Abstract
The bromodomain-containing chromatin modifying factor BRD4 is an inherited susceptibility gene for breast cancer progression and metastasis, but its functionality in these settings has yet to be explored. Here we show that deletion of either of the BRD4 bromodomains had modest effects on the metastatic suppression ability of BRD4. In contrast, expression of the natural short isoform of BRD4 that truncates the protein after the SEED domain restored progression and metastatic capacity. Unexpectedly, deletion of the proline-rich region induced mesenchymal-like conversion and acquisition of cancer stem cell-like properties, which are mediated by the carboxy terminal P-TEFb binding domain. Deletion of this proline-rich region also induced a gene expression signature that predicted poor outcome in human breast cancer datasets and that overlapped G3 grade human breast tumors. Thus, our findings suggest that BRD4 may be altering the predisposition of tumors to undergo conversion to a more-de-differentiated or primitive state during metastatic progression.
Keywords: Breast cancer, Metastasis, Gene expression, Survival, Stem cell
Introduction
Metastasis is an extremely complex process that remains a major problem in the management of cancer. For breast cancer the median survival of patients with metastatic cancer is 2–4 years, compared to an approximate survival rate of 80% for women with non-metastatic tumors (1). The ability to control the spread or effectively treat patients with or at risk of metastatic disease would therefore significantly improve overall outcome of the disease.
Part of the complexity that complicates our understanding of tumor progression is likely due to the highly genetically heterogeneous nature of human populations. Previously, our laboratory demonstrated that the genetic context on which a tumor arises significantly impacts the ability of that tumor to form pulmonary metastases (2). Subsequently, using a systems genetics approach we have identified a number of genes associated with mammary tumor metastatic progression in the mouse and validated their role in human breast cancer gene expression through analysis of publicly available breast cancer datasets (3–6).
One of these genes is the bromodomain containing gene Brd4. Ectopic expression of Brd4 in mice was found to repress primary tumor growth and metastatic capacity. Brd4 activation also induced a gene signature that predicted good outcome in human breast cancer datasets (5). BRD4 has two bromodomains within the N-terminus, an ET domain, a serine-rich SEED domain and a proline-rich C-terminal region. BRD4 interacts with acetylated chromatin via its two bromodomains (7) and also binds the metastasis modifier SIPA1 through bromodomain II (8).
Brd4 is expressed normally as two alternatively spliced variants. Both isoforms retain the amino half of the long isoform and they have a distinct 3'-UTR. To gain a better understanding of the molecular mechanism by which Brd4 suppresses tumor progression, isoform and domain analysis was performed. Here we demonstrated that the proline-rich region encoded in the carboxy half of the longer isoform is essential for the tumor- and the metastasissuppressive properties of Brd4. Unexpectedly, the extreme C-terminal domain containing the PTEFb binding region (9) was found to act as a metastasis enhancer and to induce both epithelialto-mesenchymal transition (EMT)- and cancer stem cell-like properties without the activation of the canonical pathways associated with these processes. These data suggest that the metastasis susceptibility function mediated by Brd4 may be the result of a complex series of opposing positive and negative factors that modify the ability of tumor cells to revert to a more de-differentiated state, possibly by a novel mechanism. If true, this implies that inherited metastatic susceptibility may be due in part to polymorphisms lowering the barriers preventing tumor cells from reverting to more primitive transcriptional states.
Material and Methods
Cell culture and transfection
The Mvt-1 cell line was obtained as a gift from Lalage Wakefield (NCI, Bethesda, MD) in 2003. The cell line was validated as mouse by microsatellite-based PCR and in vivo passage in FVB/NJ mice. The most recent validation by in vivo passage was performed in November 2010. The ΔI, ΔII and ΔC vectors (8, 10) were co-transfected along with pSuper.Retro.Puro (Oligoengine, Seattle, WA), as a selectable marker for transfectants, using the FuGene 6 transfection reagent (Roche, Indianapolis, IN) to develop stable cell lines and clones generated by limiting dilution. Cells expressing Brd4 or β-Galactosidase (β-Gal) were previously described (5).
Lentiviral gene transfer
The SF and STer lentiviral vectors encode the FLAG-Brd4 short isoform gene and the first 699 amino acids (aa) of BRD4, respectively, driven by the mouse Pol2 promoter The lentiviral particles were produced by transfection of HEK293T cells with 1 μg of SF vector, 750 ng psPAX2 packaging plasmid and 250 ng pMD2.G envelope plasmid using the FuGene 6 transfection reagent. Forty-eight hours later, the virus-containing media was harvested and filtered through a 0.45 μm filter. Viral particles were then used to infect the Mvt-1 cells. STer viral particles were produced by the Viral Technology Group in NCI, Frederick.
Animal studies
Orthotopic injection was performed by injecting 6-weeks old FVB/NJ female mice, into the forth mammary fat pad, with 1×105 cells/animal. Primary tumor weight was compared 25–26 days post-injection. Lungs were harvested, sectioned, H&E stained and metastasis nodules counted. Tail vein injections were performed by injecting FVB/NJ female mice with 2.5×105 cells/animal and lungs processed as described above.
All experiments were performed in compliance with the National Cancer Institute's Animal Care and Use Committee guidelines.
