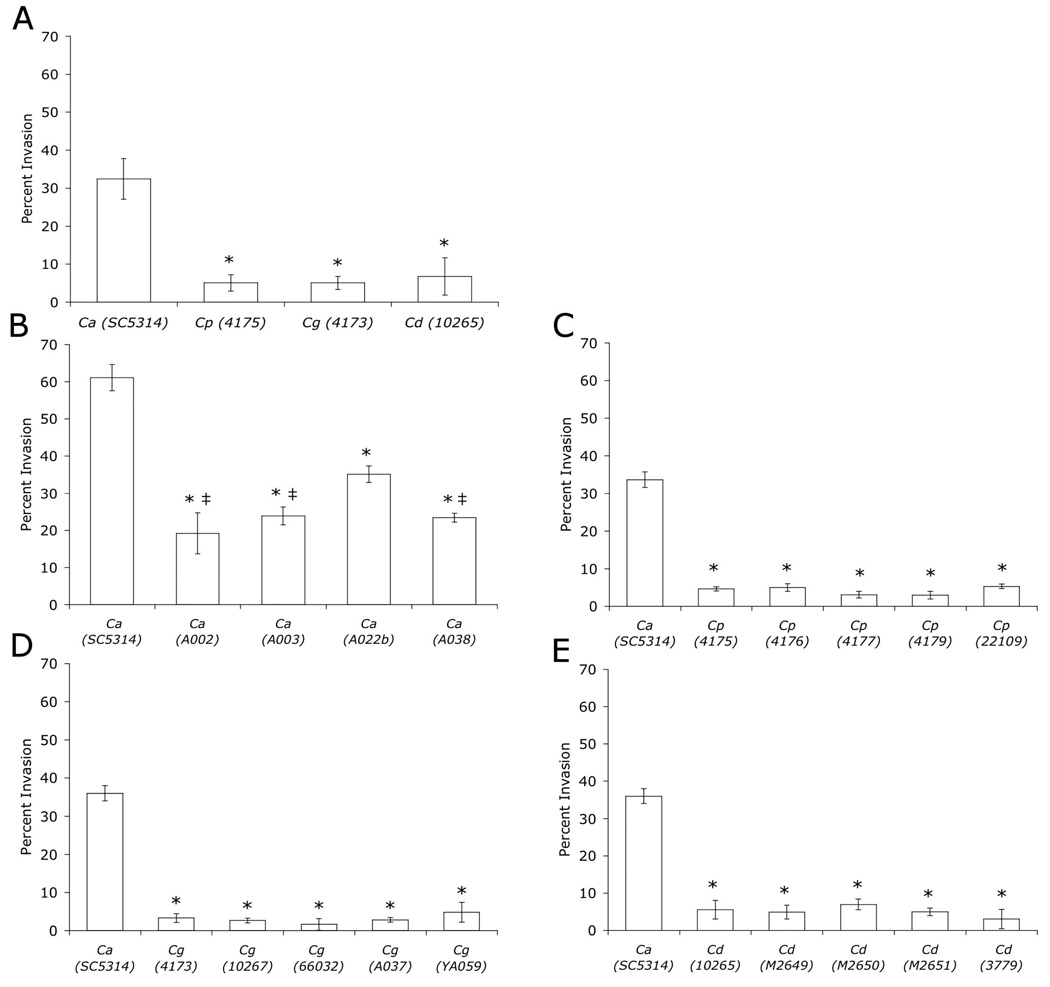
Figure 2.
Penetration of H4 enterocytes (percent of total cells invading = percent invasion) by Candida spp. C. albicans (Ca), C. parapsilosis (Cp), C. glabrata (Cg), C. dubliniensis (Cd). A. * p < 0.003, B. * p < 0.003 compared to SC5314, ‡ p < 0.05, compared to A022b, C–E. * p < 0.001.
