Abstract
1,25-dihydroxyvitamin D3 affects proliferation, differentiation and apoptosis and protects DNA against oxidative damage with a net tumorostatic and anticancerogenic effects. It acts through a specific nuclear receptor that is widely distributed through the body. Although a beneficial role of vitamin D in melanoma patients has been suggested, there is a lack of information on the changes in the expression pattern of vitamin D receptor during progression of pigmented lesions. Using immunohistochemistry, we analyzed expression of vitamin D receptor in 140 samples obtained form 82 patients, including 25 benign nevi, 70 primary cutaneous melanomas, 35 metastases, 5 re-excisions, and 5 normal skin biopsies. The strongest expression was observed in normal skin that significantly decreased in melanocytic proliferations with the following order of expression: normal skin > melanocytic nevi > melanomas = metastases. The vitamin D receptor expression in skin surrounding nevi and melanoma was also significantly reduced as compared to normal skin. Tumor-infiltrating and lymph node lymphocytes retained high levels of vitamin D receptor. There was negative correlation between tumor progression and vitamin D receptor expression with a remarkable decrease of the immunoreactivity in nuclei of melanoma cells at vertical versus radial growth phases, and with metastatic melanomas showing the lowest cytoplasmic receptor staining. Furthermore, lack of the receptor expression in primary melanomas and metastases was related to shorter overall patients’ survival. In addition, the receptor expression decreased in melanized melanoma cells in comparison to amelanotic or poorly pigmented cells. Therefore, we propose that reduction or absence of vitamin D receptor is linked to progression of melanocytic lesions, that its lack affects survival of melanoma patients, and that melanogenesis can attenuate the receptor expression. In conclusion, changes in vitamin D receptor expression pattern can serve as important variables for diagnosis, predicting clinical outcome of the disease and/or as a guidance for novel therapy of melanomas based on use of vitamin D or its derivatives.
Keywords: Melanogenesis, melanoma, tumor progression, Vitamin D, vitamin D receptor
Introduction
Secosteroid vitamin D3 is a product of ultraviolet B (UVB) induced photochemical transformation of 7-dehydrocholesterol [1], which further undergoes sequential hydroxylation at positions 25 (liver) and 1 (kidney) to form 1,25-dihydroxyvitamin D3 (1,25(OH)2D3; calcitriol), a product that is involved in broad range of biological processes [2]. 1,25(OH)2D3 is also produced locally in the skin, where it regulates functions of the epidermis, hair follicles and skin immune system [3–7]. The best known function of vitamin D3 is the regulation of calcium-phosphate homeostasis targeting the intestine, kidney and bone cells [2, 7]. However, recent investigation revealed that many other cells and organs are susceptible to pleiotropic bioregulatory activities of 1,25(OH)2D3 that are different from the calcemic action [2, 7–11]. Thus, it acts as immunomodulator, which enhances native immune activity while inhibiting local and systemic proinflammatory reactions. 1,25(OH)2D3 can also serve as an antioxidant, can affect pathways and processes essential for the maintenance of cell integrity, and it inhibits the growth and induces the differentiation of cultured normal and malignant cells by arresting cell cycling at G0/G1 and/or G2/M phases [2, 7, 8, 10, 11]. It also can inhibit in vivo growth of variety of tumors as documented by studies in animal models as well as by epidemiological analyses [12, 13]. Similar antiproliferative and differentiation inducing properties are displayed by novel vitamin D3 hydroxyderivatives generated through the action of P450scc enzyme [14–19].
The ability of vitamin D3 to regulate the above processes is dependent on the presence of the vitamin D receptor (VDR), which belongs to a large superfamily of nuclear receptors with highly conserved nuclear and ligand-binding domains [9, 11, 20]. In the cytoplasm, after binding 1,25(OH)2D3 VDR heterodimerizes with retinoic acid X receptor (RXR) and is translocated to the nucleus. This complex recruits coactivating molecules and binds to vitamin D response elements (VDRE) located in promoter of many vitamin D-responsive genes. VDR is widely distributed through the body being present in almost all tissues and cells including skin [2, 7, 9, 20]. One of the first publication reporting VDR presence outside organs involved in calcium-phosphate homeostasis (intestine, kidney and bone tissues) and showing anti-cancer properties, was the work of Colston et al [21] regarding its expression in skin and melanoma cell lines. Subsequent experiments on human melanoma cell lines confirmed that VDR is present in melanoma cells, although level of its expression was heterogeneous among different cell lines [22].
Melanoma arises through malignant transformation of melanocytes from either pre-existing melanocytic nevi or de novo from the single melanocytes that are located at the junction of the epidermis [23]. After invasion of the papillary dermis, melanoma progresses from the radial growth phase (non-tumorogenic melanoma) to the vertical growth phase [24]. It must also be noted that melanoma is a tumor with most rapidly increasing worldwide incidence and a very high mortality rate due to very low sensitivity to existing therapies once it starts to metastasize [23]. Therefore, there is a continuous effort to develop targeted and effective approaches in advanced melanomas treatment. Although there is a clear-cut correlation between exposure to solar radiation and melanoma incidence, Berwick et al. study [25] and others [26] have suggested that chronic UV-exposure provides protection against melanoma, with the most likely causal factor being through local vitamin D production. Further evidence for a putative preventive role of vitamin D in melanoma development is provided by VDR gene polymorphism epidemiological studies [27] and that levels of 25-hydroxyvitamin D3 may affect prognosis in melanoma patients [28–30]. This in conjunction with well known anti-cancerogenic effects of vitamin D3 and its reported in vitro anti-melanoma activity, led us to perform the first (in our knowledge) comprehensive evaluation of VDR in cohort of patients with benign and malignant melanocytic skin lesions. We analyzed VDR expression in correlation with clinical data, histological type, stage, morphology, proliferation and apoptotic activity (using bcl-2 staining) in pigmented nevi and melanomas in order to determine the VDR expression pattern, correlation of VDR immunostaining with proliferation, apoptosis, melanin synthesis, to verify its potential role in melanoma progression, prognosis and eventually vitamin D-based therapy in selected patients.
Materials and methods
Tissue samples
Pathological tissue samples were obtained from 82 patients (41 females, 41 males; the mean age was 37.5 ± 15.9 years, ranging from 19 to 85 years of age in patients with benign lesion and 58.8 ± 14.6 years, ranging from 22 to 90 in patients with malignant tumors) who were treated in the Lukaszczyk Oncology Center, Bydgoszcz, Poland during the period of 2003–2009. All of the participants were Caucasians. Normal skin was obtained from 5 patients undergoing other surgical operations. Tissue samples were selected from the archive randomly and included 25 benign nevi, 69 primary cutaneous melanomas, 35 metastases, 5 local recurrences and 5 normal skin samples. The sections were evaluated by two pathologists, according to World Health Organization Classification of melanocytic tumors. The representative pictures of hematoxilin and eosin-stained sections of analyzed lesions are shown in Supplemental Fig 1. The group of benign lesions contained 4 junctional, 10 intradermal, 7 compound, 4 dysplastic nevi. The primary tumors included 30 superficial spreading, 37 nodular and 2 acral lentiginous melanomas. Melanomas were classified according to Clark’s stage and Breslow’s depth and included: 4 melanomas at Clark’s stage I, 6 at Clark’s stage II, 23 at Clark’s stage III, 24 at Clark’s stage IV, 12 at Clark’s stage V. Breslow’s thickness of studied melanomas was 0–1 mm for 16 cases; 1.1mm-2mm for 10 cases, 2.1mm-3mm for 10 cases, 3.1mm-4mm for 5 cases, and more than 4.1mm for 28 cases. Characteristics of patients and clinicopathologic features are summarized in Table 1. All local recurrences and 33 out of 35 metastases were matched with primary lesions from the same patients. This study was approved by the Committee of Ethics of Scientific Research of Collegium Medicum of Nicolaus Copernicus University, Poland.
Table 1.