In vitro invasion three dimensional (3D) assay
The invasiveness of the cells was assayed by culturing the cells in a 3D culture as described (5).
Total RNA isolation
Total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen, Germantown, MD) as described (5). Samples containing high-quality total RNA with A260/A280 ratios between 1.8 and 2.1 were used for further analysis.
Microarray and survival analysis
Microarray expression analysis was performed at LMT, NCI Frederick. Microarray analysis of Brd4 and β-Gal clones was previously described (5). Normalization was performed using Partek Genomics Suite software. For survival analysis BRB array tool software was used.
Quantitative real-time PCR
cDNA was synthesized from RNA isolated from cells using the iScript cDNA synthesis kit (BioRad, Hercules, CA) per the manufacturer's protocol. Quantitative real-time PCR was performed using an ABI PRISM 7900HT Sequence Detection System as described (5).
F-actin staining
Cells were seeded in 2-well slides (Nunc, Rochester, NY) at a density of 1×105 cells/well. Twenty-four hours later, cells were fixed with paraformaldehyde containing sucrose and Triton X-100, then re-fixed with paraformaldehyde containing sucrose. Staining for F-actin was carried out by overnight incubation with Alexa-Fluor®488 Phalloidin (Molecular Probes, Eugene, Oregon). Cells were subsequently washed and mounted with VECTASHIELD (Vector Laboratories, Burlingame, CA) and examined with Zeiss LSM 510 confocal microscope.
Tumorsphere formation assay
Single cells were seeded onto Ultra-low attachment 24-well plates (Corning, Corning, NY) at a density of 3000 cells/well in serum-free growth media. Pictures were taken 3 and 6 days after seeding using a light microscope.
Flow cytometry
Cells were trypsinized to a single cell suspension, washed with PBS and 1×106 cells were resuspended in 100 μl Stain buffer (FBS) (BD Biosciences, San Diego, CA). Cells were stained for the pre-conjugated antibodies CD24a-PE and CD49f-FITC or matched isotope controls (BD Biosciences). Cells were then washed and resuspended in FBS and analyzed by FACS (LSR II Fortessa, BD Biosciences). The data were evaluated using FlowJo software.
Results
Brd4 proline-rich C-terminal domain mediates primary tumor growth suppression
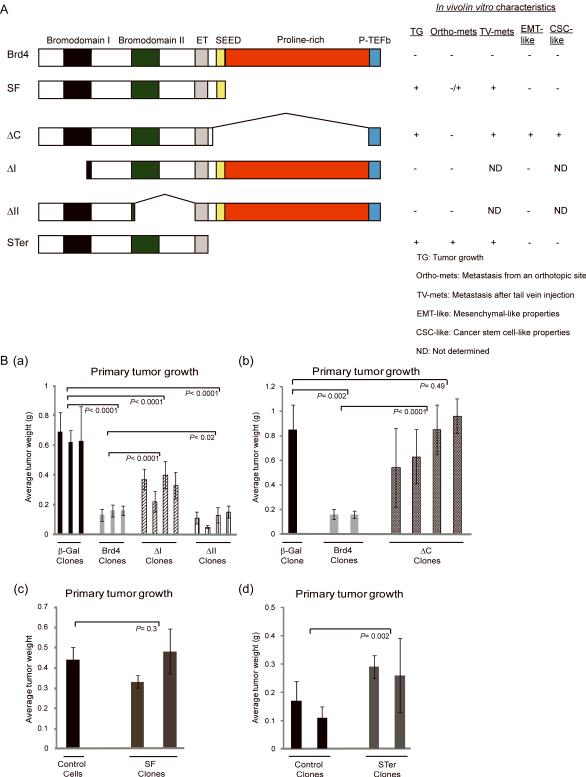
To investigate which part of Brd4 is essential for its tumor suppressive property, stable clones of the highly metastatic mouse mammary tumor cell line Mvt-1 (11) expressing full length Brd4 (5), the short isoform (SF) or mutants deleted for the bromodomains (ΔI, ΔII) (8), or for the SEED and proline-rich domains (ΔC) (10) were established (Fig. 1A). These stable cell lines were orthotopically injected into FVB/NJ mice and 25–26 days later primary tumor weight was quantified.
Figure 1.
Tumor suppressive property of Brd4 is mediated by the proline-rich domain. A, modular structure of BRD4 and BRD4 deletion mutants. In vivo/in vitro characteristics demonstrated throughout the study are summarized. EMT-like is represented here by cellular morphology. B, a, b, c, d, primary tumor weight of FVB/NJ female mice injected with 1×105 cells expressing ΔI, ΔII, Brd4 and β-Gal (a), ΔC, Brd4 and β-Gal (b), SF and controls (c), STer and controls (d) 25–26 days after orthotopic injection into the mammary fat pad. Means ± SD, n=5 for each clone were used. Mann-Whitney test was used to calculate the P-value.