Genetic variation among clinical strains of C. albicans is common and often affects phenotype (22). Thus, we analyzed multiple C. albicans clinical isolates, as well as multiple isolates of C. parapsilosis, C. glabrata, and C. dubliniensis, due to this propensity for genetic variation, which may cause variation in virulence-associated phenotypes. All C. parapsilosis, C. glabrata, and C. dubliniensis strains had similar low invasion potentials within each species and had significantly reduced ability to invade H4 cells as compared to C. albicans strain SC5314 (Fig. 2C–E). Among C. albicans isolates, H4 cell penetration differed with C. albicans SC5314 exhibiting the greatest invasion, followed by A022b and then the other C. albicans clinical isolates (Fig. 2B). Importantly, all of the C. albicans strains penetrated H4 cells to a greater extent than non-albicans spp. supporting the idea that C. albicans, because of its ability to form hyphae, has better penetrating ability.
Few clinical laboratories distinguish between members of the C. parapsilosis complex (C. parapsilosis, C. orthopsilosis and C. metapsilosis), which have been reported to vary in phenotype. To determine the identity of the clinical “parapsilosis” strains analyzed in our study, we used a PCR-based strategy to discriminate among these species based on unique sequences within the internally transcribed spacer 1 and 2 regions (19). Using this approach, we found that all of the “parapsilosis” strains that we analyzed (Table 1) were confirmed to be C. parapsilosis (data not shown).
Immature enterocyte injury by Candida spp
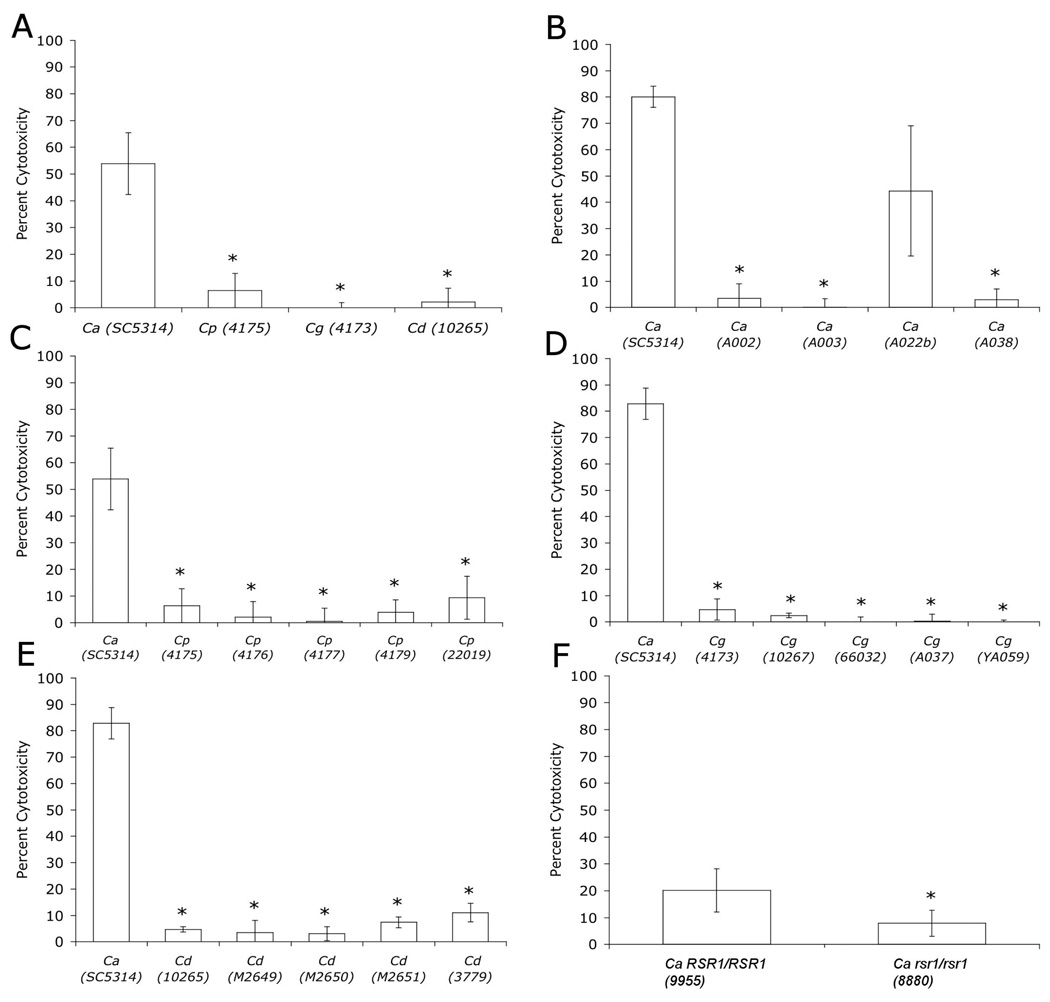
To investigate how enterocyte penetration correlates with injury, the amount of LDH released from injured H4 enterocytes was measured following incubation with Candida spp. C. albicans SC5314 caused significantly more H4 cell injury than C. parapsilosis, C. glabrata or C. dubliniensis (Fig. 3). Of note, there were no significant