Characteristics of the patients.
| Clinicopathologic features | No of patients (%) |
|---|---|
| Type of lesions | |
| All cases | 139 (100%) |
| Nevi | 25 (18.0%) |
| Primary melanomas | 69 (49.6%) |
| Metastatic melanomas | 35 (25.2%) |
| Recurrent melanomas | 5 (3.6%) |
| Normal skin | 5(3.6%) |
| Age of patients with nevi | |
| <20 years | |
| 21–40 years | 1 (4.0%) |
| 41–60 years | 15 (60.0%) |
| 61–80 years | 7 (28.0%) |
| >80 years | 1 (4.0%) |
| Age of patients with melanomas | 1 (4.0%) |
| <20 years | 0 (0.0%) |
| 21–40 years | 6 (8.7%) |
| 41–60 years | 32 (46.4%) |
| 61–80 years | 25 (36,2%) |
| >80 years | 6 (8.7%) |
| Male/female ratio | |
| All cases | 41/41 (50%/50%) |
| Nevi | 7/18 (28%/72%) |
| Melanomas | 29/40 (42.0%/58.0%) |
| Anatomical Site* | |
| Nevi | |
| All cases | 25 (100.0%) |
| Acral | 0 (0.0%) |
| Anogenital | 0 (0.0%) |
| Extremity | 5 (20.0%) |
| Head and neck | 5 (20.0%) |
| Trunk | 14 (26.0) |
| Melanomas | |
| All cases | 69 (100.0%) |
| Acral | 7 (10.1%) |
| Anogenital | 2 (2.9%) |
| Extremity | 18 (26.1) |
| Head and neck | 11 (15.9) |
| Trunk | 29 (42.0) |
| Metastases | |
| All cases | 35 (100%) |
| Lymph nodes | 31 (88.6%) |
| Liver | 1 (2.9%) |
| Skin | 1 (2.9%) |
| Parotid gland | 2 (5.7%) |
Site of lesion was not available for 1 patient with intradermal nevus and 2 patients with nodular melanomas
Immunohistochemistry
Formalin-fixed paraffin embedded samples of pathological and normal skin were used in our study. The protein expression of VDR and Bcl2 were studied by immunostaining using monoclonal antibodies (clone 97A; Abcam Inc., Cambridge, MA, USA, clone 124; Dako, Glostrup, Denmark; respectively) according the standards protocols used in our laboratories. After antigen retrieval achieved by microwave heating in Tris/EDTA (pH 9.0), sections were blocked with blocking serum (Vector Laboratories Inc., Burlingame, CA, USA), and incubated with rat monoclonal antibody (clone 9A7; Abcam Inc., Cambridge, MA, USA) at dilution 1:75 in TBS with 0.5 % BSA overnight at 4°C. In the negative controls, the primary antibody was omitted. Next, secondary anti-rat antibody was added for 30 min (Vector Laboratories Inc., Burlingame, CA, USA) followed by incubation with Vectastain® ABC-AP Reagent for 30 min and the immunoreactions detection using the alkaline phosphatase substrate (Vector Laboratories Inc., Burlingame, CA, USA). Endogenous alkaline phosphatase activity was blocked with Levamisol solution according to the manufacture’s protocol (Vector Laboratories Inc., Burlingame, CA, USA). Sections were mounted under the Faramount medium (Dako, Glostrup, Denmark).
The protein expression of Bcl2 was studied on the consecutive sections, according the manufacturer’s procedure. Briefly, antigen retrieval was achieved by microwave heating in Tris/EDTA (pH 9.0). After quenching of the endogenous peroxidase activity with 3% of H2O2, sections were incubated with primary monoclonal mouse antibodies at dilution 1:100 with TBS with 0.1 % BSA for 30 minutes at room temperature (RT). Next, anti-mouse HRP-labeled secondary, anti-mouse antibody (EnVision+System HRP Labelled Polymer Anti-Mouse, Dako, Glostrup, Denmark) was added for 30 minutes at RT, followed by rinsing with TBS. Finally, DAB (Dako, Glostrup, Denmark) was applied for 5 minutes, and then sections were counterstained with hematoxylin, dehydrated and mounted (Consul Mount, Thermo Shandon). The positive controls for Bcl-2 staining were sections of lymph node.
Immunolabeled sections were viewed under Nikon Eclipse 80i light microscope (equipped with Nikon Digital Sight DS Fi1-U2 digital camera and NIS-Elements BR 3.0 software (Nikon Instruments Europe B.V., Badhoevedorp, The Netherlands).
Morphological evaluation of immunostained sections
VDR and Bcl-2 immunostaining intensity of normal epidermis, nevi, melanoma and metastases was scored semiquantitatively by two independent observers (WJ and AB) from 0 to 3 arbitrary units (A.U.) with 0 as negative (0), weak (1), moderate (2) and strong (3). For Bcl-2, the tumor areas showing immunoreactivity and maximal staining intensity were also recorded. VDR staining intensity in pigmented lesions was evaluated with reference to intense reddish-pink basal layer of normal skin epidermis, scored as strong. Light reddish-pink and light pink stained cells were scored as cells with moderate or weak VDR expression, correspondingly. The VDR expression was assessed for cytoplasm and nuclei of cells separately. Moreover it was evaluated in the epidermis surrounding pigmented lesions. In melanomas the immunostaining of cells of radial and vertical growth phases was estimated separately.
Melanin content was graded as 0 if melanin was absent, 1 if faint or plain melanin was visible in up to 50% of cells and 2 if clear melanin was present in more than 50 % of cells. Mitotic activity and solar elastosis (SE) were assessed on sections stained with hematoxylin and eosin. Mitotic activity was assayed both as mitosis per mm2 and highest mitosis number per high powerfield view. The SE was evaluated as 0 absent, 1 if SE reached skin adnexa and 2 if SE was extensive and visible through entire dermal thickness.
Cell culture experiments
Human SKMel-188 melanoma cells were cultured in Ham’s F10 medium (low in precursors to melanin) supplemented with 5% fetal bovine serum (Sigma, St. Louis, MO, USA) and 1% penicillin/ streptomycin/ amphotericin antibiotic solution (Sigma, St. Louis, MO, USA). The induction of melanogenesis was achieved either by switch to the DMEM medium (rich in precursors to melanin) [31]. After 3 days, the cells were harvested protein extracted and submitted for western blot analyses as described previously [15]. Briefly, non pigmented and pigmented cells were lysed in RIPA cell lysis buffer (Cell Signaling, Danvers, MA, USA) and whole cell proteins isolated. The amount of proteins is determined using Bradford protein assay kit (BioRad, Hercules, CA, USA). Levels of VDR and β-actin were assessed by western blotting as described previously. The primary antibodies used were the rabbit polyclonal antibodiy of anti-VDR (H-81, Santa Cruz Inc, Santa Cruz, CA, USA) at 1:1 000 dilution and anti-β actin-peroxidase (Sigma) at 1:7 000 dilution. Secondary antibody used was anti rabbit -HRP (Santa Cruz Inc, Santa Cruz, CA, USA) 1:7 000 dilution. Resulting bands on the immunoblot were developed with the ECL developer system (Pierce, Rockford, IL, USA) and visualized on a Kodak Imager
Statistical analyses
Statistical analysis of the results was performed with the Prism 4.00 (GraphPad Software, San Diego, CA). The statistical analysis was done using t-test for comparison of two groups or with one-way analysis of variance (ANOVA) for three of more groups. To evaluate the association between VDR staining and categorical variables the Pearson’s correlation was used. Survival analysis was performed using Log-rank test. P < 0.05 was considered statistically significant.