Consistent with our previous findings Brd4-ectopic expression reduced primary tumor growth compared to controls [Fig. 1B(a, b)]. Tumorigenicity of ΔII-expressing cells, lacking the binding domain for SIPA1, was also significantly impaired [Fig. 1B(a)], suggesting that the interaction with SIPA1 was not necessary for suppression of the primary tumor by Brd4. Primary tumor growth was partially restored by deletion of bromodomain I (ΔI) [(Fig. 1B(a)]. In contrast, ΔC and SF clones generated tumors comparable in size to controls [(Fig. 1B(b, c)], suggesting that primary tumor growth suppression was mediated by the proline-rich domain between the SEED and P-TEFb binding domains. To further confirm this, a lentiviral construct containing the first 699 aa of ΔC but missing the last 83 aa P-TEFb binding domain (STer) was generated (Fig. 1A). Ectopic expression of STer resulted in a significant increase in tumor weight [(Fig. 1B(d)], confirming the contribution of the proline-rich domain in the tumor suppression property of Brd4.
Ectopic expression of the ΔC mutant results in an EMT-like phenotype
Unexpectedly, ΔC-ectopic expression profoundly changed cell morphology to a mesenchymal-like phenotype and induced a loss of contact inhibition compared to Brd4 or β-Gal cells that have an epithelial-like appearance (Fig. 2A and Supplementary Fig. S1A). In contrast, expression of SF (Fig. 2A and Supplementary Fig. S1A) or ΔI did not affect cell morphology (Fig. 2A). Expression of ΔII resulted in a more epithelial-like appearance (Fig. 2A) with reduced proliferation in vitro compared to the other cell lines, however this reduction in growth was not statistically significant [Supplementary Fig. S1B(a)]. However, ΔC-, SF- and ΔI-ectopic expression did not change cell proliferation in vitro compared to Brd4 or β-Gal cells [Supplementary Fig. S1B(a, b)].
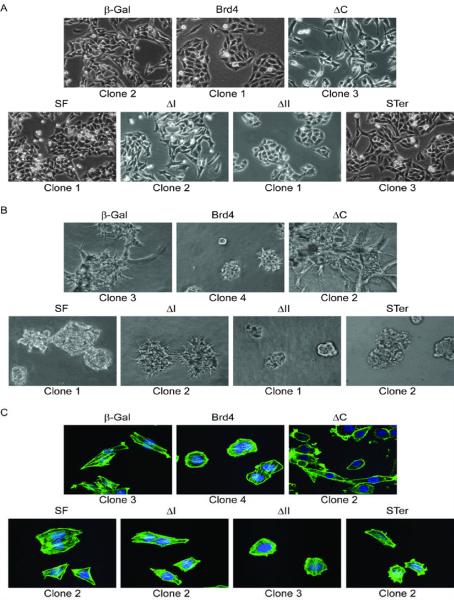
Figure 2.
In vitro characteristics of cells expressing BRD4 deletion mutants or SF. A, two-dimensional culture of one clone of the seven different cell lines. Pictures were taken at 40x magnification using light microscopy. B, F-actin fluorescence staining of one clone of the cells. Pictures were taken at 63x magnification using confocal microscopy. C, growth of one clone on top of a solidified basement membrane extract in a 3D culture. Pictures were taken at 40× magnification using light microscopy.
The ability of the cells to grow in 3D cultures was also determined by performing 3D in vitro invasion assay. Consistent with previous data (5), 3D basement membrane extract cultures showed that Brd4 clones had reduced invasion properties (Fig. 2B). The invasiveness of ΔII clone was comparable to that of Brd4, while ΔI and SF clones were also able to proliferate and invade the basement membrane extract compared to β-Gal (Fig. 2B). ΔC clones in contrast were highly invasive and had an increased ability to grow in a 3D culture compared to the other cell lines (Fig. 2B).
Both cell spreading and cell migration are highly dependent on the dynamics of the actin cytoskeleton. Staining the cells with phalloidin showed an organized actin cytoskeleton in only SF, ΔI, ΔII, Brd4 and β-Gal cells (Fig. 2C). A dramatic change in actin organization was observed upon ΔC-ectopic expression, which is characterized by the presence of cortical actin but loss of stress fibers (Fig. 2C), suggesting that while the ΔC-expressing cells had undergone some mesenchymal-like changes, complete EMT was not achieved.
Domain analysis of the deletion mutants suggested that while the proline-rich domain mediates primary tumor growth, the EMT-like phenotype was most likely due to either the loss of the SEED domain or the retention of the 83 aa P-TEFb binding C-terminus. To address this question, STer-expressing cells were further investigated. STer-ectopic expression (Supplementary Fig. S1A) did not change cellular morphology or proliferation and invasion in a 3D culture and did not affect the actin cytoskeleton organization (Fig. 2A, B and C), compared to controls. These data suggest that the EMT-like changes appear to be mediated by the P-TEFb-binding region and not by the SEED or the proline-rich domains.
ΔC suppresses metastasis in spontaneous but not experimental metastasis assays
We have previously shown that full length Brd4 profoundly reduces lung metastasis when subcutaneously implanted into mice (5). To investigate the domains essential for metastasis suppression, pulmonary metastatic lesions from the orthotopic tumors were counted. ΔI- and ΔII-expressing cells significantly suppressed metastatic burden [Fig. 3A(a)]. ΔII cells also showed reduced metastasis [Fig. 3A(a)], suggesting that the interaction with SIPA1 was not necessary for suppression. SF only partially inhibited metastatic capacity but was still significantly reduced compared to controls [Fig. 3A(b)] while STer cells were comparable to controls [Fig. 3A(c)]. Despite the ability of ΔC clones to form large primary tumors, these cells formed very few lung metastases compared to β-Gal [Fig. 3A(d)] or Brd4 cells [Fig. 3A(d)].