differences observed for H4 cell injury among the non-albicans Candida spp. (Fig. 3A) or among multiple isolates of an individual species (Fig. 3C–E). Thus, like enterocyte penetration, enterocyte injury by Candida is associated with the ability to form hyphae. The hypothesis that hyphal morphogenesis is associated with H4 injury was further tested by analyzing H4 injury by a C. albicans rsr1 mutant strain. C. albicans cells lacking Rsr1 have defects in hyphal morphogenesis (filaments are shorter and fatter), reduced invasion and injury of oral epithelial cells, and attenuated virulence in a mouse model of systemic candidiasis (14, 20). Similarly, we found that a C. albicans rsr1 strain had significantly less cytotoxicity for H4 cells as compared with its isogenic parent strain (Fig. 3F), supporting the association between hyphal morphogenesis and injury. Lastly, we compared enterocyte injury by five C. albicans strains, all of which had similar growth rates and similar extents of hyphal elongation after incubation with H4 cells (35–40 µm, n=30 for each strain, p=0.7). We reasoned that if hyphal elongation is sufficient for tissue injury, then all of the C. albicans strains would cause similar amounts of enterocyte LDH release. We found that C. albicans A022b caused similar H4 cytotoxicity as did SC5314, but the amount of enterocyte damage caused by these two strains was statistically greater than the three other C. albicans isolates tested (Fig. 4B). Together, the cytotoxicity results indicate that although hyphal morphogenesis is generally associated with cytotoxicity, it, by itself, is not sufficient for this process.
Figure 3.
H4 enterocyte damage (percent cytotoxicity as described in Materials and Methods) caused by Candida spp. C. albicans (Ca), C. parapsilosis (Cp), C. glabrata (Cg), C. dubliniensis (Cd). A. * p < 0.01, B. * p < 0.01 compared to SC5314 and A022b, , C–E. * p < 0.001, F. * p < 0.003.
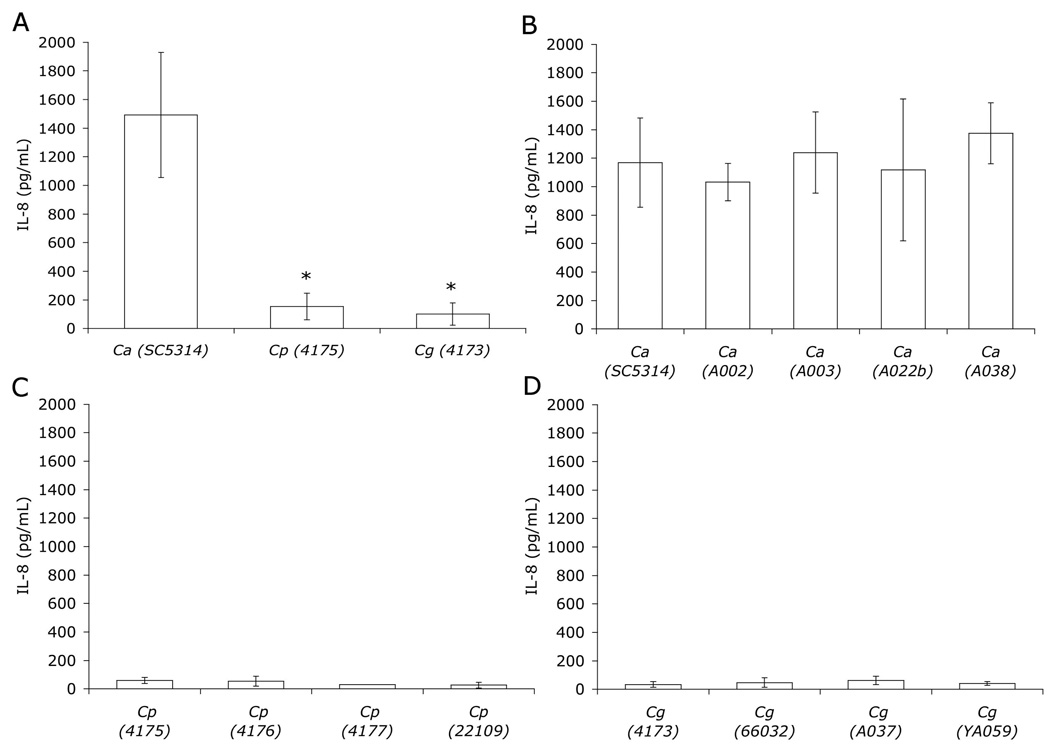
Figure 4.