Results
VDR expression in human skin melanocytic lesions
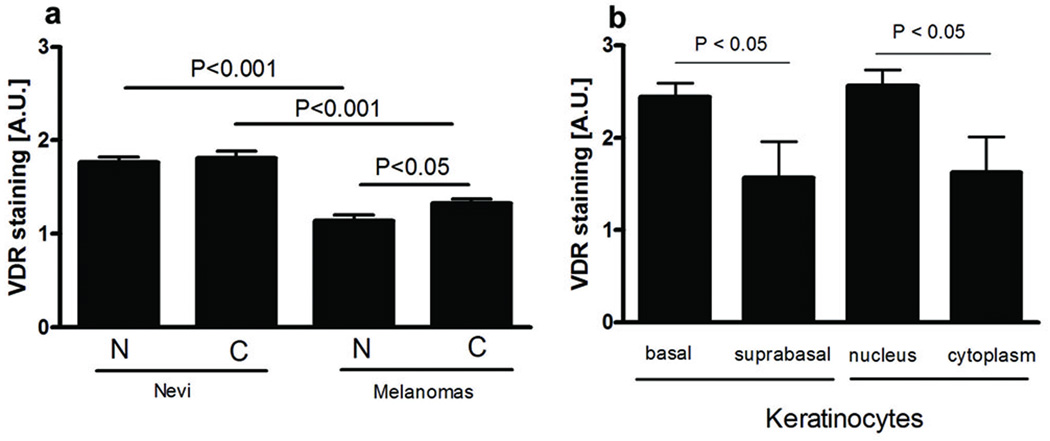
Immunohistochemistry performed on 140 formalin fixed-paraffin-embedded sections of melanocytic nevi, primary melanomas, metastases and normal skin with anti-VDR antibody showed marked differences among different lesions. In general, the stain was stronger in normal (keratinocytes and lymphocytes) than in tumor (nevocytes, melanoma) cells. In normal and tumor cells, the VDR immunoreactivity was present both in the cytoplasm and nucleus (Fig. 1 a-k, Fig. 2 a). The predominance of nuclear and cytoplasmic expression of VDR depended on histological type of skin pigmented lesions, with the significant differences observed in melanomas only. In the epidermis of normal skin, stronger intensity of VDR staining was observed in nuclei of keratinocytes. In the suprabasal layer, VDR expression was slightly but significantly lower than in the basal keratinocytes (Fig. 2 b).
Figure 1.
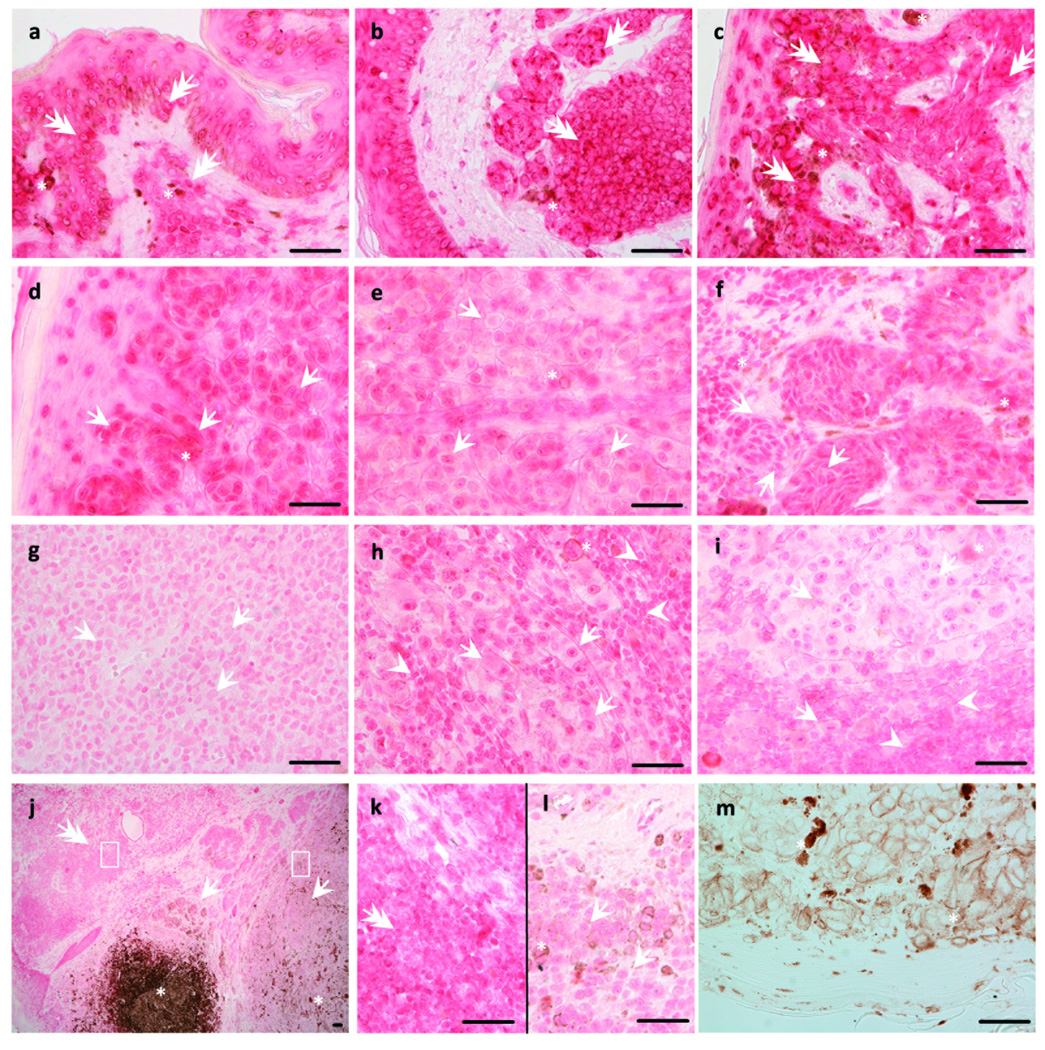
Localization of VDR in primary skin pigmented lesions and metastases of melanoma. Compound (a), dermal (b), desmoplastic (c) nevi revealed stronger VDR staining (pink-red) than radial (d) and vertical (e) growing cells of SSM and radial (f) and vertical (g) growing cells of NMM. Lymphocytes infiltrating tumor (h) and lymph nodes lymphocytes (i) also revealed stronger VDR expression than melanoma cells. Melanoma arising (j) in nevus (k) showed weaker VDR expression. Nevus section used as negative control (j). Arrows indicate melanoma cells, double arrows indicate nevi cells, asterisks indicate melanin (brown), arrow heads indicate lymphocytes. Square indicates tumor-infiltrating lymphocytes enlarged in insert. Scale bar: 50 µm.
Figure 2.
Supracellular and intraepidermal localization of VDR immunoreactivity.
a. VDR immunoreactivity in skin pigmented lesions and melanomas metastases was seen in cell cytoplasm and nuclei. N-nuclear VDR staining, C-cytoplasmic VDR staining.
b. VDR immunoreactivity in normal skin keratinocytes.
A.U. – arbitrary units.
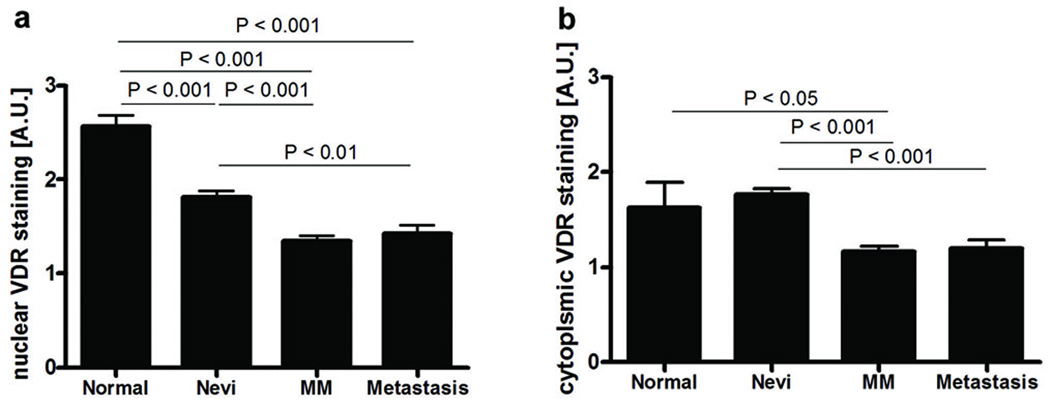
The highest expression of VDR was in nuclei of normal epidermal keratinocytes that significantly decreased in melanocytic proliferations with following order of expression: normal skin > melanocytic nevi > melanomas = metastases (Fig. 3 a). Cytoplasmic VDR expression in normal skin keratinocytes was similar to melanocytes of nevi, and it significantly decreased in primary melanomas and metastases (Fig. 3 b). Metastatic and primary tumor melanoma cells showed similar VDR immunoreactivity both in nuclei and cytoplasm (Fig. 3 a-b). VDR reduction with the development of pigmented lesions was more pronounced in cell nuclei than in cytoplasm. Five of analyzed primary melanomas (4 superficial spreading melanomas, 1 nodular melanoma) arose within the nevi. In two of them with highest Breslow’s thickness (>4mm), nevus cells showed stronger VDR expression than melanoma cells.