Figure 3.
ΔC suppresses metastasis from an orthotopic site but not when directly administered into the circulation. A, a, b, c and d, quantification of pulmonary metastasis nodules from mice injected orthotopically with clones expressing ΔI, ΔII, Brd4 and β-Gal (a), SF and controls (b), STer and controls (c) and ΔC, Brd4 and β-Gal (d) after sectioning and H&E staining of the lungs. B, a, b and c, quantification of pulmonary metastasis nodules from tail vein injection of mice with one clone of ΔC, Brd4 or β-Gal (a), two clones of SF-expressing cells and control Mvt-1 cells (b), two clones of STer or controls (c) after sectioning and H&E staining. Means ± SD, n=5 for each clone were used. Mann-Whitney test was used to calculate the P-value.
To determine whether the ΔC metastatic defect was due to inhibition of lung colonization or occurred at an earlier step in the metastatic cascade experimental metastasis assays were performed. Brd4 expression reduced the number of the metastatic lesions compared to β-Gal controls [Fig. 3B(a)] while SF expression enhanced pulmonary metastasis [Fig. 3B(b)]. STer cells also formed lung metastatic lesions after tail vein injection; however, there was no difference compared to control mice [Fig. 3B(c)]. Unexpectedly, mice injected with ΔC cells had a profoundly enhanced number of pulmonary lesions compared to mice injected with cells expressing SF, STer, Brd4 or β-Gal [Fig. 3B(a)], indicating that the spontaneous metastatic defect in ΔC cells was not due to an inability to grow in the secondary site. Furthermore, the in vivo invasive ability of ΔC cells was tested to determine whether the inability of ΔC to form spontaneous pulmonary metastases was due to an invasive defect. Histological analysis of the tumors and surrounding tissues showed that both ΔC and β-Gal clones invaded the surrounding tissues (Supplementary Fig. S2B, left panels) and grew within regional lymph nodes partially replacing lymph node parenchyma (Supplementary Fig. S2B, right panels), suggesting that the inhibition of ΔC metastasis to the lungs is not due to the inhibition of local invasion.
ΔC and SF induce distinct transcriptional signatures compared to Brd4
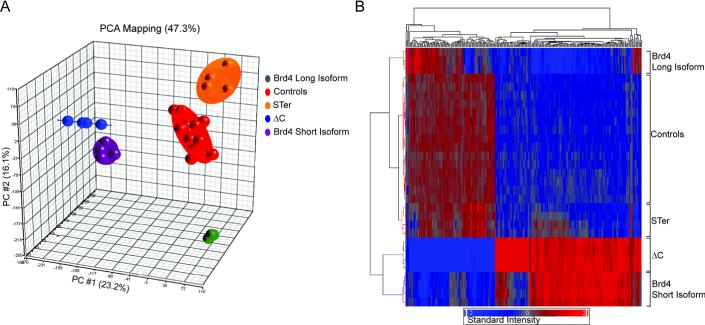
Previous studies have suggested that the short isoform opposes the function of the long isoform by competing for the target acetylated histones (12, 13). To assess the effect of SF expression on cellular transcription, Affymetrix-based gene expression analysis was performed. Principal Component Analysis (PCA) of the microarray data showed that ΔC and SF clones cluster closely to each other, while Brd4 clones were more distantly related (Fig. 4A). Hierarchical clustering analysis of the expression profiles revealed that the ΔC and SF expression signatures highly correlated and in general opposed the Brd4 expression profile (Fig. 4B). STer, which has the SEED domain absent, clustered with control cells (Fig. 4A and B). Taken together, these data are consistent with the possibility that SF negatively regulates Brd4 activity and that the SEED domain significantly contributes to SF suppressive activity. In addition, comparison of STer with ΔC suggests that the P-TEFb binding domain also contributed additional functions resulting in a SF-like transcriptional signature.
Figure 4.
Gene expression profiles of cells expressing ΔC, SF, STer, Brd4 and control cells. A, PCA of the gene expression profile. The first principal component is plotted on the X axis and captures 23.2% of the variance and the second principal component is plotted on the Y axis and captures 16.1% of the variance. B, hierarchical clustering of the expression profiles. The dendogram (top and left side) measures samples degree of relatedness in gene expression. Red and blue colors indicate intensity.