IL-8 secreted by H4 cells following incubation for 12 h with Candida spp. C. albicans (Ca), C. parapsilosis (Cp), C. glabrata (Cg). A. * p < 0.01.
IL-8 secretion by immature enterocytes in response to Candida spp
The pro-inflammatory response of immature enterocytes caused by interaction with Candida was evaluated by measuring IL-8 released by H4 cells following infection with Candida spp. During these assays, C. albicans was again noted to be predominantly hyphal by 1 h after inoculation, whereas C. parapsilosis and C. glabrata formed mostly budding yeast. IL-8 levels were increased 12 h after infection with C. albicans SC5314 as compared to uninfected controls, and C. parapsilosis- and C. glabrata- infected H4 cells (Fig. 4A and data not shown). In addition, multiple strains of each individual species behaved similarly to each other with respect to the amount of IL-8 elicited from H4 cells (p>0.9, Fig. 4B–D). These results demonstrate a pro-inflammatory response from immature human enterocytes following infection with C. albicans but not with C. parapsilosis and C. glabrata. Thus, similar to tissue penetration, hyphal morphogenesis is also associated with IL-8 secretion by H4 cells.
DISCUSSION
Despite the significant association of Candida colonization and invasive infection with diseases of the premature GI tract, little is known about how Candida spp. interact with the developing enterocyte. To our knowledge, this study is the first report of the invasive and inflammatory interactions between Candida species and immature human intestinal cells. Several studies have analyzed the interaction of a single Candida spp., C. albicans, with other epithelial cell models including immortalized oral and adult intestinal (primary and Caco-2) cells. For these epithelial cell lines, tissue penetration and injury is associated with C. albicans hyphal growth: genetic mutants with defects in hyphal morphogenesis have reduced invasiveness (20, 23, 24). Additionally, morphogenesis mutants cause less mortality and organ invasion in intravenously inoculated animal models (20, 25). In our study of immature enterocytes, we also observe an association between C. albicans hyphal morphogenesis and tissue penetration and, furthermore, tissue inflammation. In contrast to other Candida spp., C. albicans formed abundant filamentous hyphae after incubation with H4 cells and was the Candida spp. that caused the most enterocyte penetration and IL-8 release. We also observed that, in general, the ability to penetrate tissue was associated with tissue injury (LDH release by H4 cells) (compare Figs. 2 and 3). An exception to this was seen for individual C. albicans isolates. Despite their similar ability to produce hyphae, penetrate H4 cells and induce H4 IL-8 release, C. albicans strains differed in their ability to injure enterocytes. This observation indicates that hyphal penetration of host tissue is not sufficient by itself to cause tissue injury and implicates other injury mechanisms (e.g. secretion of degradative enzymes) that differ among C. albicans isolates. Furthermore, the observation that C. albicans strains all elicited similar IL-8 release despite having varied abilities to injure H4 cells suggests that C. albicans may induce enterocyte inflammation by an injury-independent mechanism. The varied cytotoxicity abilities seen with the C. albicans strains also highlight the importance of testing multiple isolates before generalizing assay results to a species as a whole.
It appears that different penetration mechanisms are employed by C. albicans depending upon the specific host cell type (26). For example, in oral epithelial cell interactions, C. albicans hyphae (live or dead) are engulfed by epithelial cell membrane evaginations and invasion is attenuated by treatment with inhibitors of endocytosis. In contrast, adult intestinal carcinoma cell (Caco-2) invasion requires live C. albicans hyphae and the secretion of C. albicans proteases and is not affected by endocytosis inhibitors. In addition, a recent study observed that, in Caco-2 cell monolayers, C. albicans degrades E-cadherin, a protein important for the maintenance of cell-cell junctions (27). Thus, it appears that C. albicans penetration of oral epithelial cells occurs via induced endocytosis whereas penetration of Caco-2 cells occurs via active penetration facilitated by degradative proteases. The mechanism of C. albicans penetration into immature enterocytes is currently not known but will be important to establish as we consider anti-candidal therapies in premature infants in the future.
