Abstract
This cross-sectional, multi-center cohort study compares the level of health-related quality of life (HRQOL) of pediatric liver transplant (LT) recipients to children with other chronic health conditions.
Design and Methods
LT sample included 873 children who survived at least 12 months following LT. Six chronic disease samples were compiled from numerous studies, including over 800 patients with Juvenile Rheumatoid Arthritis (JRA), type 1 diabetes, cancer in remission, cardiac disease, end-stage renal disease and inflammatory bowel disease. Generic HRQOL was measured from both the parental and patient perspective using the PedsQL™ 4.0 Generic Core Scales.
Results
Pediatric LT patients reported better physical health than children with JRA. According to parents, pediatric LT recipients had better HRQOL than children on renal dialysis on all domains except school functioning. Across all domains but emotional functioning, pediatric LT recipients reported significantly lower HRQOL than children with type 1 diabetes. Overall, pediatric LT patients reported comparable HRQOL to children who had undergone renal transplantation and patients with cancer in remission.
Conclusions
Pediatric LT patients manifested impaired HRQOL similar to children with chronic diseases and these data suggest they face ongoing challenges that warrant monitoring and indicate a need for interventions to improve their HRQOL.
Keywords: Pediatric Liver Transplantation, quality of life, chronic disease, PedsQL
Introduction
In an effort to identify factors associated with improved outcomes following liver transplantation (LT), health care providers are increasingly assessing health-related quality of life (HRQOL) in conjunction with traditional biological outcomes. HRQOL provides a comprehensive evaluation of a patient’s perceptions of the impact of an illness and its treatment on their own functioning and well-being (1, 2). In situations in which a child is too young, too cognitively impaired, too ill or too fatigued to complete a HRQOL measure, HRQOL may be assessed from the perspective of a parent (3).
Generic HRQOL instruments are multidimensional, consisting at minimum of the physical, psychological (including emotional and cognitive), and social health dimensions delineated by the World Health Organization (1, 4). The use of a generic HRQOL instrument enables comparisons across chronic conditions and benchmarking with healthy population samples (3, 5, 6). While generic HRQOL instruments provide important information for comparisons across groups, disease-specific measures may enhance measurement sensitivity for health domains relevant to a particular chronic condition. For example, Weissberg-Benchell et al. (2010) developed the Pediatric Quality of Life Inventory™ (PedsQL™) 3.0 Transplant Module for pediatric solid organ transplant recipients (7). In their initial validation study, which included a sample of 184 pediatric LT patients, the authors reported excellent measurement properties for the PedsQL™ Transplant Module which encompasses 8 disease-specific Scales: 1) About My Medicines I (barriers to medical regimen adherence), 2) About My Medicines II (medication side effects), 3) My Transplant and Others (social relationships and transplant), 4) Pain and Hurt (physical discomfort), 5) Worry (worries related to health status), 6) Treatment Anxiety (fears regarding medical procedures), 7) How I Look (impact of transplant on appearance), and 8) Communication (communication with medical personnel and others regarding transplant issues).
Previous studies using generic HRQOL instruments suggest pediatric liver transplant (LT) recipients manifest lower HRQOL than healthy children (8–17). Recently, in a cross-sectional study of 873 pediatric liver transplant patients ages 2 to 18 years surviving liver transplantation at least 12 months (8), we found that pediatric liver transplant patients evidenced significantly lower generic HRQOL compared to a matched healthy sample, with the largest differences demonstrated in school functioning. A sub-analysis of the school functioning items revealed that the greatest deficits for this sample pertained to missing school for doctor and hospital visits.
