Abstract
Sildenafil is a potent and selective inhibitor of the type 5 cGMP-specific phosphodiesterase that is used clinically to treat erectile dysfunction and pulmonary arterial hypertension. Here we report that sildenafil has differential effects on cell surface ABC transporters such as ABCB1, ABCC1 and ABCG2 that modulate intracompartmental and intracellular concentrations of chemotherapeutic drugs. In ABCB1-overexpressing cells, non-toxic doses of sildenafil inhibited resistance and increased the effective intracellular concentration of ABCB1 substrate drugs, such as paclitaxel. Similarly, in ABCG2-overexpressing cells, sildenafil inhibited resistance to ABCG2 substrate anticancer drugs, for example, increasing the effective intracellular concentration of mitoxantrone or the fluorescent compound BODIPY-prazosin. Sildenafil also moderately inhibited the transport of E217βG and methotrexate by the ABCG2 transporter. Mechanistic investigations revealed that sildenafil stimulated ABCB1 ATPase activity and inhibited photolabeling of ABCB1 with [125I]-IAAP, whereas it only slightly stimulated ABCG2 ATPase activity and inhibited photolabeling of ABCG2 with [125I]-IAAP. In contrast, Sildenafil did not alter the sensitivity of parental, ABCB1- or ABCG2-overexpressing cells to non-ABCB1 and non-ABCG2 substrate drugs, nor did sildenafil affect the function of another ABC drug transporter ABCC1. Homology modeling predicted the binding conformation of sildenafil within the large cavity of the transmembrane region of ABCB1. Overall, we found that sildenafil inhibits the transporter function of ABCB1 and ABCG2, with a stronger effect on ABCB1. Our findings suggest a possible strategy to enhance the distribution and potentially the activity of anti-cancer drugs by jointly using a clinically approved drug with known side effects and drug-drug interactions.
Keywords: Sildenafil, multidrug resistance, ABCB1, ABCG2, chemosensitivity
INTRODUCTION
The ABC transporters belong to a superfamily of transmembrane proteins that transport a wide variety of substrates across extra- and intracellular membranes, ranging from ions, sugars, amino acids, vitamins, lipids and drugs to larger molecules such as oligosaccharides, oligopeptides and even higher molecular weight proteins (1). Several have been identified as transporting cancer chemotherapeutics agents including taxanes, anthracyclines, vinca alkaloids, and topoisomerase inhibitors (1). These transporters were discovered in in vitro models of drug resistance and have been postulated to confer multidrug resistance (MDR) in patients. At present, the major members of the ABC transporters linked to MDR in cancer cells include ABCB1 (P-glycoprotein, ABCB1/MDR1), ABCCs (MRPs) and ABCG2 (BCRP/MXR/ABCP) (2). These proteins share the ability to transport a large number of structurally diverse, mainly hydrophobic compounds from cells, but each transporter has their own specific substrates (2). Initially, it was hoped that inhibiting these transporters would restore the sensitivity of drug resistant cancer cells to chemotherapeutic drugs and lead to a more efficacious treatment for cancer patients. As a result, a number of compounds have been identified with the ability to inhibit individual or several transporters by blocking drug efflux, increasing drug accumulation and thus sensitizing resistant cancer cells. It has been reported that cyclosporine A and VX-710 (biricodar) can inhibit the function of multiple transporters, including ABCB1, ABCC1 and ABCG2 (3-5). Verapamil, LY475776 and V-104 can block the activity of ABCB1 and ABCC1 (3, 6, 7). GF-120918 (elacridar) suppresses the function of ABCB1 and ABCG2 (3, 7). Unfortunately, most of these inhibitors were ineffective in clinical trials owing to their unfavorable side effects or toxic pharmacokinetic interactions or simply because the magnitude of improvement in therapeutic outcome of these inhibitors with conventional chemotherapeutic agents was either non-significant or inconclusive (2). Whether or not better inhibitors of the ABC transporters could be identified that would have a greater therapeutic effect is unknown, and in vitro studies continue to address this question. Meanwhile, additional data regarding a role for the ABC transporters as modulators of oral absorption and as determinants of sanctuary sites have emerged. These studies show that a number of blood-tissue barriers are mediated at least in part by ABC transporters. These include the blood-brain barrier, the maternal-fetal barrier, the blood-testicular barrier, and an apparent blood-cardiac muscle barrier (8). While these can be protective barriers, they can also prevent adequate penetration of anticancer agents into needed tissue compartments. Thus, the identification of drugs that block ABC transporters at such sites, increasing drug penetration, has potential clinical benefit well beyond a “drug resistance reversal strategy”.
Discovering new functions of drugs already used in the clinic is one of the important strategies for drug development. In our previous studies, we found that several tyrosine kinase inhibitors (erlotinib, lapatinib, nilotinib and AG1478) could reverse ABCB1- and ABCG2-mediated MDR by inhibiting their transport function, but had no effect on ABCC1 (9-12). In screening novel drugs that are already in clinical use for potential effects to reverse MDR, we found that sildenafil (Viagra®), a potent and selective inhibitor of cGMP-specific phosphodiesterase type 5 (PDE5), reverses ABCB1- and ABCG2-mediated substrate efflux. Interestingly, sildenafil was shown in previous models to increase cytotoxicity, presumably by different mechanisms than drug uptake. Our results show that sildenafil significantly inhibits ABCB1-mediated drug efflux, slightly inhibits ABCG2-mediated drug efflux, while having no effect on efflux mediated by ABCC1.
MATERIALS AND METHODS
Materials
[3H]-paclitaxel (37.9 Ci/mmol), [3H]-mitoxantrone (4 Ci/mmol) and [3H]-methotrexate (23 Ci/mmol) were purchased from Moravek Biochemicals Inc (Brea, CA). [3H]-E217βG (40.5 Ci/mmol) and [125I]-Iodoarylazidoprazosin (IAAP) (2,200 Ci/mmol) were obtained from PerkinElmer Life Sciences (Boston, MA). The fluorescent compound BODIPY-prazosin was purchased from Invitrogen Corp (Carlsbas, CA). Monoclonal antibodies C-219 (against ABCB1) and BXP-34 (against ABCG2) were acquired from Signet Laboratories Inc (Dedham, MA). Sildenafil was purified from Viagra (Indicate strength) tablets as described previously (13). Fumitremorgin C (FTC) was synthesized by Thomas McCloud Developmental Therapeutics Program, Natural Products Extraction Laboratory, NCI, NIH (Bethesda, MD). Other chemicals were purchased from Sigma Chemical Co (St. Louis, MO).
Cell Lines and Cell Culture
The ABCB1-overexpressing drug-resistant cell line KB-C2 was established by step-wise selection of the parental human epidermoid carcinoma cell line KB-3-1 in increasing concentrations of colchicine and was cultured in medium containing 2 μg/mL of colchicine (14). The ABCB1-overexpressing drug-resistant cell line KB-V1 (generously provided by Dr. Michael M. Gottesman, NCI, NIH) was established by step-by-step increasing the concentration of vinblastine to human epidermoid carcinoma cell line KB-3-1 and cultured in the medium with 1 μg/mL of vinblastine. An ABCC1-overexpressing MDR cell line, KB-CV60, was also derived from KB-3-1 cells and was maintained in medium with 1 μg/mL of cepharanthine and 60 ng/mL of vincristine (15). Both KBC2 and KB-CV60 cell lines were kindly provided by Dr. Shin-ichi Akiyama (Kagoshima University, Japan). HEK293/pcDNA3.1, ABCG2-482-R5, ABCG2-482-G2, and ABCG2-482-T7 cells were established by selection with 2 mg/mL G418 after transfecting HEK293 with either empty pcDNA3.1 vector or pcDNA3.1 vector containing full length ABCG2 coding either wild type arginine (R), or mutants - glycine (G), or threonine (T) at amino acid 482, respectively (16). The wild type ABCG2 overexpressing drug-resistant cell line MCF-7/Flv1000 was cultured in the medium with 1000 nM of flavopiridol (17). The mutated ABCG2 overexpressing drug-resistant cell line MCF-7/ADVP3000 was maintained in the medium with 5 μg/mL of verapamil and 3 μg/mL of doxorubicin (17). Another G482 mutant ABCG2 overexpressing drug-resistant cell line S1-M1-80 was maintained in the medium with 80 μM of mitoxantrone (17). The drug-resistant cell line S1/Flv5000, which does not express ABCG2, was also generated from S1 by increasing the amount of flavopiridol and was maintained in the medium with 5 μM of flavopiridol (Robert W. Robey and Susan E. Bates, unpublished data). All the cell lines were grown as adherent monolayers in flasks with DMEM culture medium (Hyclone Co., UT) containing 10% bovine serum at 37°C in a humidified atmosphere of 5% CO2.