C. albicans and C. parapsilosis are the most common colonizers of the neonatal gastrointestinal tract and, accordingly, the most common causes of neonatal invasive candidiasis (10). Candidiasis due to C. parapsilosis has been increasing in incidence since 1990, although it appears to cause less severe disease and less mortality in neonates than systemic infections due to C. albicans (11). C. parapsilosis has a propensity to grow in parenteral nutrition solutions and to form biofilms on catheters (28). Although it has been isolated from the GI tracts of neonates with systemic candidiasis (5), we found that C. parapsilosis neither demonstrates significant ability to invade and damage immature enterocytes, nor does it elicit as great of an inflammatory response from H4 cells as compared to C. albicans. Interestingly, in the only study to speciate Candida isolated from infants with systemic candidiasis and intestinal disease, C. albicans was the sole species identified (7). This observation along with the results of our study support the idea that the immature GI tract may not be the primary site of entry and/or disease for C. parapsilosis, despite evidence of GI tract colonization. Rather, C. parapsilosis may be introduced into the bloodstream via indwelling vascular catheters.
Enterocytes are part of the first line of defense against infection in the GI tract, secreting cytokines in response to tissue injury (29). In particular, IL-8 is thought to be important for the activation and recruitment of neutrophils from intravascular to interstitial sites, a paradigm that has also been proposed for the pathogenesis of necrotizing enterocolitis (30). Cytokine release by H4 cells has been evaluated in vitro in response to components of bacterial infection, namely lipopolysaccharide and IL-1β. After stimulation of the monolayer with either factor, H4 cells secrete much more of the pro-inflammatory cytokine IL-8, as compared to the adult enterocyte cell line Caco-2 (31, 32). Additionally, baseline levels of IL-8 were elevated in H4 cells as compared to Caco-2 cells indicating that these immature cells exist in a more proinflammatory state prior to infection (31, 32). Our studies further explore the H4 cell immune response by analyzing levels of IL-8 secretion induced by interaction with live Candida organisms, rather than components of an organism. We found that IL-8 was significantly elevated in response to infection with C. albicans as compared to that with other Candida spp. Of note, infection of adult enterocytes (Caco-2) with Candida spp., including C. albicans, does not elicit an IL-8 response (33). Given the exaggerated immune response demonstrated by H4 cells as compared to Caco-2 cells, the increase in cytokines induced by interaction with C. albicans may contribute to the pathophysiology of disease in the intestinal tract by causing a dysregulated inflammatory response.
Elevated serum IL-8 levels have also been observed in severe cases of necrotizing enterocolitis, from its onset through the first 24 h (34). In addition, in intestinal tissue from infants with necrotizing enterocolitis, IL-8 mRNA is up-regulated throughout the serosa, muscularis, and intestinal epithelium compared with specimens taken from infants with other inflammatory conditions or those without disease (35). At this point, it remains unclear what the pathogenic initiator of either necrotizing enterocolitis or spontaneous intestinal perforation is, although intestinal microorganisms along with an exaggerated inflammatory response and bowel injury are likely contributing mechanisms (36, 37). Investigations of the interaction of the gut flora with the premature GI tract and how this impacts the risk for the development of necrotizing enterocolitis has focused exclusively on the bacterial microbiota. Our results support the idea that Candida, and in particular C. albicans, promotes inflammation and invasion of immature enterocytes and that fungal-enterocyte interactions should be considered in models of gastrointestinal inflammation and disease in premature infants.
ACKNOWLEDGEMENTS
We thank W. Allan Walker for providing H4 cells and the Clinical Microbiology Laboratory at the University of Minnesota and the laboratory of Pete and Bebe Magee for Candida clinical isolates. We are grateful to Rebecca Pulver for assistance with statistical analysis and Jennifer Norton for technical assistance.