There is a relative absence of empirical data, however, on the extent to which the HRQOL of pediatric LT recipients compares to pediatric patients with other chronic health conditions (17). The sample sizes in previous LT studies have been relatively small and for the most part recruitment was conducted at single centers, thus limiting the statistical power to potentially find differences between groups (Type II error rate), and hence decreasing the generalizability of their findings. Understanding how a large, multi-center pediatric LT sample functions relative to other individual disease groups is an essential element of defining key outcomes for the LT population. By identifying domains of functioning that are similarly impacted across pediatric liver transplantation and other pediatric chronic health conditions, it may be possible to develop new interventions for pediatric LT patients based on treatment programs that have been shown to be efficacious in other disease populations in the domains found to be impaired. Further, such comparisons could help set expectations for parents and clinicians caring for LT recipients, by describing their level of chronic medical disability within the context of other more common pediatric diseases.
Consequently, the objective of the present study was to compare the HRQOL of LT recipients across childhood and adolescence to pediatric patients with other chronic health conditions that share similar non-categorical characteristics with LT recipients such as, a history of a major surgical procedure or life-threatening diagnosis, the need for chronic medications, and disease associated with chronic inflammatory states that may result in constitutional symptoms of fatigue or diminished physical stamina. The HRQOL outcomes of children and adolescents with Juvenile Rheumatoid Arthritis (JRA), type 1 diabetes, cancer in remission, cardiac disease, end-stage renal disease, and inflammatory bowel disease were compared to a cross-sectional sample of pediatric LT recipients from the perspective of patients and parents utilizing a widely-used well-validated generic HRQOL measure, the PedsQL™ 4.0 Generic Core Scales. Pediatric LT recipients were recruited from the Studies of Pediatric Liver Transplantation (SPLIT) registry, the largest assembled cohort of pediatric LT recipients in North America. This study includes data from the majority of children having survived liver transplantation at 22 North American transplant centers during the past ten years, thus providing a broad perspective of health outcomes in this population.
Methods
Pediatric liver transplant sample
Data collection for the pediatric LT sample was conducted as part of an ancillary study to the SPLIT registry and included 22 of the registry centers that elected to participate. Patients from the SPLIT registry at these centers were eligible if they were between 2 and 18 years of age, were recipients of liver transplantation, and had survived at least 12 months following transplant. Eligible patients and parents/guardians were also required to be fluent in either English or Spanish. In addition, children who were not maintaining regular medical follow-up with their transplant center and recipients of combined organ transplants were excluded. The study was approved by the Institutional Review Boards (IRBs) at participating centers and written informed consent was obtained from all parents or guardians, prior to participation. Assent was obtained as required by individual institutions.
The total pediatric LT sample included 873 children. Child self-report was available for 363 participants and parent proxy-report was available for 869 participants. Both child self-report and parent proxy-report were available for 359 cases. There were no significant differences in gender, age at transplant or primary disease between participants and eligible non-participants (n= 466). Participants were more likely to be White (60% vs. 49%) and less likely to be Hispanic (15% vs. 24%) compared to eligible non-participants (p=0.0001). For all participants combined, the average age of the 479 girls (54.9%) and 394 boys (45.1%) was 8.17±4.43 years. For child self-report, the average age of the 199 girls (54.8%) and 164 boys (45.2%) was 12.49±3.05 years. With regard to race/ethnicity, the sample contained 527 (60.4%) White non-Hispanic, 129 (14.8%) Hispanic, 130 (14.9%) Black non-Hispanic, 41 (4.7%) Asian/Pacific Islander, 6 (0.7%) American Indian or Alaskan Native, 40 (4.5%) Other or Missing. The patient’s primary disease was Biliary atresia in 44%, metabolic liver disease in 15%, fulminant liver failure in 13%, chronic cholestatic disease in 12% and other diagnoses in 16%. The median interval from transplant to survey was 3.10 (interquartile range 1.68–5.32) years. Thirty-two percent of the patients had been hospitalized in the preceding 12 months with a median of five hospital days, (interquartile range 3–12 days). Graft function was excellent in the vast majority of these patients with 94% having a Total bilirubin of ≤1.5 mg/dL and only 13% experiencing an episode of rejection in the 12 months prior to testing. Serious chronic co-morbidities were uncommon with 1.4% having Diabetes or glucose intolerance, 0.6% having a calculated glomerular filtration rate (cGFR) (18) of < 60 ml/min/1.73 m2, and only one patient in the sample receiving dialysis.