Figure 3.
VDR expression in human skin pigmented lesions. During development of skin pigmented lesions VDR immunostaining decreased both in nuclei (a) and cytoplasm (b) of the cells. Normal-normal skin, MM-primary melanomas. A.U. – arbitrary units.
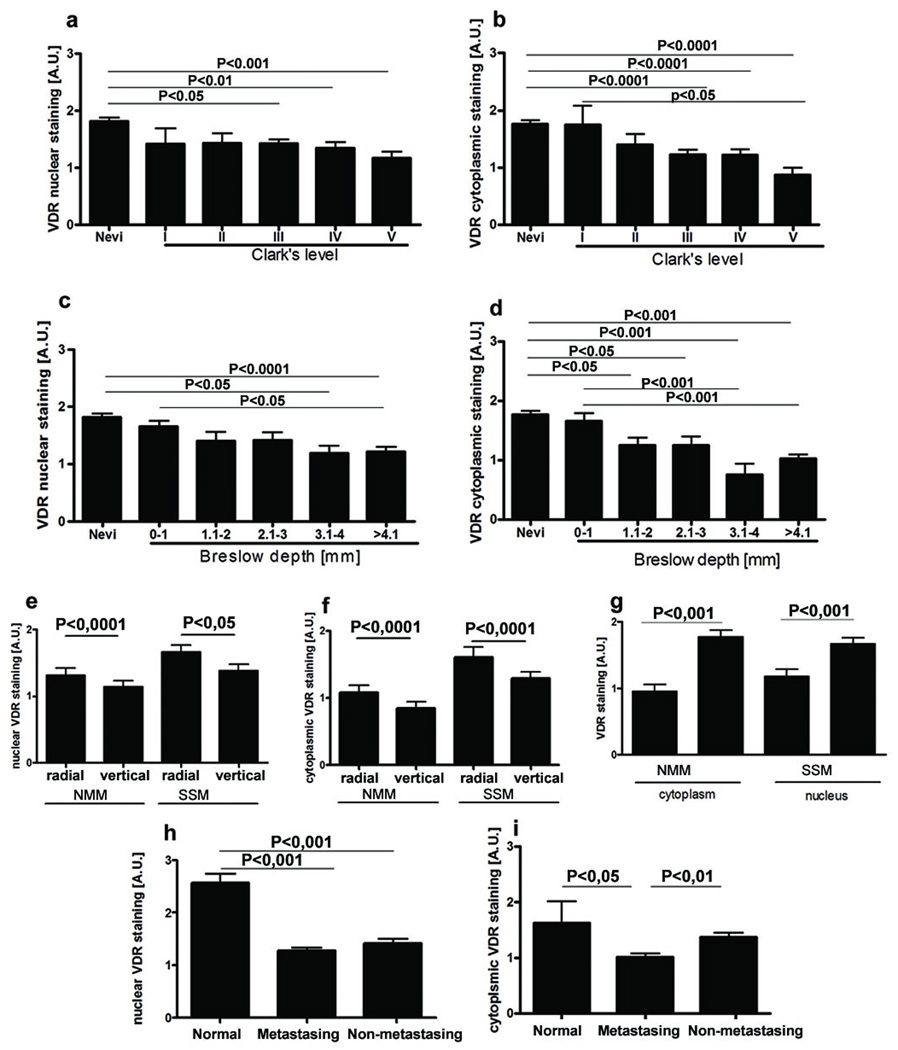
When melanomas were stratified according to the Clark’s level and Breslow’s depth, there was gradual decrease of VDR expression in comparison to melanocytic nevi with significant difference observed already for Clark’s level III or Breslow’s depth of 1.1–2 mm (cytoplasm) or 3.1–4 mm (nuclei) (Fig. 4a-d). These differences were observed both in cytoplasm and nuclei, however, stronger reduction of VDR immunoreactivity was in the cytoplasm. For melanoma progression, assessed using Clark’s level, the observed negative correlation was statistically significant for cytoplasmic VDR expression: r=−0.253, p=0.0417 in radial growth phase; r=−0.3087, p= 0.0124 for cytoplasmic in vertical growth phase. Similar negative correlation for cytoplasmic VDR was observed in melanomas assessed according Breslow’s thickness: r=−0.3392, p= 0.0075 in radial growth phase; r=−0.4336; p=0.0005 in vertical growth phase. In addition, for melanomas classified according Breslow’s scale negative correlation between progression of tumor and VDR nuclear staining was also observed: r= −0.3045; p=0.0170, and r=−0.2526; p= 0.0495 for radial and vertical growth phases, correspondingly. It should be noted that melanomas of highest Clark’s and Breslow’s stages had significantly reduced cytoplasmic VDR immunostaining as compared to less advanced tumors (Fig. 4 b, d), with similar changes in nuclear VDR expression of melanomas assessed using Breslow’s depth (Fig. 4 c). Moreover, cells at the vertical growth phase showed significantly reduced VDR immunoreactivity in comparison to the radial growth phase (Fig. 4 e, f). Interestingly, VDR expression also decreased in the deeper layer of melanocytic nevi (data not shown).
Figure 4.
VDR expression changes during progression of melanocytic lesions and is determined by histological type and aggressiveness of melanomas.
a. VDR nuclear staining in nevi and melanomas assessed according to Clark’s level.
b. VDR cytoplasmic staining in nevi and melanomas assessed according Clark’s level.
c. VDR nuclear staining in nevi and melanomas assessed according Breslow’s thickness.
d. VDR cytoplasmic staining in nevi and melanomas assessed according Breslow’s thickness.
e. VDR immunoreactivity in nuclei of radial and vertical growth phases of nodular (NMM) and superficial spreading (SSM) melanomas.
f. VDR immunoreactivity in cytoplasm of radial and vertical growth phases of nodular (NMM) and superficial spreading (SSM) melanomas.
g. Comparison of VDR expression in nodular (NMM) and superficial spreading (SSM) melanomas.
h. Comparison of VDR expression in nuclei of normal skin and primary metastasing and non-metastasing melanomas.
i. Comparison of VDR expression in cytoplasm of normal skin and primary metastasing and non-metastasing melanomas.
A.U. – arbitrary units.
VDR expression was also depended on the type of tumor. Both the nuclear and cytoplasmic immunoreactivities were significantly lower in more aggressive nodular melanomas than in superficial spreading melanomas (Fig. 4 g). Moreover, cytoplasmic VDR immunoreactivity of primary melanomas that metastasized (metastasizing melanomas) was significantly lower than in primary non-metastasizing tumors (Fig. 4 h, i). When metastasizing and non-metastasing primary melanomas were stratified according growth pattern of primary tumors (SSM or NMM), these differences were more pronounced and also included stain in the nuclei of NMM (data not shown). However, we have not found any correlation between VDR expression and other markers of poor prognosis such as satellitosis, ulceration or vascular invasion [24]. Concerning melanocytic nevi, the nuclear and cytoplasmic immunoreactivities of VDR were similar among their different histological types (data not shown).