MTT Cytotoxicity Assay
Cells in 96-well plates were preincubated with or without the reversing agents for 1 hour, then different concentrations of chemotherapeutic drugs were added into designated wells. After 68 hours of incubation, MTT solution (4 mg/mL) was added to each well, and the plate was further incubated for 4 hours, allowing viable cells to change the yellow-colored MTT into dark-blue formazan crystals. Subsequently the medium was discarded, and 100 μL of dimethylsulfoxide (DMSO) was added into each well to dissolve the formazan crystals. The absorbance was determined at 570 nm by an OPSYS microplate Reader from DYNEX Technologies, Inc (Chantilly, VA).
Paclitaxel and Mitoxantrone Accumulation
Cells in 24-well plates were preincubated with or without the reversing agents for 1 hour at 37°C, then incubated with 0.1 μM [3H]-paclitaxel or 0.2 μM [3H]-mitoxantrone for 2 hours in the presence or absence of the reversing agents at 37°C. After washing three times with ice-cold PBS, the cells were typsinized and lysed in 10 mM lysis buffer (pH 7.4, containing 1% Triton X-100 and 0.2% SDS). Each sample was placed in scintillation fluid and radioactivity was measured in a Packard TRI-CARB ® 1900CA liquid scintillation analyzer from Packard Instrument Company, Inc (Downers Grove, IL).
Flow Cytometry Assays
Flow cytometry assays were performed as previously described (16). Briefly, cells were trypsinized and then resuspended in complete media (phenol red-free IMEM with 10% fetal calf serum) containing 250 nM BODIPY-prazosin alone or with various concentrations of the inhibitors for 30 minutes at 37°C. Cells were then washed once in cold complete medium and then incubated for another 1 hour at 37°C in substrate-free media continuing with or without the described concentrations of the inhibitors to generate the efflux histograms. Subsequently, cells were washed twice with cold DPBS and placed on ice in a dark environment until ready for analysis. Cells were analyzed on a FACSort flow cytometer equipped with a 488 nm argon laser. For all samples, at least 10,000 events were collected. Cell debris was eliminated by gating on forward vs side scatter, and dead cells were excluded based on propidium iodide staining.
In Vitro Transport Assays
Transport assays were performed using the rapid filtration method as previously described (18). Membrane vesicles were incubated with various concentrations of inhibitors for 1 hour on ice, and then transport reactions were carried out at 37°C for 10 minutes in a total volume of 50 μL medium (membrane vesicles 10 μg, 0.25 M sucrose, 10 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 4 mM ATP or 4 mM AMP, 10 mM phosphocreatine, 100 μg/mL creatine phosphokinase, and 0.25 μM [3H]-E217βG or 0.5 μM [3H]-methotrexate). Reactions were stopped by the addition of 3 mL of ice-cold stop solution (0.25 M sucrose, 100 mM NaCl, and 10 mM Tris-HCl, pH 7.4). During the rapid filtration step, samples were passed through 0.22 μm GVWP filters (Millipore Corporation, Billerica, MA) presoaked in the stop solution. The filters were washed three times with 3 mL of ice-cold stop solution. Radioactivity was measured by the use of a liquid scintillation counter.
ATPase Assay of ABCB1 and ABCG2
The Vi-sensitive ATPase activity of ABCB1 or ABCG2 in the membrane vesicles of High Five insect cells was measured as previously described (19). The membrane vesicles (100 μg of protein/mL) were incubated in ATPase assay buffer (50 mM MES, pH 6.8, 50 mM KCl, 5 mM sodium azide, 2 mM EGTA, 2 mM dithiothreitol, 1 mM ouabain, and 10 mM MgCl2) with or without 0.3 mM vanadate at 37°C for 5 minutes, then incubated with different concentrations of drugs at 37°C for 3 minutes. The ATPase reaction was incubated by the addition of 5 mM Mg-ATP. After incubating at 37°C for 20 minutes, the reactions were stopped by adding 0.1 mL of 5% SDS solution. The liberated Pi was measured as previously described (20). In the inhibition assays, the decrease in maximum Vi-sensitive ABCB1 or ABCG2 activity by sildenafil was measured in the presence of verapamil at 50 μM or FTC at 10 μM, respectively.
Photoaffinity Labeling of ABCB1 and ABCG2 with [125I]-IAAP
The photoaffinity labeling of ABCB1 and ABCG2 with [125I]-IAAP was performed as previously described (21). The crude membranes from High Five insect cells expressing ABCB1 and MCF7/FLV1000 cells expressing R482 ABCG2 (50 μg of protein) were incubated at room temperature with different concentrations of drugs in 50 mM Tris-HCl (pH 7.5) with [125I]-IAAP (5-7 nM) for 5 minutes under subdued light. The samples were photo-cross-linked by exposure to a 365 nm UV light for 10 minutes at room temperature. ABCB1 and ABCG2 were immunoprecipitated using BXP21 antibodies respectively as described previously (20). Both ABCB1 and ABCG2 samples were subjected to SDS-PAGE in a 7% Tris-acetate NuPAGE gel, the gel was dried and exposed to Bio-Max MR film (Eastman Kodak Co.) at -70°C for 8-12 hours. The radioactivity incorporated into the ABCB1 or ABCG2 band was quantified using the STORM 860 PhosphorImager system and ImageQuaNT (Molecular Dynamics, CA).
Ligand structure preparation
Sildenafil and IAAP were built using the fragment dictionary of Maestro 9.0 and energy minimized by Macromodel program v9.7 (Schrödinger, Inc., New York, NY, 2009) using the OPLSAA force field (22) with the steepest descent followed by truncated Newton conjugate gradient protocol. Partial atomic charges were computed using the OPLS-AA force field. The low-energy 3D structures of sildenafil and IAAP were generated with the following parameters present in LigPrep v2.3: different protonation states at physiological pH, all possible tautomers and ring conformations.
Protein structure preparation
The X-ray crystal structure of ABCB1 in apoprotein state (PDB ID: 3G5U) and in complex with inhibitors QZ59-RRR (PDB ID: 3G6O) and QZ59-SSS (PDB ID: 3G61) obtained from the RCSB Protein Data Bank were used to build the homology model of human ABCB1 (23). Homology modeling was carried out using the default parameters of Prime v2.1 as implemented in Maestro 9.0. The input file for amino acid sequence of human ABCB1 in Prime structure prediction application was obtained as fasta file (uniprot accession number P08183.3) extracted from http://www.uniprot.org. The co-crystal structures of ABCB1 from mouse model in complex with QZ59-RRR and QZ59-SSS inhibitors were used as template for modeling site-1 and site-2, respectively; while apoprotein-ABCB1 was used as a template for modeling the site-3 and site-4. The resultant alignment of human ABCB1 and mouse ABCB1 sequences produced 87% sequence identity and 93% similarity. On the resultant alignment built using default parameters, side chains were optimized and residues were minimized. The initial structure thus obtained was refined by means of default parameters mentioned in protein preparation facility implemented in Maestro v9.0 and Impact program v5.5 (Schrödinger, Inc., New York, NY, 2009), in which the protonation states of residues were adjusted to the dominant ionic forms at pH 7.4. Refined human ABCB1 homology model was further used to generate four different receptor grids by selecting QZ59-RRR (site-1) and QZ59-SSS (site-2) bound ligands, all amino acid residues known to contribute to verapamil binding (site-3) and two residues known to be common to three previous sites (site-4) as shown in supplemental Table 1.