Selection of chronic disease comparison samples
Six chronic disease samples were compiled from a number of studies which utilized the PedsQL™ 4.0 Generic Core Scales (19–25), and consisted of over 800 pediatric patients with a history of JRA, type 1 diabetes, cancer in remission, cardiac disease, renal disease, and inflammatory bowel disease. Table 1 displays the demographic characteristics of the samples. Patients from the cited studies were chosen to create comparison samples of patients that were primarily being managed in an ambulatory setting and who had not had a recent major surgical intervention, but who had serious chronic health conditions that required ongoing medical monitoring and/or therapy. The cancer in remission sample included only patients who had been off cancer treatment (chemotherapy or radiation) for 12 months or greater. The cardiac disease sample included those children who had cardiac malformations that were judged to have caused at least moderate compromise in cardiac function and which had been surgically corrected or who were in the process of staged surgical procedures. Cardiac patients with mild disease that was treated non-operatively and those with severe, complex disease which was deemed uncorrectable (including single ventricle anatomy) were not included. Cardiac patients who had undergone cardiac surgery within the year prior to assessment were also not included since the LT sample had recovered from the transplant procedure by at least one year. The renal group included 45 patients that had undergone renal transplantation and 51 who had been on dialysis for at least two months at the time of assessment.
Table 1.
Characteristics of Comparison Groups
| Sample | Gender (Female) | Age (mean±SD) | |
|---|---|---|---|
| Liver Transplant | |||
| Total | 873 | 55% | 8.2±4.4 |
| Self-report | 363 | 55% | 12.5±3.0 |
| Juvenile Rheumatoid Arthritis | |||
| Total | 100 | 77% | 9.5±4.4 |
| Self-report | 82 | 82%* | 10.9±3.8 |
| Type 1 Diabetes | |||
| Total | 320 | 54% | 12.3±3.9 |
| Self-report | 292 | 54% | 12.8±3.5 |
| Cancer Survivors | |||
| Total | 94 | 40% | 10.1±4.2 |
| Self-report | 72 | 36% | 11.2±3.5 |
| Cardiac Disease | |||
| Total | 223 | 42% | 9.7±4.8 |
| Self-report | 157 | 44% | 12.2±3.5 |
| Renal Transplant | |||
| Total | 45 | 11%** | 12.8±4.9 |
| Self-report | 39 | 13%*** | 14.0±3.9 |
| Renal Dialysis | |||
| Total | 51 | 39%**** | 12.5±4.3 |
| Self-report | 46 | 35%***** | 13.2±3.7 |
| Inflammatory Bowel Disease | |||
| Total****** | 70 | 44.3% | 14.1±2.1 |
6% of sample missing gender information
27% of sample with missing gender information
28% of sample with missing gender information
14% of sample with missing gender information
13% of sample with missing gender information
All patients had self-reported data
Procedures
Eligible patient and parent or guardian dyads were recruited during a routine follow-up visit at their transplant center between 2005 and 2008. The parent/guardian and patient (if age 8 years or older) were instructed to complete one of four age-specific versions of the PedsQL™ 4.0 Generic Core Scales. Only parents/guardians familiar with the child’s general functioning were included as proxy respondents for the study. After completing the questionnaires, the patients continued with the clinical part of the routine visit.