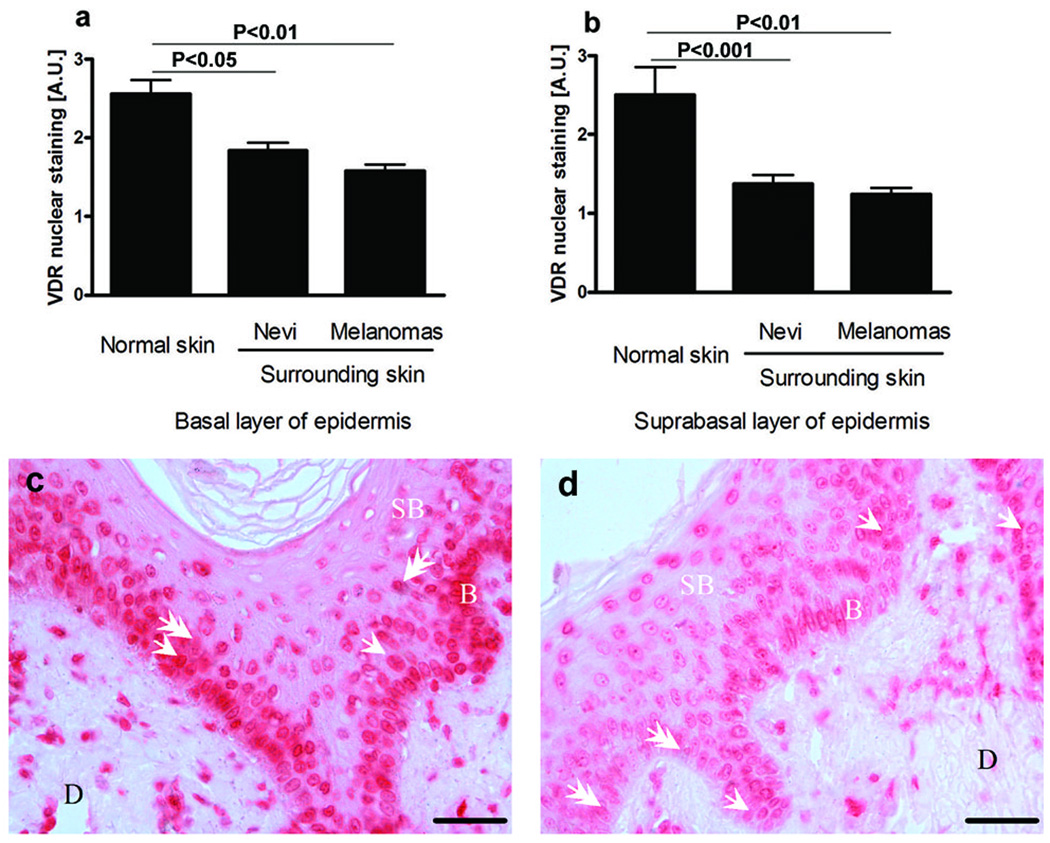
Nevertheless, the presence and behavior of melanocytic lesions considerably affected the nearest environment (Fig. 5 a-d). Thus, VDR expression in skin surrounding melanocytic tumors was markedly lower in comparison to normal skin (Fig. 5 c, d). This reduction was seen both in basal and suprabasal keratinocytes of the epidermis (Fig. 5 a, b).
Figure 5.
Type of pigmented lesion affects VDR expression in epidermal keratinocytes. Comparison of VDR expression in basal (a) and suprabasal (b) keratinocytes surrounding tumor. Representative VDR immunocytochemistry in normal skin (c) and skin adjacent to melanoma (d). Arrows point to cytoplasmic VDR staining; arrowheads point to nuclear VDR staining, D: dermis, SB: suprabasal keratinocytes; B: basal keratinocytes; scale bar: 50 µm. A.U. – arbitrary units.
To better understand the dynamic of disease progression in individual patients, we performed a matched analysis of VDR expression in recurrent versus primary melanomas and found a heterogenous pattern. Nuclear VDR staining decreased only in two recurrent lesions, while in the remaining three lesions, the VDR expression was slightly higher (but statistically not significant) in recurrent melanomas. Paired analysis of metastases and primary tumors revealed similar VDR staining intensity in the cytoplasm of two and in nuclei of nine patients. Lower VDR expression was found in the cytoplasm of fourteen and in the nuclei of five cases of the metastatic lesions as in comparison to primary melanomas. However, in eighteen and seventeen cases VDR expression in nuclei and cytoplasm, respectively, was higher in metastases than in primary tumors. However, this expression was still lower in comparison to normal skin, melanocytic nevi or tumor infiltrating lymphocytes. Statistically significant differences for VDR expression were for VGP cells of primary melanoma vs metastases or recurrent disease (p=0.0094 and p=0.0036, correspondingly). There were no significant differences in VDR expression for RGP cells and corresponding metastatic or recurrent disease.
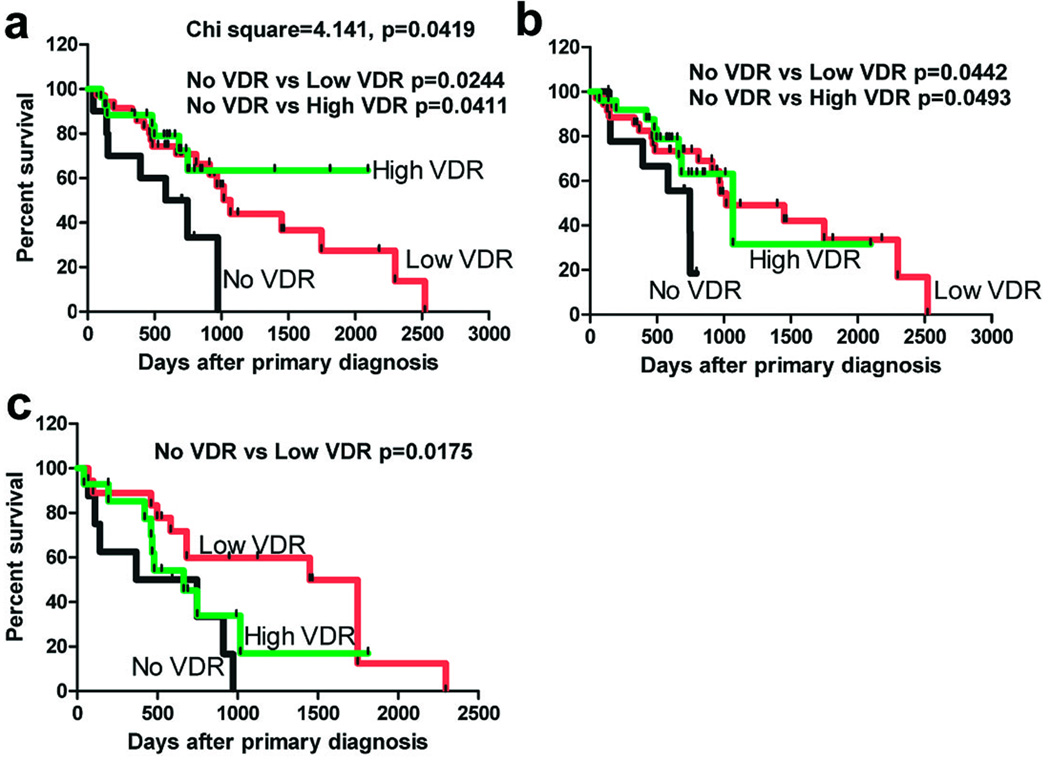
Importantly, we analyzed VDR expression in relation to overall survival (OS) of the patients and found that high VDR expression was a factor that favorably influenced the OS in melanoma cohort (Fig. 6, Table 2). Thus, the VDR expression in both primary and metastatic melanomas was important for survival of the patients (Fig. 6 a-c). Lack of VDR in primary lesions and metastases related to shorter overall survival time. Specifically, differences in the survival of melanoma patients with, respectively, low and high VDR staining intensity in nuclei of cells of RGP in comparison to cases without VDR (Fig. 6a) were statistically significant (p=0.0244 and p=0.0411). The Log rank test showed that the trend was significant (Chi square = 4.141; p=0.0419). Patients with lack of cytoplasmic VDR expression in VGP also had significantly shorter overall survival time than patients with low and high VDR expression (p=0.0442; p=0.0493, respectively; Fig. 6b). Similarly, the low nuclear VDR expression in metastases had the significant impact (p=0.0175) on survival of melanoma patients. The median overall survival for melanoma cohort in relation to cytoplasmic and nuclear VDR expression is also summarized in Table 2. The t-student test analysis of the data summarized in table 2 shows significantly shorter OS for the patients with lack of nuclear VDR in melanoma cells, confirming the log-rank (Mantel-Cox) test analysis listed in figure 6. There were no differences between VDR expression both in cytoplasm and nucleus and diseases free survival.