Docking protocol
All docking calculations were performed using the “Extra Precision” (XP) mode of Glide program v5.5 (Schrödinger, Inc., New York, NY, 2009) and the default parameters. The top scoring pose-ABCB1 complex was then subjected to energy minimization using Macromodel program v9.7 using the OPLS-AA force field (22) and used for graphical analysis. All computations were carried out on a Dell Precision 470n dual processor with the Linux OS (Red Hat Enterprise WS 4.0).
Statistical Analysis
All experiments were repeated at least three times and the differences were determined by using the Student's t-test. The statistical significance was determined at p < 0.05.
RESULTS
Sildenafil sensitizes ABCB1- and ABCG2-overexpressing cells to chemotherapeutic drugs
To investigate the effect of sildenafil on ABC transporters, we first examined the sensitivity of ABCB1-, ABCC1-, and ABCG2- overexpressing cells to sildenafil. Notably, the results of the MTT assay showed that sildenafil did not inhibit the growth of any of the cell lines used in this study at concentrations up to 50 μM (data not shown). Next, we examined whether sildenafil could increase the sensitivity of ABCB1-expressing drug resistant cells to substrate drugs. As shown in Table 1, the ABCB1-overexpressing cells KB-C2 and KB-V1 showed much higher IC50 values to ABCB1 substrates colchicine, vinblastine, and paclitaxel than parent KB-3-1 cells did. Sildenafil decreased the IC50 values of these drugs in KB-C2 and KB-V1 cells. Sildenafil at 2.5 μΜ was able to moderately increase sensitivity to these three drugs. At 10 μM concentration, sildenafil further increased the sensitivity of cells, and its efficacy was comparable to that of the known ABCB1 inhibitor verapamil used at the same concentration (24). Neither sildenafil nor verapamil alter the cytotoxicity of these chemotherapeutic drugs in parental KB-3-1 cells. However, the IC50 values of cisplatin, which is not a substrate of ABCB1 and exhibited equal sensitivity in KB-3-1, KB-C2 and KB-V1 cells, were not affected by either sildenafil or verapamil in these three cells. Subsequently, the effects of sildenafil on ABCC1- and ABCG2-mediated drug resistance were also determined. In the ABCC1-overexpressing KB-CV60 cells, sildenafil at 10 μΜ concentration had no significant reduction of the IC50 value of vincristine, a known substrate of ABCC1 (data not shown). It has been reported that mutations at amino acid 482 in ABCG2 alter the substrate and antagonist specificity of ABCG2 (16, 25), therefore we examined the reversing effect of sildenafil on both wild-type (R482) and mutant (R482G and R482T) ABCG2-overexpressing cells. As shown in Table 2, compared to parental MCF-7 cells, MCF-7/Flv1000 and MCF-7/AdVp3000 cells exhibited high levels of resistance to ABCG2 substrates flavopiridol, mitoxantrone and SN-38, but not to the non-ABCG2 substrate cisplatin. Similarly, the IC50 values for flavopiridol, mitoxantrone and SN-38 in S1-M1-80 were significantly higher than those in parental S1 cells. Sildenafil was able to slightly decrease the IC50 values for flavopiridol, mitoxantrone and SN-38 in cells expressing wild-type or mutant ABCG2. At a concentration of 50 μM, sildenafil decreased IC50 values for these three drugs in the ABCG2-expressing cell lines down to levels that were observed when cytotoxicity assays were performed in the presence of the known specific ABCG2 inhibitor FTC at 2.5 μM (17). However, the IC50 values of these ABCG2 substrate drugs showed no significant difference in the parental cells or the S1/FLV5000 cells, which do not express ABCG2 but also are resistant to flavopiridol in the presence or absence of sildenafil. Furthermore, when the non-ABCG2 substrate drug cisplatin was used, either sildenafil or FTC in any of the cell lines did not affect its IC50 values. A similar phenomenon was observed in both wild-type or mutant ABCG2-transfected HEK293 cells (Table 3). The representative cell survival curves are shown in supplemental Figure 1, which demonstrate that the survival curves in the presence of sildenafil were remarkably shifted to left in the ABCB1 or ABCG2-overexpressing cells. Based on the above results, it appears that sildenafil significantly inhibits ABCB1-mediated drug efflux and only partially reverses ABCG2-mediated efflux.
Table 1.
The reversal effect of sildenafil and verapamil on ABCBl-mediated resistancea
| IC50 ± SDb (μM) |
|||
|---|---|---|---|
| Compounds | KB-3-1 | KB-C2 | KB-V1 |
| Colchicine | 0.0057±0.0015(1.0)c | 4.292±1.493(747.5) | 0.240±0.035(41.8) |
| +Sildenafil (2.5μM) | 0.0062±0.0013(1.1) | 0.721±0.236(125.5) | 0.059±0.035(10.3) |
| +Sildenafil (5μM) | 0.0058±0.0021(1.0) | 0.163±0.031(28.4) | 0.052±0.022(9.1) |
| +Sildenafil (10μM) | 0.0051±0.00148(0.9) | 0.060±0.004(10.5) | 0.044±0.024(7.6) |
| +Verapamil (2.5μM) | 0.0054±0.0016(0.9) | 0.264±0.132(46.0) | 0.168±0.036(29.2) |
| +Verapamil (5μM) | 0.0047±0.0021(0.8) | 0.101±0.026(17.6) | 0.081±0.037(14.1) |
| +Verapamil (10μM) | 0.0032±0.0001(0.6) | 0.055±0.009(9.6) | 0.055±0.038(9.6) |
| Vinblastine | 0.0452±0.0010(1.0)c | 0.464±0.234(10.3) | 5.427±3.362(120.1) |
| +Sildenafil (2.5μM) | 0.0445±0.0114(1.0) | 0.139±0.086(3.1) | 0.295±0.211(6.5) |
| +Sildenafil (5μM) | 0.0443±0.0076(1.0) | 0.092±0.085(2.0) | 0.131±0.091(2.9) |
| +Sildenafil (10μM) | 0.0437±0.0173(1.0) | 0.036±0.016(0.8) | 0.084±0.049(1.9) |
| +Verapamil (2.5μM) | 0.0400±0.0148(0.9) | 0.123±0.109(2.7) | 0.238±0.085(5.3) |
| +Verapamil (5μM) | 0.0250±0.0038(0.6) | 0.056±0.035(1.2) | 0.107±0.015(2.4) |
| +Verapamil (10μM) | 0.0222±0.0002(0.5) | 0.038±0.021(0.8) | 0.057±0.013(1.3) |
| Paclitaxel | 0.0056±0.0006(1.0)c | 4.380±0.802(788.5) | 5.997±1.952(1079.6) |
| +Sildenafil (2.5μM) | 0.0059±0.0014(1.1) | 0.056±0.027(10.0) | 0.293±0.337(52.7) |
| +Sildenafil (5μM) | 0.0057±0.0010(1.0) | 0.024±0.016(4.4) | 0.144±0.171(25.8) |
| +Sildenafil (10μM) | 0.0056±0.0021(1.0) | 0.013±0.007(2.4) | 0.071±0.040(12.8) |
| +Verapamil (2.5μM) | 0.0048±0.0021(0.9) | 0.049±0.039(8.9) | 0.237±0.308(42.7) |
| +Verapamil (5μM) | 0.0042±0.0011(0.7) | 0.012±0.003(2.1) | 0.055±0.044(9.9) |
| +Verapamil (10μM) | 0.0034±0.0007(0.6) | 0.009±0.004(1.7) | 0.042±0.017(7.5) |
| Cisplatin | 1.745±0.161(1.0)c | 1.726±0.083(1.0) | 1.756±0.178(1.0) |
| +Sildenafil (2.5μM) | 1.637±0.088(0.9) | 1.884±0.320(1.1) | 1.835±0.071(1.1) |
| +Sildenafil (5μM) | 1.601±0.030(0.9) | 1.838±0.206(1.1) | 1.865±0.006(1.1) |
| +Sildenafil (10μM) | 1.628±0.157(0.9) | 1.651±0.104(0.9) | 1.811±0.081(1.0) |
| +Verapamil (2.5μM) | 1.729±0.231(1.0) | 1.790±0.250(1.0) | 1.784±0.165(1.0) |
| +Verapamil (5μM) | 1.623±0.001(0.9) | 1.762±0.142(1.0) | 1.747±0.056(1.0) |
| +Verapamil (10μM) | 1.457±0.007(0.8) | 1.738±0.042(1.0) | 1.797±0.106(1.0) |
Cell survival was determined by MTT assay as described in “Materials and Methods”.