Measures
The PedsQL™ 4.0 (Pediatric Quality of Life Inventory™ Version 4.0) Generic Core Scales
The 23-item PedsQL™ 4.0 Generic Core Scales encompass: 1) Physical Functioning (8 items), 2) Emotional Functioning (5 items), 3) Social Functioning (5 items), and 4) School Functioning (5 items) (6, 26). The instructions ask how much of a problem each item has been during the past one month. A 5-point categorical response scale is utilized across child self-report for ages 8–18 (0 = never a problem; 1 = almost never a problem; 2 = sometimes a problem; 3 = often a problem; 4 = almost always a problem). Items are reverse-scored and linearly transformed to a 0–100 scale (0=100, 1=75, 2=50, 3=25, 4=0), so that higher scores indicate better HRQOL. The Total Scale Score is computed as the sum of all the items on the PedsQL™ divided by the number of items answered. The Physical Health Summary Score (8 items) is the same as the Physical Functioning Scale. To create the Psychosocial Health Summary Score (15 items), the mean is computed as the sum of the items divided by the number of items answered in the Emotional, Social, and School Functioning Scales. Scale Scores are computed as the sum of the items divided by the number of items answered (this accounts for missing data). If more than 50% of the items in the scale are missing, the Scale Score is not computed. This accounts for the differences in sample sizes reported across Tables 1 and 2.
Table 2.
Scale Descriptives for the PedsQL™ 4.0 Generic Core Scales for Child Self-Report and Parent Proxy-Report and Comparisons with Pediatric Chronic Disease Scores
| Liver Transplant | Juvenile Rheumatoid Arthritis | Type 1 Diabetes | Cancer (Off-Treatment > 12 months) | Cardiac | Renal Transplant | Renal Dialysis | IBD | |
|---|---|---|---|---|---|---|---|---|
| Scale | Mean ± SD (adj. sig. level) | Mean ± SD (adj. sig. level) | Mean ± SD (adj. sig. level) | Mean ± SD (adj. sig. level) | Mean ± SD (adj. sig. level) | Mean ± SD (adj. sig. level) | Mean ± SD (adj. sig. level) | Mean ± SD (adj. sig. level) |
| Child Self-Report | (n = 363) | (n = 82) | (n =292 ) | (n =72 ) | (n = 157) | (n =39 ) | (n =46 ) | (n =70 ) |
| Total Score | 77.21 ± 14.28 | 73.50 ± 16.53 (NS) | 80.79 ± 12.78 (p<0.004) | 77.50 ± 15.30 (NS) | 78.83 ± 13.80 (NS) | 78.94 ± 14.24 (NS) | 69.77 ± 14.90 (p<0.05) | 76.85 ± 14.31 (NS) |
| Physical Health | 82.29 ± 15.62 | 70.60 ± 21.73 (p<0.001) | 85.80 ± 13.16 (p<0.006) | 79.95 ± 18.78 (NS) | 83.02 ± 15.69 (NS) | 80.76 ± 20.53 (NS) | 69.62 ± 19.11 (p<0.001) | 77.90 ± 17.32 (NS) |
| Psychosocial Health | 74.51 ± 15.83 | 74.99 ± 16.57 (NS) | 78.06 ± 14.55 (p<0.006) | 76.30 ± 16.04 (NS) | 76.56 ± 14.90 (NS) | 77.86 ± 13.46 (NS) | 69.88 ± 15.04 (NS) | 76.29 ± 14.44 (NS) |