Figure 6.
The dependence of overall survival time (OS) on VDR expression in nuclei of radial growth phase (a) and cytoplasm of vetrical growi phase (b) of primary melanomas and in nuclei of metastases (c). Data were analyzed using log-rank (Mantel-Cox) test.
Table 2.
Overall survival in patients with melanoma in relation to VDR expression.
| Level of expression of VDR in melanomas |
No of cases (% of total) |
No of deaths (% of total) |
Median/mean survival (days) |
*Differences versus absent VDR |
|---|---|---|---|---|
| nuclei of cells in RGP | ||||
| Absent | 9 (13.0%) | 6 (66.7%) | 746/510 | - |
| Low VDR | 33 (47.8%) | 17 (51.5%) | 1018/874 | p=0.0122 |
| High VDR | 27 (39.1%) | 9 (33.3%) | 1068/761 | p=0.044 |
| nuclei of cells in VGP | ||||
| Absent | 14 (20.3%) | 7 (50.%) | 746/544 | - |
| Low VDR | 41 (59.4%) | 19 (46.3%) | 1449/834 | p=0.0031 |
| High VDR | 14 (20.3%) | 6 (42.9%) | 1018/667 | p=0.0488 |
| nuclei of cells in metastases | ||||
| Absent | 8 (20.0%) | 7 (87.5%) | 557/489 | - |
| Low VDR | 18 (45.0%) | 12 (66.7%) | 1449/1001 | p=0.0320 |
| High VDR | 14 (35.0%) | 9 (64.3%) | 663/622 | p=0.0211 |
| cytoplasm of cells in RGP | ||||
| Absent | 18 (26.1%) | 12 (66.7%) | 810/794 | - |
| Low VDR | 29 (42,0%) | 15 (51.7%) | 966/753 | p>0.05 |
| High VDR | 22 (31.9%) | 6 (27.3%) | 1018/689 | p>0.05 |
| cytoplasm of cells in VGP | ||||
| Absent | 10 (26.1%) | 7 (70.0%) | 664/523 | - |
| Low VDR | 32 (42.0%) | 19 (59.4%) | 1018/873 | p=0.0407 |
| High VDR | 27 (31.9%) | 8 (29.6%) | 1068/672 | p>0.05 |
| cytoplasm of cells in metastases | ||||
| Absent | 10 (25.0%) | 8 (80.0%) | 498/760 | - |
| Low VDR | 28 (75.0%) | 19 (67.9%) | 682/778 | p>0.05 |
| High VDR | 2 (5.0%) | 1 (50.0%) | 663/628 | p>0.05 |
The statistical analyses were performed using t-student test.
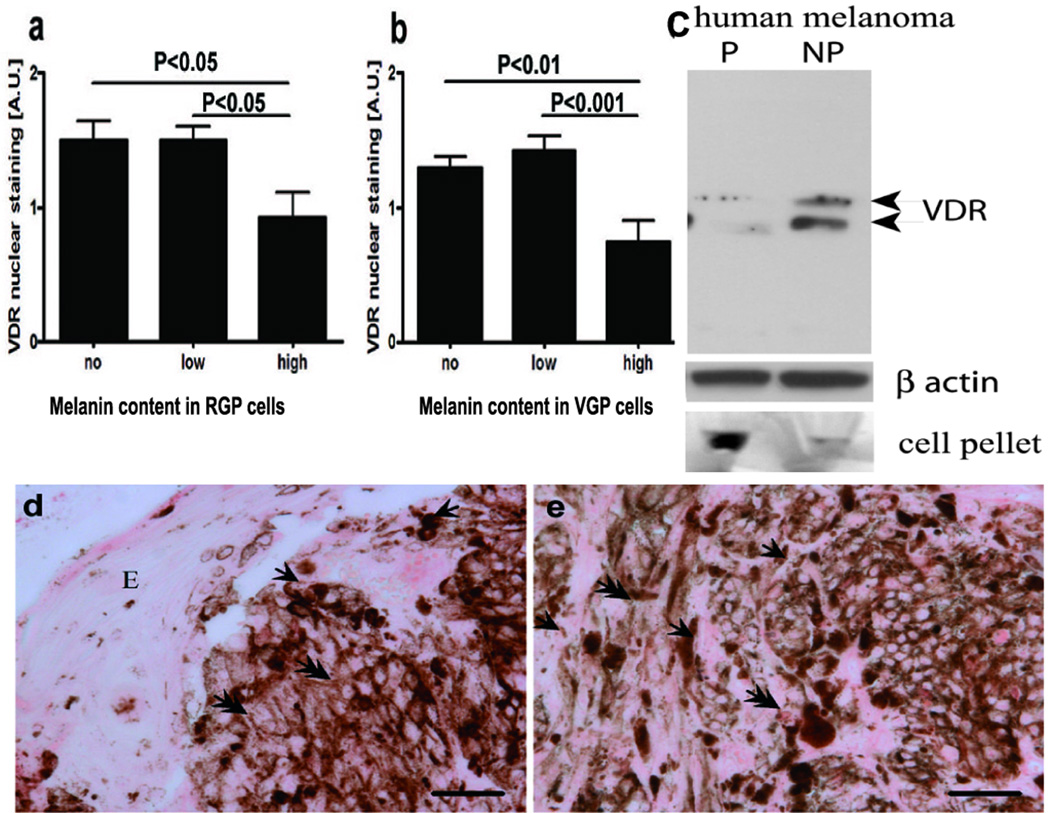
Nuclear VDR expression depends on melanin synthesis
The nuclear, but not cytoplasmic expression of the VDR in primary melanomas was significantly lower in highly melanized melanoma cells in comparison to amelanotic or poorly melanized melanomas (Fig. 7 a-e). This pattern was common for both radial and vertical growth phases (Fig. 7 a, b, d, e). There was no difference in VDR expression between amelanotic and poorly melanized melanoma cells. Also there was no correlation between melanin content and VDR expression in tested melanocytic nevi (not shown), which suggests the selectivity for malignant lesions.
Figure 7.
VDR expression is inversely correlated to melanin synthesis in melanoma cells.
a. Comparison of VDR expression in radial growth phase.
b. Comparison of VDR expression in vertical growth phase.
c. WB analysis shows decreased expression of VDR in pigmented cells (P) in comparison to non-pigmented cells (NP). Beta-actin stain shows the equal loading of the protein. The relative melanization of cells is visualized in cell pellets on the bottom of the panel.
d. Illustration of lack or low expression of VDR at the radial growth phase
e. Illustration of lack or low expression of VDR at the vertical growth phase.
Arrows point to cytoplasm, arrowheads point to nuclei; E: epidermis; Scale bar: 50 µm. A.U. – arbitrary units.
To confirm the negative correlation between melanogenesis and VDR expression we induced melanin synthesis in human SKMel-188 melanoma cells (31), by using media riche in melanin precursors. Induction of melanin production was observed and it was accompanied by a marked decrease of VDR expression as evaluated by WB using antibodies against VDR (Fig. 7c).
Mitotic and anti-apoptotic activity and VDR expression in skin melanocytic lesions
In general, nodular melanomas demonstrated significantly higher mitotic activity as compared to superficial spreading melanomas. The mean mitotic activity in all tumors was 4.5 per mm2 (ranging from 0 to 57) and 6.8 per high microscopic power field view (ranging from 0 to 60) and was inversely correlated with VDR cytoplasmic immunoreactivity (r=−0.3529, p=0.0027). The mitotic activity was highest in melanomas with lowest or no VDR expression in the cytoplasm. The mitotic activity was not linked to nuclear VDR expression (Supplemental Fig. 2 a, b), but it correlated with melanoma progression assessed with Clark’s level (r=0.3015, p=0.0106) and Breslow’s thickness (r=0.4200, p=0.0003), although no statistically significant differences were observed between primary metastasizing and non-metastasizing nodular melanomas. On the contrary, superficial spreading melanomas that metastasized revealed significantly higher mitotic activity. Mitotic activity was lower in melanomas with high melanin synthesis, but this relationship was not statistically significant, most likely due to large scatter of the mitotic index among cases.