Data are means ±SD of at least three independent experiments performed in triplicate.
Fold-resistance was the value of that IC50 value for colchicines, vinblastine, paclitaxel and cisplatin of KB-3-1 cells was divided by IC50 value for colchicines, vinblastine, paclitaxel and cisplatin of KB-3-1, KB-C2 and KB-V1 cells in the absence or presence of sildenafil and verapamil.
Table 2.
The reversal effect of sildenafil and FTC on ABCG2-mediated resistance in drug selected cell linesa
| IC50 ± SDb (μM) |
||||||
|---|---|---|---|---|---|---|
| Compounds | S1 | S1/FLV5000 | S1-M1-80 | MCF-7 | MCF-7/Flv1000 | MCF-7/ADVP3000 |
| Flavopiridol | 0.0961±0.0096(1.0)c | 6.2046±0.4631(64.9) | 0.6992±0.0470(7.3) | 0.3443±0.0021(1.0)c | 6.8556±0.4794(19.9) | 3.9005±0.4139(11.3) |
| + Sildenafil (10μM) | 0.0854±0.0291(0.9) | 5.4169±0.1866(56.4) | 0.1310±0.0192(1.4) | 0.3445±0.0342(1.0) | 3.3609±0.2038(9.8) | 2.0516±0.2546(6.0) |
| + Sildenafil (50μM) | 0.0767±0.0292(0.8) | 5.1281±0.3575(53.4) | 0.0951±0.0351(1.0) | 0.3605±0.0445(1.0) | 0.4531±0.0151(1.3) | 0.5682±0.0321(1.7) |
| + FTC (2.5μM) | 0.1239±0.0388(1.3) | 5.6540±0.1843(58.8) | 0.0948±0.0148(1.0) | 0.3441±0.0148(1.0) | 0.2777±0.0197(0.8) | 0.4166±0.0147(1.2) |
| Mitoxantrone | 0.3796±0.0416(1.0)c | 1.1218±0.1437(3.0) | 223.60±8.60(559.3) | 7.6649±0.1934(1.0)c | 511.35±16.71(66.7) | 279.25±6.03(36.4) |
| + Sildenafil (10μM) | 0.1917±0.0006(0.5) | 0.4212±0.1297(1.1) | 113.65±12.80(284.3) | 2.6792±0.0243(0.3) | 127.21±2.62(16.6) | 96.77±3.01(12.6) |
| + Sildenafil (50μM) | 0.1085±0.0344(0.3) | 0.3469±0.0330(0.9) | 32.95±2.33(82.4) | 1.3434±0.0843(0.2) | 36.93±2.12(4.8) | 24.39±3.66(3.2) |
| + FTC (2.5μM) | 0.3119±0.0310(0.8) | 0.9986±0.0218(2.6) | 3.55±1.91(8.9) | 1.4795±0.2029(0.2) | 18.45±1.63(2.4) | 13.82±0.67(1.8) |
| SN-38 | 0.2449±0.0170(1.0)c | 0.3442±0.0874(1.4) | 3.5533±1.3962(14.4) | 1.2218±0.2626(1.0)c | 22.827±2.001(18.7) | 14.301±1.617(11.3) |
| + Sildenafil (10μM) | 0.2488±0.0598(1.0) | 0.1647±0.0063(0.7) | 1.4886±0.5598(6.0) | 0.6009±0.0071(0.5) | 10.639±1.537(8.7) | 3.861±0.173(3.2) |
| + Sildenafil (50μM) | 0.2070±0.0244(0.8) | 0.1584±0.0151(0.6) | 0.4924±0.0281(2.0) | 0.3137±0.0346(0.3) | 4.214±0.277(3.4) | 1.784±0.086(1.5) |
| + FTC (2.5μM) | 0.2808±0.0280(1.1) | 0.2933±0.0265(1.2) | 0.1014±0.0310(0.4) | 0.2993±0.0272(0.2) | 2.580±0.132(2.1) | 0.198±0.049(0.2) |
| Cisplatin | 14.70±2.23(1.0)c | 53.45±5.80(3.6) | 18.00±2.59(1.2) | 34.41±1.76(1.0)c | 35.12±1.86(1.0) | 31.12±3.20(0.9) |
| + Sildenafil (10μM) | 15.39±2.94(1.0) | 56.27±1.93(3.8) | 20.08±1.43(1.4) | 34.76±0.80(1.0) | 34.43±1.32(1.0) | 30.72±3.79(0.9) |
| + Sildenafil (50μM) | 15.90±3.35(1.1) | 56.88±4.19(3.9) | 20.64±2.24(1.4) | 32.27±1.70(1.0) | 34.47±5.58(1.0) | 28.01±4.65(0.8) |
| + FTC (2.5μM) | 13.27±0.52(0.9) | 47.35±4.48(3.2) | 15.30±1.38(1.0) | 33.24±1.41(1.0) | 35.02±1.04(1.0) | 31.13±0.33(0.9) |
Cell survival was determined by MTT assay as described in “Materials and Methods”.
Data are means ±SD of at least three independent experiments performed in triplicate.
Fold-resistance was the value of that IC50 value for flavopiridol, mitoxantrone, SN-38, and cisplatin of S1 or MCF-7 cells was divided by IC50 value for flavopiridol, mitoxantrone, SN-38, and cisplatin of S1, S1/FLV5000 and S1-M1-80 cells or MCF-7, MCF-7/Flv1000 and MCF-7/ADVP3000 in the absence or presence of sildenafil and FTC.
Table 3.
The reversal effect of sildenafil and FTC on ABCG2-mediated resistance in ABCG2-transfected cell linesa
| IC50 ± SDb (μM) |
||||
|---|---|---|---|---|
| Compounds | HEK293/pcDNA3 | HEK/ABCG2-G2 | HEK/ABCG2-R5 | HEK/ ABCG2-T7 |
| Flavopiridol | 0.1640±0.0171(1.0)c | 0.3976±0.0910(2.4) | 0.2821±0.0148(1.7) | 0.7578±0.0423(4.6) |
| + Sildenafil (10μM) | 0.1493±0.0059(0.9) | 0.1216±0.0112(0.7) | 0.1670±0.0107(1.0) | 0.2256±0.0108(1.4) |
| + Sildenafil (50μM) | 0.1599±0.0180(1.0) | 0.1122±0.0117(0.7) | 0.1466±0.0030(0.9) | 0.1916±0.0316(1.2) |
| + FTC (2.5μM) | 0.1276±0.0033(0.8) | 0.0988±0.0386(0.6) | 0.1643±0.0236(1.0) | 0.1643±0.0296(1.0) |
| Mitoxantrone | 0.0356±0.0030(1.0)c | 1.1042±0.6023(31.0) | 0.4574±0.3924(12.8) | 0.8424±0.1858(23.7) |
| + Sildenafil (10μM) | 0.0115±0.0007(0.3) | 0.0875±0.0045(2.5) | 0.1142±0.0004(3.2) | 0.1168±0.0118(3.3) |
| + Sildenafil (50μM) | 0.0057±0.0006(0.2) | 0.0429±0.0061(1.2) | 0.0784±0.0207(2.2) | 0.0418±0.0020(1.2) |
| + FTC (2.5μM) | 0.0315±0.0092(0.9) | 0.0696±0.0054(2.0) | 0.0420±0.0156(1.2) | 0.0919±0.0208(2.6) |
| SN-38 | 0.0058±0.0002(1.0)c | 0.2042±0.1362(2.3) | 0.1282±0.0882(22.1) | 0.1171±0.0704(20.2) |
| + Sildenafil (10μM) | 0.0034±0.0010(0.6) | 0.0101±0.0021(1.7) | 0.0175±0.0018(3.0) | 0.0488±0.0186(8.4) |
| + Sildenafil (50μM) | 0.0019±0.0003(0.3) | 0.0044±0.0100(0.8) | 0.0063±0.0029(1.1) | 0.0139±0.0025(2.4) |
| + FTC (2.5μM) | 0.0036±0.0092(0.8) | 0.0050±0.0009(1.9) | 0.0081±0.0051(1.4) | 0.0082±0.0023(1.4) |
| Cisplatin | 1.6300±0.1697(1.0)c | 1.2305±0.0841(0.8) | 1.3265±0.2001(0.8) | 1.8435±0.0233(1.1) |
| + Sildenafil (10μM) | 1.5640±0.0156(1.0) | 1.1371±0.1577(0.7) | 1.3855±0.0955(0.9) | 1.8115±0.0403(1.1) |
| + Sildenafil (50μM) | 2.0180±0.4285(1.2) | 1.1993±0.1429(0.7) | 1.9820±0.0438(1.2) | 1.9680±0.0665(1.2) |
| + FTC (2.5μM) | 1.1535±0.0488(0.7) | 1.0466±0.028(0.6) | 1.3495±0.2072(0.8) | 1.9530±0.0834(1.2) |
Cell survival was determined by MTT assay as described in “Materials and Methods”.