| Emotional Functioning | 74.00 ± 19.90 | 72.10 ± 21.46 (NS) | 74.16 ± 19.77 (NS) | 77.57 ± 20.31 (NS) | 74.17 ± 18.42 (NS) | 79.04 ± 18.70 (NS) | 71.88 ± 18.61 (NS) | 74.00 ± 20.49 (NS) |
| Social Functioning | 80.95 ± 19.09 | 78.58 ± 19.34 (NS) | 85.93 ± 16.42 (p<0.002) | 79.36 ± 19.77 (NS) | 82.53 ± 16.97 (NS) | 82.31 ± 17.54 (NS) | 75.19 ± 17.52 (NS) | 86.43 ± 14.67 (p<0.05) |
| School Functioning | 68.53 ± 18.56 | 74.61 ± 18.35 (p<0.05) | 74.27 ± 18.16 (p<0.001) | 71.11 ± 18.11 (NS) | 73.13 ± 18.20 (NS) | 72.31 ± 16.13 (NS) | 62.34 ± 20.45 (NS) | 68.43 ± 18.21 (NS) |
| Parent Proxy-Report | (n = 869) | (n = 97 ) | (n = 310 ) | (n = 94 ) | (n = 222 ) | (n = 45 ) | (n =50 ) | (n = 70 ) |
| Total Score | 77.26 ± 17.58 | 73.43 ± 18.96 (NS) | 77.48 ± 14.33 (NS) | 73.64 ± 18.72 (NS) | 81.94 ± 14.84 (p<0.001) | 75.57 ± 17.75 (NS) | 64.27 ± 16.84 (p<0.001) | 70.54 ± 16.81 (p<0.05) |
| Physical Health | 79.33 ± 22.07 | 69.69 ± 23.68 (p<0.002) | 82.19 ± 17.97 (NS) | 74.80 ± 24.08 (NS) | 86.13 ± 16.90 (p<0.001) | 78.38 ± 24.65 (NS) | 64.75 ± 22.18 (p<0.001) | 71.70 ± 20.37 (p<0.05) |
| Psychosocial Health | 75.72 ± 17.33 | 75.48 ± 18.50 (NS) | 74.91 ± 15.12 (NS) | 72.95 ± 18.07 (NS) | 79.55 ± 15.54 (p<0.003) | 74.14 ± 17.50 (NS) | 64.08 ± 16.48 (p<0.001) | 69.93 ± 16.56 (p<0.05) |
| Emotional Functioning | 73.27 ± 19.28 | 74.47 ± 20.32 (NS) | 70.38 ± 18.35 (NS) | 72.93 ± 19.94 (NS) | 76.94 ± 20.11 (p<0.05) | 75.67 ± 18.36 (NS) | 62.50 ± 18.91 (p<0.001) | 66.00 ± 20.55 (p<0.05) |
| Social Functioning | 78.99 ± 20.63 | 76.08 ± 21.31 (NS) | 82.81 ± 18.49 (p<0.05) | 76.28 ± 21.71 (NS) | 85.54 ± 17.24 (p<0.001) | 78.56 ± 22.20 (NS) | 68.90 ± 18.93 (p<0.002) | 78.14 ± 19.26 (NS) |
| School Functioning | 67.42 ± 22.43 | 75.78 ± 21.55 (p<0.003) | 71.35 ± 19.04 (p<0.05) | 68.87 ± 23.08 (NS) | 74.78 ± 18.94 (p<0.001) | 66.06 ± 18.53 (NS) | 60.66 ± 23.52 (NS) | 65.64 ± 21.95 (NS) |
Note: Adjusted significance levels used the Hochberg method.
NS denotes no statistically significant differences.
Statistical analysis
Mean PedsQL™ Scale and Summary Scores were calculated for the pediatric LT sample and for each chronic disease sample. PedsQL™ Scores between the LT sample and chronic disease samples were compared using independent samples t-tests. The overall Type I error rate was maintained at 0.05 by the Hochberg adjustment for multiple comparisons (27). This adjustment was made separately for child self-report and parent-proxy report. Effect size as utilized in these analyses was calculated by subtracting the LT mean from the chronic disease sample mean, and then dividing by the pooled standard deviation. Effect sizes are designated as small (.20), medium (.50), and large (.80) in magnitude (28). Statistical analyses were conducted using SPSS Version 16.0 for Windows (29, 30) or the SAS System for Windows, v 9.2 (SAS Institute, Cary, NC).