Bcl-2 expression was highest in nevi, decreased in melanomas and was the lowest in metastases (Supplemental Fig. 2 c, d). There were no differences in Bcl-2 expression between radial and vertical growth phases (not shown). Furthermore, we did not observe any significant relationship between Bcl-2 and cytoplasmic VDR expression in melanoma cells. However, we found that maximal Bcl-2 expression was the highest in melanomas with highest VDR nuclear expression at the vertical growth phase as compared to maximal Bcl-2 in melanomas with no VDR expression (data not shown).
Solar elastosis and VDR expression in skin pigmented lesions
The solar elastosis as an indicator of UV-induced skin damages or absorbed UV radiation was analyzed in primary melanomas and nevi. In melanoma patients solar elastosis was slightly correlated with age (r=0.2363, p=0.0198). In nevi we did not observe such relationship. Solar elastosis was significantly higher in melanomas arising in extremities as compared to nevi (Supplemental Fig. 2e). Solar elastosis in other parts of the body was similar in the analyzed pigmented lesions. Among melanoma cases, the highest solar elastosis was observed in head and neck being significantly higher than in the trunk (Supplemental Fig. 2f). There was no correlation between solar elastosis and VDR expression in melanomas. In nevi arising in extremities the solar elastosis was significantly lower as compared to these in head and neck (Supplemental Fig. 2g).
Discussion
The data above on the correlation between VDR expression and clinical parameters, histological type, stage, morphology, proliferation and apoptotic activity (using bcl-2 staining) represents in our knowledge the first comprehensive evaluation of VDR in cohort of patients with melanocytic nevi and melanomas. The VDR expression was the strongest for normal non involved skin and it decreased with following orders of expression: normal skin> melanocytic nevi > melanomas = metastases for nuclear localization, and normal skin= melanocytic nevi > melanomas = metastases for the cytoplasmic location. The VDR expression also decreased along the tumor progression stage and there was marked negative correlation between strong melanin pigmentation and VDR expression. Also there was negative correlation between cytoplasmic VDR stain and mitotic activity and melanoma cells with high expression of nuclear VDR had the highest expression of bcl-2. There was no correlation between VDR levels and solar elastosis. Finally, lack of VDR expression in primary melanomas and metastases correlated with shorter overall survival time. These results are in agreement with cell culture-based studies that suggested a role for VDR in biology of melanomas [21, 22] as well as with population-based studies showing that VDR gene polymorphism [27, 30, 32, 33] or local generation of vitamin D or levels of circulating 25(OH)D3 [6, 25, 28, 29] can alter disease susceptibility and prognosis of melanoma patients.
The lower expression of VDR in melanocytic nevi than in normal or perilesional skin, represented the opposite pattern reported for the basal and squamous cell carcinomas vs normal skin [34, 35]. The VDR expression in melanomas correlated with histological type, staging and mitotic activity, with more aggressive forms having lower expression of VDR. Such reduction of receptor expression can affect some of malignant features of melanoma cells including unlimited replicative potential or an ability to invade the dermis [36, 37]. In our studies, we observed significant mitotic rate in melanomas without VDR. This is consistent with published data showing that VDR can regulate target genes related to cell cycle control [2, 10]. Lack or very low VDR expression in vertical growth phase is consistent with tumor progression leading to a decreased tumorostatic activity of vitamin D3 due to shortage of its receptor that in turn could promote an aggressive behavior of the tumor. In accordance, lack of either nuclear or cytoplasmic VDR in primary lesions or metastases correlated well with shorter overall survival time (Figure 6, Table 2). Thus, testing for VDR expression not only can provide mechanistic explanation for some aspects of melanoma progression but also may be of clinical value, predicting the outcome of the disease.
In the progression of melanocytic lesions, the alterations of both cytoplasmic and nuclear VDR was observed suggesting that balance between both compartments is relevant for the regulatory actions of vitamin D3. For example, nuclear localization of VDR is in accordance with the receptor binding to the VDRE in target genes. Therefore, the more pronounced reduction in nuclear as compared to cytoplasmic VDR staining along progression of pigmented lesions could contribute to the escape of melanoma cells from homeostatic surveillance and growth control, especially of the regulatory mechanisms dependent on the ligand activated receptor activity. In other neoplasms alternations in cytoplasmic and nuclear VDR-staining was also found along different stages of tumorogenesis [38–40], which, for example, included decreased nuclear VDR expression during development of colorectal tumors [38].
In melanomas regulation of apoptosis represents a balance between expression of pro-and antiapoptotic proteins. Interestingly, our studies have shown high expression of Bcl-2 in benign melanocytic nevi with its decrease in melanomas with the lowest expression in more malignant tumors. This somewhat counterintuitive observation is in agreement with other reports showing a similar trend in Bcl-2 expression (reviewed in [24]). Vitamin D3 decreases anti-apoptotic proteins including Bcl-2 [41]. Interestingly, the described pattern of VDR expression was opposite to theoretically expected, e.g., maximal Bcl-2 expression was essentially the highest in melanomas with high VDR nuclear expression in VGP cells as compared to melanomas lacking VDR. This may suggest uncoupling of VDR activity and regulation of Bcl-2 or more complex interactions, which would require further experimental testing. For example, presence of VDR could represent a target for 1,25(OH)2D3 that, when delivered to intratumoral environment, could indeed down-regulate the expression of Bcl-2 and induce apoptosis. Accordingly, the highest expression of VDR in benign melanocytic nevi or less aggressive melanomas would allow control of proliferation and apoptosis at earlier stages of progression of pigmented lesions. In this context, very low or lack of VDR would facilitate maintenance of malignant phenotype.
Skin keratinocytes and fibroblasts create a microenvironment essential for melanocytes survival [42, 43]. Tumor cells and surrounding tissue environment interact, enabling tumor development. Already in nevi we observed decreased VDR expression in surrounding skin. In melanomas these interactions could be even stronger with tumor regulating the microenvironment to promote its progression. Accordingly a decrease of VDR expression in surrounding skin cells could represent a manifestation of such tumor regulatory actions.
Active melanogenesis can affect behavior of melanocytes and surrounding cellular environment [43–45]. It can also attenuate the sensitivity of melanoma cells to chemo- and radiotherapy and have immunosuppressive effect [46–48]. In present studies, we observed significant impact of melanin synthesis on VDR expression in highly melanized vs amelanotic or poorly melanized tumors. This was further substantiated by experimental induction of melanogenesis in melanoma cells in which high melanogenic activity correlated with a decrease of VDR protein. Therefore, we suggest that melanogenesis can also affect vitamin D anti-tumor activity by decreasing intracellular concentrations of its receptor. This is consistent with a concept that metabolic intermediates generated during melanin synthesis could contribute to melanoma progression and melanogenesis could affect the outcome of radio-, chemo- or immunosensitivity of melanotic melanomas [46–48].
As related to histopathology, the decreased VDR expression along the tumor progression, and differences between nevi and early stages of melanomas suggest that VDR could be useful as an auxiliary, immunohistochemical marker in melanoma diagnosis. Furthermore, VDR expression in primary and metastatic lesions could help in predicting the clinical outcome of the disease since lack of VDR was related to the shorter survival time. We also suggest that vitamin D-based therapy could be added as an adjuvant strategy in melanoma patients with immunohistochemistry helping in selection/stratification of patients for treatment with active forms of vitamin D. This conception is supported by our matched analysis showing the presence of VDR in both recurrent and primary melanomas and in both primary and metastatic lesions, showing the presence of target (VDR) for vitamin D-based therapy. For melanotic melanomas, we suggest that combined therapy based on vitamin D analogs and inhibition of melanin synthesis could be more effective, since melanogenesis attenuated the expression of VDR. In addition, the high level of VDR in benign melanocytic lesions suggests that vitamin D itself and/or vitamin D analogues may play a protective role against tumor progression.
In conclusion, we propose that progression of melanocytic lesions is linked to reduction of VDR expression, that lack of VDR expression in melanomas leads to shorter overall survival time and that melanogenesis can attenuate the VDR expression. These findings should have important implications for the diagnosis, for predicting clinical outcome of the disease or for designing novel forms of therapy based on treatment with vitamin D or with vitamin D analogs.