Data are means ±SD of at least three independent experiments performed in triplicate.
Fold-resistance was the value of that IC50 value for flavopiridol, mitoxantrone, SN-38, and cisplatin of HEK293/pcDNA3 cells was divided by IC50 value for flavopiridol, mitoxantrone, SN-38, and cisplatin of HEK293/pcDNA3, HEK293/ABCG2-G2, HEK293/ABCG2-R5, and HEK293/ ABCG2-T7 cells in the absence or presence of sildenafil and FTC.
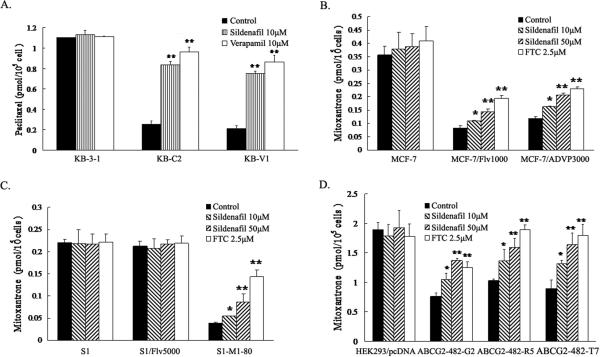
Sildenafil increases the accumulation of [3H]-paclitaxel in ABCB1 overexpressing cells, [3H]-mitoxantrone and BODIPY-prazosin in ABCG2 overexpressing cells
To investigate the potential mechanism by which sildenafil sensitizes ABCB1- and ABCG2-overexpressing cells to chemotherapeutic drugs, we examined the effect of sildenafil on the accumulation of chemotherapeutic drugs in ABCB1- or ABCG2-overexpressing cells. Intracellular [3H]-paclitaxel was measured in ABCB1-overexpressing cells in the presence or absence of sildenafil, and the results are shown in Figure 1A. After 2 hours of incubation, the intracellular level of [3H]-paclitaxel in ABCB1-overexpressing KB-C2 and KB-V1 cells were significantly lower than that of the parental KB-3-1 cells. Sildenafil at 10 μM concentration significantly increased the intracellular level of [3H]-paclitaxel close to the effect of verapamil at 10 μM in KB-C2 and KB-V1 cells. Neither sildenafil nor verapamil altered the intracellular levels of [3H]-paclitaxel in parental KB-3-1 cells. Similarly, the intracellular levels of [3H]-mitoxantrone were measured in the ABCG2-overexpressing cells in the presence or absence of sildenafil (Figure. 1B, C and D). The intracellular level of [3H]-mitoxantrone in cells expressing either wild type or mutant ABCG2 were significantly less than that in parental cells. In the presence of sildenafil, either at 10 μM or 50 μM concentration, all ABCG2-overexpressing cell lines displayed elevated intracellular [3H]-mitoxantrone levels, and intracellular levels of [3H]-mitoxantrone increased with increasing concentrations of sildenafil. However, the effects of sildenafil at 50 μM were less than those observed for FTC at 2.5 μM. Neither sildenafil nor FTC affected the intracellular levels of [3H]-mitoxantrone in parental cells.
Figure 1. Sildenafil increased the accumulation of [3H]-paclitaxel or [3H]-mitoxantrone in the ABCB1 or ABCG2 overexpressing cells.
The accumulation of [3H]-paclitaxel (A) or [3H]-mitoxantrone (B, C and D) was measured after cells were pre-incubated with or without sildenafil, verapamil or FTC for 1 h at 37°C and then incubated with 0.1 μM [3H]-paclitaxel or 0.02 μM [3H]-mitoxantrone for another 2 h at 37°C. Data points represent the means ± SD of triplicate determinations. * and ** represent p < 0.05 and p < 0.01, respectively, for values versus those in the control group. Independent experiments were performed at least three times, and a representative experiment is shown.
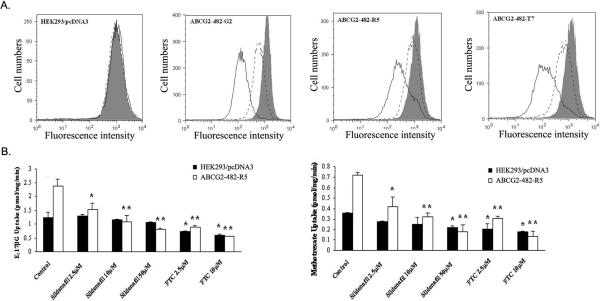
Additionally, we evaluated the effect of sildenafil on the accumulation of the fluorescent compound (16), a known fluorescent substrate of ABCG2 , in the ABCG2-overexpressing cells. Sildenafil enhanced the accumulation of BODIPY-prazosin at 50 μM concentration in cells expressing either wild-type or mutant ABCG2, but was weaker than that of FTC at 2.5 μM. Representative histograms for BODIPY-prazosin are shown in Figure 2A. Taken together, these data are in agreement with our cytotoxicity results and suggest that sildenafil is able to inhibit the efflux function of ABCB1 and ABCG2 leading to the significant increase of intracellular accumulation of [3H]-paclitaxel in ABCB1-overexpressing cells and [3H]- mitoxantrone as well as BODIPY-prazosin in ABCG2-overexpressing cells.
Figure 2. Sildenafil inhibited the efllux of BODIPY-prazosin and transport of [3H]-E217βG as well as [3H]-methotrexate by ABCG2.
(A) HEK293/pcDNA3.1, ABCG2-482-G2, ABCG2-482-R5, and ABCG2-482-T7 cells were incubated in 250 nM BODIPY-prazosin alone (heavy solid line) or with 2.5 μM sildenafil (solid line), 10 μM sildenafil (dashed line) and 10 μM FTC (shaded histogram) for 30 min at 37°C, after which the cells were washed, and allowed to efflux for 1 h at 37°C in substrate-free media continuing with or without inhibitors. All samples were analyzed on a flow cytometer. (B) Membrane vesicles were prepared from HEK293/pcDNA3.1 and ABCG2-482-R5 cells. The rates of the uptake of [3H]-E217βG (A) and [3H]-methotrexate into membrane vesicles (10 μg protein/reaction) were measured for 10 min at 37°C in uptake media containing either 4 mM ATP or 4 mM AMP with various concentrations of sildenafil or FTC for 1 h on ice, and then transport reactions were carried out for 10 min at 37°C in uptake medium containing 4 mM ATP. Data points represent the means ± SD of triplicate determinations. * and ** represent p < 0.05 and p < 0.01 respectively, for values versus those in the control group. At least three independent experiments were performed, and a representative experiment is shown.