ΔC and SF induce signatures of poor survival in human breast cancer datasets
Ectopic expression of Brd4 induces a gene signature associated with good prognosis (5). The in vivo results for ΔC and SF would predict that signatures associated with these genes should be predictive of poor outcome. To test this, a human gene expression signature was generated by mapping the most significantly differentially regulated genes obtained by comparing ΔC or SF versus the control cells (P< 10−9) from the mouse array data to human Affymetrix annotations. The ΔC or SF signatures were then used to cluster patients in publicly available human breast cancer datasets into two groups using unsupervised hierarchal clustering and Kaplan-Meier survival analysis performed. ΔC-ectopic expression induced a gene signature that predicted poor outcome in four independent human breast cancer datasets (Fig. 5A). Ectopic expression of SF also induced a gene signature that predicted poor outcome in three of the four datasets, and trending toward significance (P=0.06) in the fourth dataset (GSE3494) (Fig. 5B). Furthermore, the ΔC and SF signatures were found to overlap a signature that defines poorly differentiated and highly malignant G3 grade breast cancers (14) (Table 1; Supplementary Table S1), consistent with the in vitro data, suggesting that ΔC is inducing a less differentiated phenotype in the Mvt-1 cells.
Figure 5.
ΔC and SF microarray expression signatures predict poor outcome in human breast cancer datasets. A and B, Kaplan-Meier curves of four independent human breast cancer microarray datasets performed in Affymetrix GeneChips for ΔC-expressing cells or controls (A) and SF-expressing cells or controls (B). The endpoint for the GSE2034 and GSE4922 datasets differ in that disease-free survival was measured. Log rank test was used to calculate the P-value.
Table 1.
Ectopic expression of ΔC in the highly metastatic mouse mammary tumor cell line Mvt-1 up-regulates the expression of genes up-regulated in G3-grade tumors
| Gene Symbol | Mouse Homolog Symbol | Mouse Gene Full Name | Probe Set | Fold Change* |
|---|---|---|---|---|
| ANLN | Anln | Anillin, actin binding protein | 1433543 at | 15.36 |
| BRRN1 | Ncaph | Non-SMC condensin I complex, subunit H | 1423920 at | 2.61 |
| C6ORF173 | 2610036L11Rik | RIKEN cDNA 2610036L11 gene | 1453053 at | 2.16 |
| CDCA3 | CdcA3 | Cell division cycle associated 3 | 1452040 a at | 10.85 |
| CDCA5 | CdcA5 | Cell division cycle associated 5 | 1416802 a at | 8.98 |
| CDCA8 | Cdca8 | Cell division cycle associated 8 | 1428480 at | 7.68 |
| CENPE | Cenpe | Centromere protein E | 1439040 at | 10.22 |
| FJL11029 | Prr11 | Proline rich 11 | 1444257 at | 5.35 |
| FOXM1 | Foxm1 | Forkhead box M1 | 1448834 at | 11.90 |
| MELK | Melk | Maternal embryonic leucine zipper kinase | 1416558 at | |
| MYBL2 | Mybl2 | Myeloblastosis oncogene-like 2 | 1417656 at | 8.98 |
| SPC24 | Spc24 | SPC24, NDC80 kinetochore complex component, homolog (S. cerevisiae) | 1431087 at | 8.16 |
| STK6 | Aurka | Aurora kinase A | 1424511 at | 13.91 |
| TPX2 | Tpx2 | TPX2, microtubule-associated protein homolog (Xenopus laevis) | 1428104 at | 12.03 |
| TTK | Ttk | Ttk protein kinase | 1449171 at | 12.78 |
Fold difference between Mvt-1/ΔC and Mvt-1 control cell lines
ΔC-ectopic expression modulates the expression of a subset of EMT markers
EMT has been shown to be associated with cancer progression and metastasis (15–18). Given the fact that ΔC-expressing cells have a mesenchymal-like phenotype the microarray gene expression data of ΔC and β-Gal cells were examined for expression of EMT markers. ΔC-ectopic expression was found to modulate the expression of some previously described EMT markers (Supplementary Table S2). To confirm this, the expression of six EMT markers was validated in ΔC and β-Gal cells using real-time PCR (Supplementary Table S3). Fbn1, Vim and Foxc2 were up-regulated, and Ocln was down-regulated, by ΔC-ectopic expression consistent with an EMT phenotype. However, expression of Snai1 and Twist1 was down-regulated by ΔC-ectopic expression, opposite to what is usually seen by a standard EMT, suggesting that the ΔC cells had not fully undergone EMT.
ΔC-expressing cells form tumorspheres in non-adherent conditions and regulate a subset of stem cell markers
Human breast tumors have been found to contain cells that have the ability to undergo anchorage-independent growth in vitro and form spheres (19), akin to behaviors characterizing normal mammary stem cells (20). The EMT process has been linked to the ability of self-renewal (21). To determine whether ΔC cells have the ability to form spheres, a representative clone of ΔC, SF, Brd4, β-Gal cells was seeded onto Ultra-low attachment plates using serum-free medium. ΔC cells formed tumorspheres when cultured in serum-free and non-adherent conditions (Fig. 6A), while SF, Brd4 and β-Gal cells could not form spheres under these conditions (Fig. 6A; Supplementary Fig. S3A and data not shown).
Figure 6.
ΔC-expressing cells exhibit a cancer stem cell-like phenotype. A, ΔC- or β-Gal-expressing clones were seeded at a density of 3×103 cells/well onto 24-well Ultra-low attachment plates in serum-free media for 3 and 6 days. Pictures were taken at 40x magnification using light microscopy. B, quantitation of Abcb1a and Abcb1b expression by qRT-PCR. Expression of Abcb1a and Abcb1b was compared in four clones expressing ΔC, Brd4 or β-Gal. Ppib was used for normalization. Mann-Whitney test was used to calculate the P-value. C, expression of Cd24a in clones expressing ΔC, Brd4 or β-Gal was measured by FACS analysis (upper panel) and qRTPCR (lower panel). Unpaired t-test was used to calculate the P-value.