Financial support: This study was funded by NIH grant AI057440 to C. A. Gale and by March of Dimes Birth Defects Foundation Grant #6-FY04-53 to C. M. Bendel.
ABBREVIATIONS
- GI
Gastrointestinal
- LDH
Lactate Dehydrogenase
REFERENCES
- 1.Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995–2004. Pediatrics. 2006;117:1680–1687. doi: 10.1542/peds.2005-1996. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DA. Epidemiology and prevention of neonatal candidiasis: fluconazole for all neonates? Adv Exp Med Biol. 2010;659:99–119. doi: 10.1007/978-1-4419-0981-7_9. [DOI] [PubMed] [Google Scholar]
- 3.Friedman S, Richardson SE, Jacobs SE, O'Brien K. Systemic Candida infection in extremely low birth weight infants: short term morbidity and long term neurodevelopmental outcome. Pediatr Infect Dis J. 2000;19:499–504. doi: 10.1097/00006454-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, Duara S, Poole K, Laptook A, Goldberg R. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 5.Saiman L, Ludington E, Dawson JD, Patterson JE, Rangel-Frausto S, Wiblin RT, Blumberg HM, Pfaller M, Rinaldi M, Edwards JE, Wenzel RP, Jarvis W. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J. 2001;20:1119–1124. doi: 10.1097/00006454-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Coates EW, Karlowicz MG, Croitoru DP, Buescher ES. Distinctive distribution of pathogens associated with peritonitis in neonates with focal intestinal perforation compared with necrotizing enterocolitis. Pediatrics. 2005;116:e241–e246. doi: 10.1542/peds.2004-2537. [DOI] [PubMed] [Google Scholar]
- 7.Ragouilliaux CJ, Keeney SE, Hawkins HK, Rowen JL. Maternal factors in extremely low birth weight infants who develop spontaneous intestinal perforation. Pediatrics. 2007;120:e1458–e1464. doi: 10.1542/peds.2006-2804. [DOI] [PubMed] [Google Scholar]
- 8.Mintz AC, Applebaum H. Focal gastrointestinal perforations not associated with necrotizing enterocolitis in very low birth weight neonates. J Pediatr Surg. 1993;28:857–860. doi: 10.1016/0022-3468(93)90345-l. [DOI] [PubMed] [Google Scholar]
- 9.Andrutis KA, Riggle PJ, Kumamoto CA, Tzipori S. Intestinal lesions associated with disseminated candidiasis in an experimental animal model. J Clin Microbiol. 2000;38:2317–2323. doi: 10.1128/jcm.38.6.2317-2323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendel CM. Colonization and epithelial adhesion in the pathogenesis of neonatal candidiasis. Semin Perinatol. 2003;27:357–364. doi: 10.1016/s0146-0005(03)00059-4. [DOI] [PubMed] [Google Scholar]
- 11.Faix RG. Invasive neonatal candidiasis: comparison of albicans and parapsilosis infection. Pediatr Infect Dis J. 1992;11:88–93. [PubMed] [Google Scholar]
- 12.Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu ZX, Pringault E, Leon-Robine S, Louvard D, Walker WA. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci U S A. 1996;93:7717–7722. doi: 10.1073/pnas.93.15.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 14.Hausauer DL, Gerami-Nejad M, Kistler-Anderson C, Gale CA. Hyphal guidance and invasive growth in Candida albicans require the Ras-like GTPase Rsr1p and its GTPase-activating protein Bud2p. Eukaryot Cell. 2005;4:1273–1286. doi: 10.1128/EC.4.7.1273-1286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidel PL, Jr, Cutright JL, Tait L, Sobel JD. A murine model of Candida glabrata vaginitis. J Infect Dis. 1996;173:425–431. doi: 10.1093/infdis/173.2.425. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan DJ, Westerneng TJ, Haynes KA, Bennett DE, Coleman DC. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141(Pt 7):1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 17.Timmins EM, Howell SA, Alsberg BK, Noble WC, Goodacre R. Rapid differentiation of closely related Candida species and strains by pyrolysis-mass spectrometry and Fourier transform-infrared spectroscopy. J Clin Microbiol. 1998;36:367–374. doi: 10.1128/jcm.36.2.367-374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherman F. Getting started with yeast. In: Fink G, Guthrie C, editors. Methods in Enzymology: Guide to Yeast Genetics and Molecular Biology. San Diego: Harcourt Brace Jovanovich; 1991. pp. 3–20. [Google Scholar]