Supplementary Material
Acknowledgments
Funding Source:
The work was supported in part by a grant # AR052190 to AS from National Institutes of Health (NIAMS).
References
- 1.Holick M, MacLaughlin JA, Clark MB, Holick SA, Potts JT, Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 2.Holick M. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 3.Bikle DD, Nemanic MK, Gee E, Elias P. 1, 25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986;78:557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann B. Role of the vitamin D3 pathway in healthy and diseased skin-facts, contradictions and hypotheses. Exp Dermatol. 2009;18:97–108. doi: 10.1111/j.1600-0625.2008.00810.x. [DOI] [PubMed] [Google Scholar]
- 5.Kramer C, Seltmann H, Seifert M, Tilgen W, Zouboulis CC, Reichrath J. Characterization of the vitamin D endocrine system in human sebocytes in vitro. J Steroid Biochem Mol Biol. 2009;113:9–16. doi: 10.1016/j.jsbmb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Slominski A. Are suberythemal doses of ultraviolet B good for your skin? Pigment Cell Melanoma Res. 2009;22:154–155. doi: 10.1111/j.1755-148X.2008.00540.x. [DOI] [PubMed] [Google Scholar]
- 7.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–384. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton AL, MacDonald PN. Vitamin D: more than a "bone-a-fide" hormone. Mol Endocrinol. 2003;17:777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 9.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 10.Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004;26:21–28. doi: 10.1002/bies.10368. [DOI] [PubMed] [Google Scholar]
- 11.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tangpricha V, Spina C, Yao M, Chen TC, Wolfe MM, Holick MF. Vitamin D deficiency enhances the growth of MC-26 colon cancer xenografts in Balb/c mice. J Nutr. 2005;135:2350–2354. doi: 10.1093/jn/135.10.2350. [DOI] [PubMed] [Google Scholar]
- 13.Beer T, Myrthue A. Calcitriol in cancer treatment: from the lab to the clinic. Mol Cancer Ther. 2004;3:373–381. [PubMed] [Google Scholar]
- 14.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, Slominski AT. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–2280. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. Febs J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74:218–228. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell MJ, Adorini L. The vitamin D receptor as a therapeutic target. Expert Opin Ther Targets. 2006;10:735–748. doi: 10.1517/14728222.10.5.735. [DOI] [PubMed] [Google Scholar]
- 21.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 22.Reichrath J, Rech M, Moeini M, Meese E, Tilgen W, Seifert M. In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D3-responsive and -resistant melanoma cells. Cancer Biol Ther. 2007;6:48–55. doi: 10.4161/cbt.6.1.3493. [DOI] [PubMed] [Google Scholar]
- 23.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 24.Carlson JA, Ross JS, Slominski A, Linette G, Mysliborski J, Hill J, Mihm M., Jr Molecular diagnostics in melanoma. J Am Acad Dermatol. 2005;52:743–775. doi: 10.1016/j.jaad.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Berwick M, Armstrong BK, Ben-Porat L, Fine J, Kricker A, Eberle C, Barnhill R. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97:195–199. doi: 10.1093/jnci/dji019. [DOI] [PubMed] [Google Scholar]
- 26.Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58:S129–S132. doi: 10.1016/j.jaad.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 27.Randerson-Moor J, Taylor JC, Elliott F, Chang YM, Beswick S, Kukalizch K, Affleck P, Leake S, Haynes S, Karpavicius B, Marsden J, Gerry E, Bale L, Bertram C, Field H, Barth JH, Silva Idos S, Swerdlow A, Kanetsky PA, Barrett JH, Bishop DT, Bishop JA. Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. Eur J Cancer. 2009;45:3271–3281. doi: 10.1016/j.ejca.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nurnberg B, Graber S, Gartner B, Geisel J, Pfohler C, Schadendorf D, Tilgen W, Reichrath J. Reduced serum 25-hydroxyvitamin D levels in stage IV melanoma patients. Anticancer Res. 2009;29:3669–3674. [PubMed] [Google Scholar]
- 29.Newton-Bishop J, Beswick S, Randerson-Moor J, Chang YM, Affleck P, Elliott F, Chan M, Leake S, Karpavicius B, Haynes S, Kukalizch K, Whitaker L, Jackson S, Gerry E, Nolan C, Bertram C, Marsden J, Elder DE, Barrett JH, Bishop DT. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27:5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mocellin S, Nitti D. Vitamin D receptor polymorphisms and the risk of cutaneous melanoma: a systematic review and meta-analysis. Cancer. 2008;113:2398–2407. doi: 10.1002/cncr.23867. [DOI] [PubMed] [Google Scholar]
- 31.Slominski A, Ermak G, Wortsman J. Modification of melanogenesis in cultured human melanoma cells. In Vitro Cell Dev Biol Anim. 1999;35:564–565. doi: 10.1007/s11626-999-0093-6. [DOI] [PubMed] [Google Scholar]
- 32.Halsall JA, Osborne JE, Potter L, Pringle JH, Hutchinson PE. A novel polymorphism in the 1A promoter region of the vitamin D receptor is associated with altered susceptibilty and prognosis in malignant melanoma. Br J Cancer. 2004;91:765–770. doi: 10.1038/sj.bjc.6602006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Liu Z, Zhang Z, Strom SS, Gershenwald JE, Prieto VG, Lee JE, Ross MI, Mansfield PF, Cormier JN, Duvic M, Grimm EA, Wei Q. Genetic variants of the vitamin D receptor gene alter risk of cutaneous melanoma. J Invest Dermatol. 2007;127:276–280. doi: 10.1038/sj.jid.5700544. [DOI] [PubMed] [Google Scholar]
- 34.Mitschele T, Diesel B, Friedrich M, Meineke V, Maas RM, Gartner BC, Kamradt J, Meese E, Tilgen W, Reichrath J. Analysis of the vitamin D system in basal cell carcinomas (BCCs) Lab Invest. 2004;84:693–702. doi: 10.1038/labinvest.3700096. [DOI] [PubMed] [Google Scholar]
- 35.Reichrath J, Rafi L, Rech M, Mitschele T, Meineke V, Gartner BC, Tilgen W, Holick MF. Analysis of the vitamin D system in cutaneous squamous cell carcinomas. J Cutan Pathol. 2004;31:224–231. doi: 10.1111/j.0303-6987.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 37.Gould Rothberg BE, Bracken MB, Rimm DL. Tissue biomarkers for prognosis in cutaneous melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2009;101:452–474. doi: 10.1093/jnci/djp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14:2370–2376. doi: 10.1158/1055-9965.EPI-05-0257. [DOI] [PubMed] [Google Scholar]
- 39.Menezes RJ, Cheney RT, Husain A, Tretiakova M, Loewen G, Johnson CS, Jayaprakash V, Moysich KB, Salgia R, Reid ME. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol Biomarkers Prev. 2008;17:1104–1110. doi: 10.1158/1055-9965.EPI-07-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tokumoto M, Tsuruya K, Fukuda K, Kanai H, Kuroki S, Hirakata H. Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int. 2002;62:1196–1207. doi: 10.1111/j.1523-1755.2002.kid585.x. [DOI] [PubMed] [Google Scholar]
- 41.Guzey M, Kitada S, Reed JC. Apoptosis induction by 1alpha,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1:667–677. [PubMed] [Google Scholar]
- 42.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 43.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 44.Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18:760–763. doi: 10.1111/j.1600-0625.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slominski A, Paus R, Schadendorf D. Melanocytes as "sensory" and regulatory cells in the epidermis. J Theor Biol. 1993;164:103–120. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- 46.Brożyna A, VanMiddlesworth L, Slominski A. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int J Cancer. 2008;123:1448–1456. doi: 10.1002/ijc.23664. [DOI] [PubMed] [Google Scholar]
- 47.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124:1470–1477. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slominski A, Paus R, Mihm MC. Inhibition of melanogenesis as an adjuvant strategy in the treatment of melanotic melanomas: selective review and hypothesis. Anticancer Res. 1998;18:3709–3715. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.