In addition, microarray analysis showed that ΔC-ectopic expression modulated the expression of a subset of stem cell markers such as Abcb1a and Abcb1b drug-resistance transporters (Supplementary Table S4). Quantitative RT-PCR validated this finding demonstrating that ΔC cells up-regulated Abcb1a and Abcb1b 120- and 180-fold, respectively (Fig. 6B). In contrast, SF cells up-regulated these genes ~2-fold compared to controls (Supplementary Fig. S3B). Furthermore, the expression of Cd24a stem cell marker was highly expressed in the ΔC-expressing clone compared to Brd4 and β-Gal clones both on the protein and mRNA levels (Fig. 6C). These results are consistent with the acquisition of cancer stem cell-like properties by ectopic expression of ΔC.
Discussion
The metastatic cascade is a complex multifactor process that involves both tumor autonomous as well as non-autonomous events. Recent studies have provided an increasing understanding of many of the mechanisms associated with somatic and acquired phenotypes involved in metastatic progression. In contrast, little is known about the molecular mechanisms involved in inherited predisposition to metastasis. This study was therefore designed to gain a better understanding of one of the most significant metastasis susceptibility genes, Brd4, that was identified in our previously described genetic screens (5, 6).
To do so we have taken advantage of a naturally occurring alternative isoform of Brd4 as well as a series of pre-existing well characterized Brd4 deletion mutants (8, 10). Brd4 has two alternatively spliced isoforms that differ in the 3`-UTR and the coding region. The shorter isoform retains both chromatin binding bromodomains but lacks the C-terminal proline-rich and P-TEFb binding domains (9), suggesting that the short isoform might compete with the longer isoform for binding to acetylated histones (12) and thus interfering with the long isoform function. This is consistent with our in vivo experiments that showed that Brd4 short isoformectopic expression does not affect primary tumor growth but enhances metastatic colonization. Furthermore, transcriptional analysis of the short isoform demonstrated that it induced a gene signature indicative of poor survival in breast cancer datasets, consistent with the possibility that the shorter isoform inhibits the longer isoform and that metastatic susceptibility might in part be encoded by a ratio between the two isoforms. Further investigations, however, will be required to validate this possibility.
This possibility is further suggested by examination of the alternative 3'-UTRs. Computational predictions suggest that the 3`-UTR of BRD4 short isoform is a potential target of multiple microRNAs including some previously shown to be associated with aggressive breast cancer, such as the mir-200 and the let7 families (22–25). Loss of these miRNAs during tumor progression would result in an increase in transcription of the shorter isoform, consistent with its role in a more aggressive disease. In addition, in rare midline carcinomas BRD4 short isoform is frequently fused to the NUT (nuclear protein in testis) oncogene via an intronic translocation (26–29). Midline carcinomas are highly aggressive tumors with poor prognosis, consistent with the possibility that competitive inhibition of the longer Brd4 isoform would increase the ability of tumors to progress to metastatic disease.
These data also suggest that the C-terminal half of the full length isoform mediates the ability to suppress progression and metastasis. To gain further understanding of the mechanism, deletion analysis was performed. Deletion of bromodomain I had a least suppressive effect on tumor growth. Deletion of bromodomain II however resulted in further suppression of tumor growth and the conversion to a more epithelial morphology. The bromodomain II deletion, in addition to eliminating the chromatin binding domain, also deletes the binding domain for SIPA1 (8). Previous studies demonstrated that increased levels of SIPA1 were associated with greater malignancy (30) and relocalization of BRD4 from the nucleus to the cytoplasm (8). Our results are consistent with the possibility that interaction between BRD4 and SIPA1 contributes to tumor progression, though the mechanism by how this occurs is currently not fully understood.
In contrast, the opposite phenotype was observed during the analysis of the BRD4 C-terminal half. The C-terminus of the long isoform contains only a single defined domain, the P-TEFb binding domain at the extreme C-terminus (9), and regions of high serine and proline content of unknown function. Unexpectedly, introduction of a deletion mutant (ΔC) (10) that deletes the serine-rich SEED and the proline-rich regions but retains the carboxy-terminal 83 aa, including the P-TEFb binding domain, induced significant morphological and physiological changes reminiscent of EMT-like and cancer stem cell-like properties. In contrast, the STer mutant that deletes the entire C-terminus before the SEED domain was not able to induce these phenotypes, suggesting that the 83 aa P-TEFb binding region is the domain responsible for the EMT-like and the stem cell-like conversion.