Sildenafil inhibits the transport of E217βG and methotrexate by ABCG2
To assess the potency of sildenafil as an in vitro inhibitor of ABCG2, the ability of sildenafil to inhibit the transport activity of ABCG2 was analyzed using the chemotherapeutic drug substrate [3H]-methotrexate and the physiological substrate [3H]-E217βG. In our previous study, only wild type ABCG2 was shown being able to transport methotrexate and E217βG in the in vitro transport system (11). Thus, we used membrane vesicles prepared from HEK293/pcDNA3.1 and ABCG2482-R5 cells (Figure 2B). Sildenafil significantly inhibited the rates of both methotrexate and E217βG uptake in the membrane vesicles of wild type ABCG2 in a concentration-dependent manner, but its inhibitory effect was weaker than that of FTC at the same concentration. FTC also moderately decreased the uptake rates of both methotrexate and E217βG in the membrane vesicles of HEK293/pcDNA3.1, but sildenafil did not. These in vitro transport results suggest that sildenafil is able to directly inhibit the transport function of E217βG and methotrexate in wild-type ABCG2 expressing cells.
Sildenafil stimulates the ATPase activity and affects the [125I]-IAAP photo-labeling of ABCB1 and ABCG2
Generally, the substrates of ABC transporters stimulate their ATPase activity, and among the reversal agents, some (e.g., verapamil) stimulate the activity whereas others (e.g., cyclosporin A) inhibit ATP hydrolysis (26). To assess the effect of sildenafil on the ATPase activity of ABCB1 and ABCG2, the membrane vesicles of High Five insect cells overexpressing ABCB1 or ABCG2 were used in the presence of various concentrations of sildenafil under conditions that suppressed the activity of other major membrane ATPases. As shown in Figure 3A and B, sildenafil at the indicated concentrations potently stimulated the ATPase activity of ABCB1, but mildly stimulated the ATPase activity of ABCG2. The concentrations of sildenafil required for 50% stimulation of ATPase activity of ABCB1 and ABCG2 were 5-10 μM and 0.25-0.5 μM, respectively. These data indicate that sildenafil may potentially be a weak substrate for ABCB1 compared to ABCG2.
Figure 3. Effect of sildenafil on the ATPase activity and [125I]-IAAP photoaffinity labeling of ABCB1 and ABCG2.
The Vi-sensitive ATPase activity of ABCB1 (A) and ABCG2 (B) in membrane vesicles was determined with different concentrations of sildenafil as described in “Materials and Methods”. Mean values are given, and the error bars represent standard error from at least three independent experiments. The photoaffinity labeling of ABCB1 (C) andABCG2 (D) with [125I]-IAAP was performed in the presence of different concentrations of sildenafil. The radioactivity incorporated into ABCB1 and ABCG2 was determined by exposing the gel to an X-ray film at -70ºC. The lower panels show the autoradiograms and quantification of incorporation of IAAP into ABCB1 and ABCG2 band, respectively, from at least two independent experiments. Cyclosporine A and verapamil were used as positive controls for inhibition of [125I]-IAAP photolabeling of ABCB1 and FTC was used as a positive control for ABCG2. CSA: cyclosporine A; Vera: verapamil. * and ** represent p < 0.05 and p < 0.01, respectively, for values versus those in the control group.
The photoaffinity analogue of prazosin, [125I]-IAAP, which is transported by both ABCB1 and ABCG2 (20), has been widely used to determine the binding regions of ABCB1 and ABCG2 that interact with substrates and inhibitors. To test whether sildenafil interacts at the prazosin binding site of ABCB1 or ABCG2, the ability of sildenafil to prevent photolabeling of ABCB1 and ABCG2 with [125I]-IAAP was examined by using the membrane vesicles from High Five insect cells transfected with ABCB1 or ABCG2. As showed in Figure 3C and D, sildenafil dose-dependently inhibited the photoaffinity labeling of ABCB1 or ABCG2 with [125I]-IAAP. The 50% inhibition of the photoaffinity labeling of ABCB1 and ABCG2 with [125I]-IAAP were inhibited by sildenafil at 10 μM and 100 μM, respectively. Meanwhile, the ABCB1 inhibitors cyclosporine A at 10 μM and verapamil at 20 μM inhibited the [125I]-IAAP photo-labeling of ABCB1 up to ~95% and ~65% respectively, and the ABCG2 inhibitor FTC at 20 μM suppressed the [125I]-IAAP photo-labeling of ABCG2 to ~40% compared to membranes incubated with no inhibitor. Thus, these data suggest that sildenafil competes with IAAP to bind both ABCB1 and ABCG2.
Model for binding of sildenafil to ABCB1
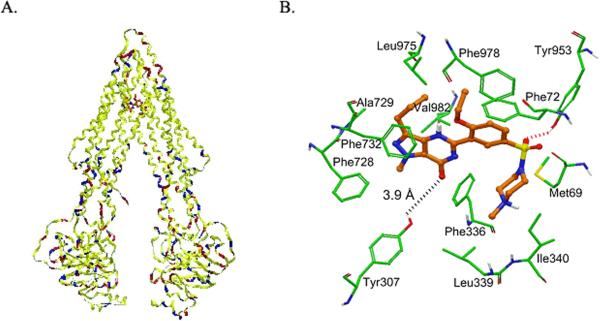
Since sildenafil has been described as ABCB1 inhibitor for the first time, its predicted binding conformation within the large cavity of the transmembrane region of ABCB1 is yet to be determined. Since, the crystal structure of human ABCB1 is yet to be determined; we sought to develop a homology model of human ABCB1 based on the mouse ABCB1-QZ59-RRR co-crystal structure as a template. Human ABCB1 homology model (Fig. 4A) was then used to dock sildenafil using Glide docking software to investigate its potential binding mode. Three binding sites were reported in the crystal structure of mouse ABCB1 as represented by ABCB1-QZ59-RRR (site-1), ABCB1-QZ59-SSS (site-2), and ABCB1-verapamil (site-3) (23). To identify favorable binding site on ABCB1 for sildenafil, we performed docking experiments using these sites. Since our photoaffinity labeling data suggested that sildenafil displaces IAAP in a dose dependent manner, we also docked IAAP to these sites for comparison. This data also indicated that sildenafil and IAAP share same binding site on ABCB1 i.e., site-1. Binding energy data for the docked poses of sildenafil and IAAP was compared at each of the binding sites (supplemental Table 1). Based on binding energy data analysis, both ligands (sildenafil and IAAP) were found to bind most favorably within the QZ59-RRR binding site of ABCB1. In the following section, bound conformation of sildenafil is discussed in context of site-1.
Figure 4. Model for binding of sildenafil to ABCB1.
(A) Ribbon diagram of open to the cytoplasm-3D structure conformation of a homology model of human ABCB1 based on the crystal structure coordinates of mouse ABCB1. The color codes for the ribbons are according to the residue charge: yellow-neutral, blue-basic and red-acidic. Sildenafil is shown as ball and stick model within the large hydrophobic cavity of ABCB1 characterized by QZ59-RRR inhibitor binding site. (B) XP-Glide predicted binding mode of sildenafil within QZ59-RRR binding site. Important amino acids are depicted as sticks with the atoms colored as carbon – green, hydrogen – white, nitrogen – blue, oxygen – red and sulfur – yellow) whereas the inhibitor is shown as ball and stick model with the same color scheme as above except carbon atoms are represented in orange. Dotted red line indicates hydrogen bonding interaction whereas dotted black line indicates interacting distance.