- 19.Asadzadeh M, Ahmad S, Al-Sweih N, Khan ZU. Rapid molecular differentiation and genotypic heterogeneity among Candida parapsilosis and Candida orthopsilosis strains isolated from clinical specimens in Kuwait. J Med Microbiol. 2009;58:745–752. doi: 10.1099/jmm.0.008235-0. [DOI] [PubMed] [Google Scholar]
- 20.Brand A, Vacharaksa A, Bendel C, Norton J, Haynes P, Henry-Stanley M, Wells C, Ross K, Gow NA, Gale CA. An internal polarity landmark is important for externally induced hyphal behaviors in Candida albicans. Eukaryot Cell. 2008;7:712–720. doi: 10.1128/EC.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokes C, Moran GP, Spiering MJ, Cole GT, Coleman DC, Sullivan DJ. Lower filamentation rates of Candida dubliniensis contribute to its lower virulence in comparison with Candida albicans. Fungal Genet Biol. 2007;44:920–931. doi: 10.1016/j.fgb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Calderone RA, Braun PC. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991;55:1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, Edwards JE, Filler SG. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol. 2005;7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 24.Dieterich C, Schandar M, Noll M, Johannes FJ, Brunner H, Graeve T, Rupp S. In vitro reconstructed human epithelia reveal contributions of Candida albicans EFG1 and CPH1 to adhesion and invasion. Microbiology. 2002;148:497–506. doi: 10.1099/00221287-148-2-497. [DOI] [PubMed] [Google Scholar]
- 25.Saville SP, Lazzell AL, Chaturvedi AK, Monteagudo C, Lopez-Ribot JL. Use of a genetically engineered strain to evaluate the pathogenic potential of yeast cell and filamentous forms during Candida albicans systemic infection in immunodeficient mice. Infect Immun. 2008;76:97–102. doi: 10.1128/IAI.00982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalle F, Wachtler B, L'Ollivier C, Holland G, Bannert N, Wilson D, Labruere C, Bonnin A, Hube B. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12:248–271. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 27.Frank CF, Hostetter MK. Cleavage of E-cadherin: a mechanism for disruption of the intestinal epithelial barrier by Candida albicans. Transl Res. 2007;149:211–222. doi: 10.1016/j.trsl.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Clark TA, Slavinski SA, Morgan J, Lott T, Arthington-Skaggs BA, Brandt ME, Webb RM, Currier M, Flowers RH, Fridkin SK, Hajjeh RA. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J Clin Microbiol. 2004;42:4468–4472. doi: 10.1128/JCM.42.10.4468-4472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molmenti EP, Ziambaras T, Perlmutter DH. Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem. 1993;268:14116–14124. [PubMed] [Google Scholar]
- 30.Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claud EC, Savidge T, Walker WA. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr Res. 2003;53:419–425. doi: 10.1203/01.PDR.0000050141.73528.AD. [DOI] [PubMed] [Google Scholar]
- 33.Saegusa S, Totsuka M, Kaminogawa S, Hosoi T. Cytokine responses of intestinal epithelial-like Caco-2 cells to non-pathogenic and opportunistic pathogenic yeasts in the presence of butyric acid. Biosci Biotechnol Biochem. 2007;71:2428–2434. doi: 10.1271/bbb.70172. [DOI] [PubMed] [Google Scholar]
- 34.Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103:766–771. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 35.Nadler EP, Stanford A, Zhang XR, Schall LC, Alber SM, Watkins SC, Ford HR. Intestinal cytokine gene expression in infants with acute necrotizing enterocolitis: interleukin-11 mRNA expression inversely correlates with extent of disease. J Pediatr Surg. 2001;36:1122–1129. doi: 10.1053/jpsu.2001.25726. [DOI] [PubMed] [Google Scholar]
- 36.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. Faseb J. 2001;15:1398–1403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 37.De Plaen IG, Liu SX, Tian R, Neequaye I, May MJ, Han XB, Hsueh W, Jilling T, Lu J, Caplan MS. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr Res. 2007;61:716–721. doi: 10.1203/pdr.0b013e3180534219. [DOI] [PubMed] [Google Scholar]