The EMT process has been implicated as an important intermediate in tumor progression (31, 32) and thought to be required during the later steps of the metastatic cascade (33, 34). Recently it has also been linked to the ability of self-renewal (21). Current thinking suggests that disseminated cancer cells may need to acquire self-renewal properties similar to those exhibited by the stem cells, in order to achieve formation of macroscopic metastases (21). The ΔC data implies that one of the mechanisms by which BRD4 suppresses tumor growth and metastasis is by preventing de-differentiation to a stem cell-like state, mediated by the proline-rich domains. Consistent with this, microarray expression analysis of ΔC, like Brd4 short isoform, converted the Brd4 gene signature from being a predictor of good outcome to being a predictor of poor outcome. It is important to mention here that the cell phenotypes induced by ΔC are not the conventional EMT or stem cell transitions since ΔC cells do not fully exhibit the EMT and the cancer stem cell properties. The expression of genes, thought to be critical for EMT, was not up-regulated in ΔC-expressing cells as normally seen (eg. Twist, Snail). Furthermore, the stem cell markers Nanog, Oct4 and Sox2 were not detected in ΔC cells, as measured by microarray analysis and quantitative PCR (data not shown). These data suggest that although ΔC-expressing cells have undergone the morphological transformation and some of the molecular changes associated with EMT and stemness either these transformations are incomplete or the ΔC transformation is a phenocopy of EMT and stemness induced by an alternative mechanism. Further investigations will be required to resolve this. As a result, we have opted to describe the ΔC transformation as EMT-like and stem cell-like until these discrepancies can be resolved.
Experimental metastasis assays further support the role of both Brd4 short isoform and ΔC as metastatic modifiers. ΔC significantly increased the metastatic capacity when introduced into the circulation of mice via tail vein injection, consistent with the more malignant in vitro phenotypes and gene expression signature. Unexpectedly however, although the short isoform was able to successfully colonize the lungs from an orthotopic site, ΔC cells were not capable of forming pulmonary metastatic lesions, despite being capable of forming aggressively growing locally invasive tumors after orthotopic implantation. Similar results have been observed with other EMT-like spindle cell shaped tumors (35). A possible explanation may rest in the permanent mesenchymal phenotype of ΔC-expressing cells. Both EMT and its converse, mesenchymal-to-epithelial transition (MET), are thought to be necessary for tumors to successfully disseminate from the primary tumor and colonize the secondary site (36, 37). ΔC cells, due to the stable expression of the Brd4 deletion mutant, appear to have permanently acquired the mesenchymal phenotype and no longer possess the plasticity to revert through MET to a more epithelial state that appears to be necessary for colonization from an orthotopic site (35).
Finally, these results begin to address the potential mechanisms by which inherited polymorphisms mediate metastatic susceptibility. Gene expression analyses of human breast cancer cohorts (4, 5, 38) have suggested that BRD4 may play an important central role in the genetically defined network of genes that define the Diasporin metastasis susceptibility pathway (6). Our current results suggest several things. First, BRD4 appears to play a significant role in establishing transcriptional programs that predict breast cancer outcome via a balance between the tumor- and metastasis-suppressive long isoform and the pro-tumorigenic short isoform. Second, the long isoform contains both negative (proline-rich) and positive (extreme carboxy-terminal) metastatic progression domains. The mechanisms by which these domains act are currently under investigation. More interestingly, the data also suggest that BRD4 is an important determinant in the reversible epigenetic plasticity of an EMT- and MET-like transition that is thought to be an obligate intermediate in the metastatic cascade. This implies that BRD4 and the other metastatic susceptibility genes may be altering the risk of developing distant metastases by predisposing the tumors of high risk patients to undergo conversion to a more de-differentiated or primitive state. If true, these results suggest that BRD4 may be an important component in the maintenance of the differentiated mammary cell transcriptional program. Further investigations into the targets of BRD4 regulation may therefore reveal important factors associated with mammary epithelial development as well as malignant progression.
Supplementary Material
Acknowledgments
We thank Drs. Lalage Wakefield and Glenn Merlino for critical reading of the manuscript. We also thank Dr. Binwu Tang for her assistance in performing the tumorsphere formation assay. We thank Dr. Dominique Esposito and his team at the Protein Expression Laboratory, SAICFrederick, for providing the necessary cloning services. This work was supported in part by the Intramural Research Program of the National Institutes of Health National Cancer Institute Center for Cancer Research.