The XP-Glide predicted binding mode of sildenafil clearly shows the importance of hydrophobic interactions within the large drug binding cavity of ABCB1 (Fig. 4B). The N-methylpiperazine (D-ring) of sildenafil was found to be stabilized by hydrophobic contacts with the side chains of Met69 of Transmembrane1 (TM1), Phe336, Leu339 and Ile340 of TM6. The C-ring along with ethoxy substituent enters into favorable hydrophobic interactions with residues Phe72 of TM1, Leu975 and Phe978 of TM12. The A-ring along with its methyl and propyl substituents and the B-ring forms hydrophobic interactions with the side chains of Phe728, Ala729, Phe732 of TM7 and Val982 of TM12. In addition to the hydrophobic contacts, sildenafil also seem to be stabilized by electrostatic interactions with key residues Tyr953 of TM11 and Tyr307 of TM5. For example, the sulfonyl oxygen atom enters into a hydrogen bonding interaction with the hydroxyl group of Tyr953 (-SO2---HO-Tyr953), whereas the carbonyl oxygen atom present in the B-ring is located at a distance of 3.9 Å from the side chain hydroxyl group of Tyr307. Transmembrane domain numbering is according to the reported literature by Aller et al (23). It may be noted that the binding energy data for sildenafil (supplemental Table 1) within each of the predicted ABCB1 sites is yet to be experimentally validated. Although docking results are not verified by site-directed mutagenesis or co-crystal complex of sildenafil-ABCB1, however, in the interim the binding model of sildenafil within site-1 of ABCB1 may serve as a guide for future ABCB1 inhibitor development.
DISCUSSION
In this study, we examined the effect of sildenafil on ABCB1-, ABCC1- and ABCG2-overexpressing cancer cells. Our data shows sildenafil has different effects on these three ABC transporters. Cytotoxicity assays showed that sildenafil potently sensitized ABCB1-overexpressing cells to ABCB1 substrates colchicine, vinblastine and paclitaxel; mildly sensitized wild-type or mutant ABCG2-overexpressing cells to the ABCG2 substrates flavopiridol, mitoxantrone and SN-38. However, sildenafil had no significant effect on sensitivity of ABCC1-overexpressing cells to its substrate vincristine. Furthermore, sildenafil did not sensitize any of the cells examined to cisplatin and had no effect on the sensitivity of any of the parental cell lines. These data suggest that the reversing ability of sildenafil could be specific to ABCB1 and ABCG2, although sildenafil appeared to have greater affinity for ABCB1 than for ABCG2. Consistent with the cytotoxicity data, the results of drug accumulation studies showed that sildenafil significantly enhances the intracellular accumulation of paclitaxel in ABCB1-overexpressing cells, and slightly enhances the intracellular accumulation of mitoxantrone and BODIPY-prazosin in cells overexpressing either wild type or mutant ABCG2. These results confirm that sildenafil has a greater affinity for ABCB1 than for ABCG2. In addition, the results of membrane vesicles transport experiments demonstrated that sildenafil directly inhibits ABCG2-mediated transport of E217βG and methotrexate. The results of drug accumulation studies and membrane vesicles transport study indicate that sildenafil reduces ABCB1- and ABCG2-mediated drug resistance by directly inhibiting the drug-transport function of ABCB1 and ABCG2. Additionally, we also investigated the interaction of sildenafil with ABCB1 as well as with ABCG2 by using the Vi-sensitive ATPase assay and photoaffinity labeling with [125I]-IAAP. Generally, the ATPase activity of ABC transporters is stimulated in the presence of transport substrates, and the fact that sildenafil powerfully stimulated the ATPase activity of ABCB1 and mildly stimulated the ATPase activity of ABCG2 suggests that it might potentially be a substrate of ABCB1 and ABCG2. Most agents block drug transport by acting as competitive or noncompetitive inhibitors that bind either to drug interaction sites or to other modulator binding sites, leading to allosteric changes that prevent outward transport of drugs (26). Therefore, sildenafil may act as a competitive inhibitor of ABCB1 and ABCG2 by competing with other drug substrates since sildenafil itself may be a substrate. Interestingly, when [125I]-IAAP was used to photolabel ABCB1 and ABCG2, we found that sildenafil inhibited the photoaffinity labeling of ABCB1 and ABCG2 with [125I]-IAAP. This indicates that sildenafil competes with IAAP to bind to drug substrate site in both ABCB1 and ABCG2, suggesting further that sildenafil interacts in the transmemberane regions of both transporters.
Quantitative structure-activity relationship (QSAR) studies on several ABCB1 inhibitors have clearly indicated the major contribution of lipophilicity towards potent ABCB1 inhibitory activity (27, 28). This is not surprising considering the fact that these inhibitors mostly bind to the large hydrophobic cavity located within the membrane bilayer portion of the ABCB1. In addition, a number of pharmacophore models for ABCB1 inhibitors have identified features such as hydrophobic, hydrogen bond acceptor, aromatic ring center and positive ionizable groups (29). Importantly, although these properties are present in several ABCB1 inhibitors, they seem to be only partially overlapping in the spatial arrangement of the phramacophoric groups, pointing towards the existence of multiple binding sites on ABCB1 (30). Interestingly, sildenafil exhibits all of these pharmacophoric features (hydrophobic, hydrogen bond acceptor, aromatic ring center and positive ionizable groups) and thus can explain its affinity for the large central hydrophobic cavity of ABCB1. ABCB1 favors positively charged amphipathic molecules, which suggests the involvement of acidic residues such as Asp and Glu in drug binding. However, analysis of a human ABCB1 homology model did not reveal the presence of acidic residues in the 8 Å sphere around the docked pose of sildenafil indicating the cationic selectivity of ABCB1 is not determined by site-1. Among the 1280 residues, 15 residues represented by Glu74 (TM1), Asp77 (TM1), Glu86 (TM1/EC1), Asp87 (EC1), Asp97 (EC1), Asp100 (TM2), Glu108 (TM2), Glu109 (TM2), Asp110 (TM2), Glu325 (EC3), Asp743 (EC4), Asp744 (EC4), Glu746 (TM8/EC4), Glu972 (EC6/TM12), and Asp973 (TM12) were found to be located close to the membrane surface and are accessible from within the site-1 may provide selectivity for cationic amphipathic drug molecules through ionic interactions during their entry into the site-1 and these regions are akin to “portals” described by Aller et al in mouse Mdr3 protein (26).
Sildenafil is a highly selective inhibitor of cGMP-specific PDE5 and is widely used to treat erectile dysfunction and pulmonary arterial hypertension. The mechanism of action of sildenafil involves the protection of cGMP from degradation by PDE5. Sildenafil acts as a competitive binding agent of PDE5 with cGMP due to their similar structures, which results in increased levels of cGMP leading to vascular smooth muscle relaxation (vasodilation) and increased inflow of blood (31). However, in this study we did not examine and could not exclude the effects of sildenafil on the cGMP level. Although the concentrations of sildenafil used in the current study were a little higher than those found in patients in the clinical setting and sufficient to block the PDE5 activity (32), we did not observe any significant effect on the growth and survival of cells. Up to now, several groups have evaluated the effect of sildenafil on cancer treatment due to the fact that some cancers showed higher level of PDE5 activity. It has been reported that sildenafil induced caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells in vitro (33). Sildenafil also augmented the endogenous antitumor immunity by reducing the myeloid-derived suppressor cell function (34). Black et al. reported sildenafil was able to increase blood-brain tumor barrier permeability and enhance efficacy of chemotherapy in a rat brain tumor model (35). Recently, sildenafil was reported to enhance the sensitivity of doxorubicin in breast cancer cells, but not exacerbate doxorubicin toxicity to either bone marrow cells or macrophages (36). While this manuscript was in preparation, Das et al. reported that sildenafil in combination with doxorubicin induced apoptosis by upregulating caspase-3 and caspase-9 activity, by reducing the phosphorylation of Bad and expression of Bcl-xl in prostate cancer cells (37). In addition, the sildenafil-doxorubicin combination reduced the tumor burden in mice prostate tumor xenograft model (37). Further study is required to ascertain whether there is expression of ABC-transporters in the Das et al. model or, on the contrary, if apoptotic mechanism is involved in sildenafil induced sensitization of ABC-transporters needs to be determined. Based on these recent studies and our data, it is reasonable to suggest that sildenafil may have the potential to improve the chemotherapeutic outcome of cancer patients, with possibly several mechanisms of action.
Strategies to develop ABC transporter proteins as a therapeutic target, to inhibit drug efflux and thereby overcome drug resistance have to date failed in the clinic. Although this was ascribed in many trials to the use of an inhibitor that was not potent or one with marked pharmacokinetic interactions, a number of trials with third generation agents have also proven to have negative outcomes. Nonetheless, there is increasing evidence that ABC transporters are important in regulating oral bioavailability, pharmacology and in sanctuary site protection. In pharmacology, single nucleotide variants that result in impaired protein expression or function have been shown to associate with higher drug levels or increased toxicitiy. The expanding awareness of these proteins in noncancer cells offers the possibility of using knowledge gained in the development of ABC transporter inhibitors to develop strategies to improve oral bioavailability or to improve drug delivery.
Additionally, it has been reported that the most common side effects of sildenafil use included flushing, headache, dyspepsia, unintentional incomplete sexual arousal and palpitation, etc (38). Theoretically, by determining the structure activity relationship (SAR) of sildenafil on inhibiting the cGMP hydrolase and ABC transporters, it is possible to synthesize a better sildenafil analogue, with a more potent ABC transporter inhibition without affecting the cGMP hydrolase activity.
In conclusion, the present study demonstrates that sildenafil inhibits ABCB1- and ABCG2-mediated drug efflux, resulting in an increase in the intracellular concentrations of anticancer drugs and ensuing drug sensitivity. Further study showed that sildenafil stimulated the ATPase activity of these two pumps and inhibited the photoaffinity labeling of the transporters with [125I]-IAAP. Whether sildenafil could contribute to improving clinical outcome in patients receiving chemotherapy, perhaps inhibiting drug efflux mediated by ABCB1 and/or ABCG2, remains to be determined. At a minimum, the study of the interaction of sildenafil with ABCB1 and ABCG2 presented herein provides important clues to potential drug-drug interactions, provides a potential approach to increasing oral drug bioavailability, and a method to increase substrate drug accumulation in sanctuary sites.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Sharron H. Francis (Vanderbilt University School of Medicine, Nashville, Tennessee) for kindly providing purified sildenafil and critical reading of the manuscript. We thank Drs. Michael M. Gottesman (NIH, USA) for providing KB-3-1 and their drug resistant cell lines. We thank students at Dr. Chen's lab, Kamlesh Sodani, Chun-ling Dai, Atish Patel, for their comments and critical reading of the manuscript. This work was supported by funds from National Institutes of Health (No.1R15CA143701 to Z.S.C.), St. John's University Research Seed Grant (No.579-1110-7002 to Z.S.C.) and the Chinese National Natural Science Foundation (No.81061160507 to L.W.F.). IW Kim, S. Shukla, RW Robey, SE Bates and SV Ambudkar were supported by the Intramural Research Program, NIH, National Cancer Institute, Center for Cancer Research. Z. Shi is a recipient of Kaisi fellowship for overseas study at St. John's University from Sun Yat-Sen University.
Abbreviations
- MDR
multidrug resistance
- ABC
ATP-binding cassette
- FTC
Fumitremorgin C
- IAAP
Iodoarylazidoprazosin
- PDE5
phosphodiesterase type 5
REFERENCES
- 1.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–66. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 2.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 3.Germann UA, Ford PJ, Shlyakhter D, Mason VS, Harding MW. Chemosensitization and drug accumulation effects of VX-710, verapamil, cyclosporin A, MS-209 and GF120918 in multidrug resistant HL60/ADR cells expressing the multidrug resistance-associated protein MRP. Anticancer Drugs. 1997;8:141–55. doi: 10.1097/00001813-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Qadir M, O'Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–6. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 5.Minderman H, O'Loughlin KL, Pendyala L, Baer MR. VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res. 2004;10:1826–34. doi: 10.1158/1078-0432.ccr-0914-3. [DOI] [PubMed] [Google Scholar]
- 6.Dantzig AH, Shepard RL, Pratt SE, Tabas LB, Lander PA, Ma L, et al. Evaluation of the binding of the tricyclic isoxazole photoaffinity label LY475776 to multidrug resistance associated protein 1 (MRP1) orthologs and several ATP- binding cassette (ABC) drug transporters. Biochem Pharmacol. 2004;67:1111–21. doi: 10.1016/j.bcp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Evers R, Kool M, Smith AJ, van Deemter L, de Haas M, Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br J Cancer. 2000;83:366–74. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sissung TM, Gardner ER, Piekarz RL, Howden R, Chen X, Woo S, et al. Impact of ABCB1 Allelic Variants on QTc Interval Prolongation. Clin Cancer Res. 2011;17:937–46. doi: 10.1158/1078-0432.CCR-10-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Z, Tiwari AK, Shukla S, Robey RW, Kim IW, Parmar S, et al. Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478. Biochem Pharmacol. 2009;77:781–93. doi: 10.1016/j.bcp.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–14. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–20. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- 12.Tiwari AK, Sodani K, Wang SR, Kuang YH, Ashby CR, Jr., Chen X, et al. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009;78:153–61. doi: 10.1016/j.bcp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Francis SH, Sekhar KR, Rouse AB, Grimes KA, Corbin JD. Single step isolation of sildenafil from commercially available Viagra tablets. Int J Impot Res. 2003;15:369–72. doi: 10.1038/sj.ijir.3901040. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama S, Fojo A, Hanover JA, Pastan I, Gottesman MM. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985;11:117–26. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi Y, Yoshida A, Takada Y, Komano T, Ueda K. Anti-cancer drugs and glutathione stimulate vanadate-induced trapping of nucleotide in multidrug resistance-associated protein (MRP). FEBS Lett. 1997;401:11–4. doi: 10.1016/s0014-5793(96)01421-4. [DOI] [PubMed] [Google Scholar]
- 16.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–8. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–52. [PubMed] [Google Scholar]
- 18.Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, et al. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63:4048–54. [PubMed] [Google Scholar]
- 19.Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–14. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 20.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–51. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 21.Sauna ZE, Ambudkar SV. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc Natl Acad Sci U S A. 2000;97:2515–20. doi: 10.1073/pnas.97.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen WL, Maxwell DS, Tirado-Rives JJ. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J Am Chem Soc. 1996;118:11225–36. [Google Scholar]
- 23.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–22. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornwell MM, Gottesman MM, Pastan IH. Increased vinblastine binding to membrane vesicles from multidrug-resistant KB cells. J Biol Chem. 1986;261:7921–8. [PubMed] [Google Scholar]
- 25.Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, et al. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–9. [PubMed] [Google Scholar]
- 26.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–98. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 27.Raub TJ. P-glycoprotein recognition of substrates and circumvention through rational drug design. Mol Pharm. 2006;3:3–25. doi: 10.1021/mp0500871. [DOI] [PubMed] [Google Scholar]
- 28.Pleban K, Ecker GF. Inhibitors of p-glycoprotein--lead identification and optimisation. Mini Rev Med Chem. 2005;5:153–63. doi: 10.2174/1389557053402729. [DOI] [PubMed] [Google Scholar]
- 29.Ekins S. Drug Transporter Pharmacophores. Transporters as Drug Carriers. 2009;44:215–21. [Google Scholar]
- 30.Loo TW, Clarke DM. Mutational analysis of ABC proteins. Arch Biochem Biophys. 2008;476:51–64. doi: 10.1016/j.abb.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 32.Milligan PA, Marshall SF, Karlsson MO. A population pharmacokinetic analysis of sildenafil citrate in patients with erectile dysfunction. Br J Clin Pharmacol. 2002;53(Suppl 1):45S–52S. doi: 10.1046/j.0306-5251.2001.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarfati M, Mateo V, Baudet S, Rubio M, Fernandez C, Davi F, et al. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2003;101:265–9. doi: 10.1182/blood-2002-01-0075. [DOI] [PubMed] [Google Scholar]
- 34.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Black KL, Yin D, Ong JM, Hu J, Konda BM, Wang X, et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008;1230:290–302. doi: 10.1016/j.brainres.2008.06.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di X, Gennings C, Bear HD, Graham LJ, Sheth CM, White KL, Jr., et al. Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-0765-7. [DOI] [PubMed] [Google Scholar]
- 37.Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci U S A. 2010;107:18202–7. doi: 10.1073/pnas.1006965107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dundar M, Kocak I, Dundar SO, Erol H. Evaluation of side effects of sildenafil in group of young healthy volunteers. Int Urol Nephrol. 2001;32:705–8. doi: 10.1023/a:1015056416501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.