Reference List
- (1).Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. Oncologist. 2003;8:514–20. doi: 10.1634/theoncologist.8-6-514. [DOI] [PubMed] [Google Scholar]
- (2).Lifsted T, Le VT, Williams M, Muller W, Klein-Szanto A, Buetow KH, et al. Identification of inbred mouse strains harboring genetic modifiers of mammary tumor age of onset and metastatic progression. Int J Cancer. 1998;77:640–4. doi: 10.1002/(sici)1097-0215(19980812)77:4<640::aid-ijc26>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- (3).Crawford NP, Ziogas A, Peel DJ, Hess J, nton-Culver H, Hunter KW. Germline polymorphisms in SIPA1 are associated with metastasis and other indicators of poor prognosis in breast cancer. Breast Cancer Res. 2006;8:R16. doi: 10.1186/bcr1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Crawford NP, Qian X, Ziogas A, Papageorge AG, Boersma BJ, Walker RC, et al. Rrp1b, a new candidate susceptibility gene for breast cancer progression and metastasis. PLoS Genet. 2007;3:e214. doi: 10.1371/journal.pgen.0030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Crawford NP, Alsarraj J, Lukes L, Walker RC, Officewala JS, Yang HH, et al. Bromodomain 4 activation predicts breast cancer survival. Proc Natl Acad Sci U S A. 2008;105:6380–5. doi: 10.1073/pnas.0710331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Crawford NP, Walker RC, Lukes L, Officewala JS, Williams RW, Hunter KW. The Diasporin Pathway: a tumor progression-related transcriptional network that predicts breast cancer survival. Clin Exp Metastasis. 2008;25:357–69. doi: 10.1007/s10585-008-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc Natl Acad Sci U S A. 2003;100:8758–63. doi: 10.1073/pnas.1433065100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Farina A, Hattori M, Qin J, Nakatani Y, Minato N, Ozato K. Bromodomain protein Brd4 binds to GTPase-activating SPA-1, modulating its activity and subcellular localization. Mol Cell Biol. 2004;24:9059–69. doi: 10.1128/MCB.24.20.9059-9069.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bisgrove DA, Mahmoudi T, Henklein P, Verdin E. Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proc Natl Acad Sci U S A. 2007;104:13690–5. doi: 10.1073/pnas.0705053104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell. 2005;19:523–34. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- (11).Pei XF, Noble MS, Davoli MA, Rosfjord E, Tilli MT, Furth PA, et al. Explant-cell culture of primary mammary tumors from MMTV-c-Myc transgenic mice. In Vitro Cell Dev Biol Anim. 2004;40:14–21. doi: 10.1290/1543-706X(2004)40<14:ECOPMT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- (12).French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–7. [PubMed] [Google Scholar]
- (13).Haruki N, Kawaguchi KS, Eichenberger S, Massion PP, Gonzalez A, Gazdar AF, et al. Cloned fusion product from a rare t(15;19)(q13.2;p13.1) inhibit S phase in vitro. J Med Genet. 2005;42:558–64. doi: 10.1136/jmg.2004.029686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66:10292–301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- (15).Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- (16).Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable betacatenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008;9:808. doi: 10.1016/S1470-2045(08)70201-8. [DOI] [PubMed] [Google Scholar]
- (18).Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- (19).Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- (20).Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelialmesenchymal transition. Cell Cycle. 2008;7:3112–8. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- (23).Worley LA, Long MD, Onken MD, Harbour JW. Micro-RNAs associated with metastasis in uveal melanoma identified by multiplexed microarray profiling. Melanoma Res. 2008;18:184–90. doi: 10.1097/CMR.0b013e3282feeac6. [DOI] [PubMed] [Google Scholar]
- (24).Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- (25).Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–60. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).French CA, Miyoshi I, Aster JC, Kubonishi I, Kroll TG, Dal CP, et al. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19) Am J Pathol. 2001;159:1987–92. doi: 10.1016/S0002-9440(10)63049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Haruki N, Kawaguchi KS, Eichenberger S, Massion PP, Gonzalez A, Gazdar AF, et al. Cloned fusion product from a rare t(15;19)(q13.2;p13.1) inhibit S phase in vitro. J Med Genet. 2005;42:558–64. doi: 10.1136/jmg.2004.029686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).French CA, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–42. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- (29).den Bakker MA, Beverloo BH, van den Heuvel-Eibrink MM, Meeuwis CA, Tan LM, Johnson LA, et al. NUT midline carcinoma of the parotid gland with mesenchymal differentiation. Am J Surg Pathol. 2009;33:1253–8. doi: 10.1097/PAS.0b013e3181abe120. [DOI] [PubMed] [Google Scholar]
- (30).Park YG, Zhao X, Lesueur F, Lowy DR, Lancaster M, Pharoah P, et al. Sipa1 is a candidate for underlying the metastasis efficiency modifier locus Mtes1. Nat Genet. 2005;37:1055–62. doi: 10.1038/ng1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Thiery JP, Tucker GC, Boyer B, Valles AM, Gavrilovic J, Moens G, et al. [Mechanisms of induced epithelium-fibroblast conversion in a line of vesical cancer in the rat] Pathol Biol (Paris) 1989;37:1034. [PubMed] [Google Scholar]
- (32).Boyer B, Tucker GC, Valles AM, Gavrilovic J, Thiery JP. Reversible transition towards a fibroblastic phenotype in a rat carcinoma cell line. Int J Cancer Suppl. 1989;4:69–75. doi: 10.1002/ijc.2910440719. [DOI] [PubMed] [Google Scholar]
- (33).Jechlinger M, Grunert S, Tamir IH, Janda E, Ludemann S, Waerner T, et al. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22:7155–69. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- (34).Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- (35).Cardiff RD. The pathology of EMT in mouse mammary tumorigenesis. J Mammary Gland Biol Neoplasia. 2010;15:225–33. doi: 10.1007/s10911-010-9184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- (37).Monteiro J, Fodde R. Cancer stemness and metastasis: therapeutic consequences and perspectives. Eur J Cancer. 2010;46:1198–203. doi: 10.1016/j.ejca.2010.02.030. [DOI] [PubMed] [Google Scholar]
- (38).Crawford NP, Yang H, Mattaini KR, Hunter KW. The metastasis efficiency modifier ribosomal RNA processing 1 homolog B (RRP1B) is a chromatin-associated factor. J Biol Chem. 2009;284:28660–73. doi: 10.1074/jbc.M109.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.