Results
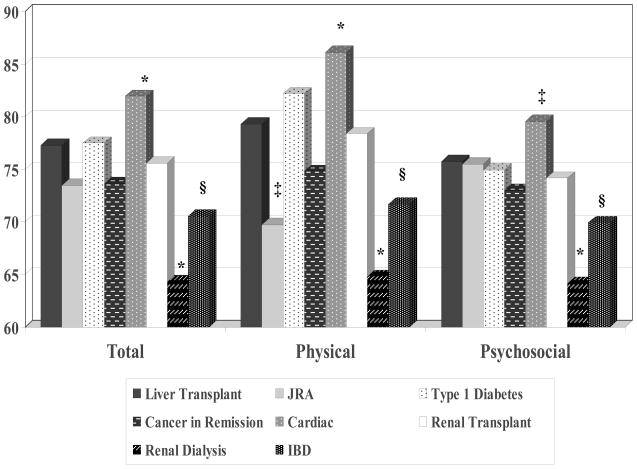
Figures 1 and 2 present the PedsQL™ Total Scale Scores, Physical Health Summary Scores, and Psychosocial Health Summary Scores for the LT sample and the chronic disease groups. Table 2 displays the comparisons of the subscale scores between the LT sample and the chronic disease groups.
Figure 1.
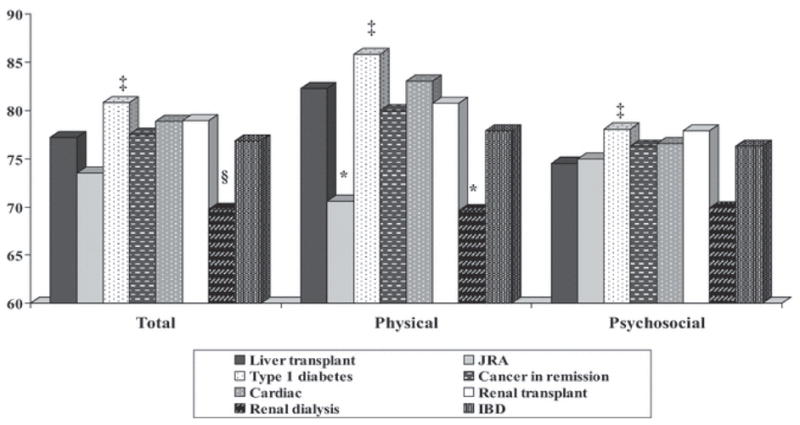
Figure 2. Parent Proxy-Reported PedsQL™ Scores for Liver Transplant Sample and Chronic Disease Groups.
Note: § p ≤ .05, ‡ p ≤ .01, * p ≤ .001; Significant differences indicate comparisons between the pediatric liver transplant sample and other pediatric chronic disease groups; Liver transplant n = 869, Juvenile rheumatoid arthritis n = 97, Type 1 diabetes n = 310, Cancer off-treatment n = 94, Cardiac n = 222, Renal transplant n = 45, Renal dialysis n = 50, Inflammatory bowel disease n = 70.
Juvenile Rheumatoid Arthritis
The LT patients and their parents reported significantly higher physical health than patients with JRA (child self-report effect size = 0.69; parent proxy-report effect size = 0.43). The LT patients and their parents reported significantly lower school functioning compared to patients with JRA (child self-report effect size = −0.33; parent proxy-report effect size = −0.37).
Type 1 Diabetes
The LT patients self-reported significantly lower overall HRQOL, physical health, psychosocial health, social functioning, and school functioning compared to pediatric patients with type 1 diabetes (overall HRQOL effect size =−0.26; physical health effect size=−0.24; psychosocial health effect size=−0.23; social functioning effect size=0.28; school functioning effect size=−0.31). In contrast to the self-report results, parents of LT patients did not report lower overall HRQOL for their children compared to parents of children with diabetes (effect size = 0.01); however, they did report lower social functioning and school functioning for their children compared to parents of pediatric patients with type 1 diabetes (social functioning effect size = −0.19; school functioning effect size=0.18).
Cancer Survivors
The mean PedsQL™ subscale and summary scores reported by the LT patients and their parents were similar to those reported for patients with cancer in remission. None of the child self-report or parent proxy-report PedsQL™ comparisons between the liver transplant and cancer survivor samples were statistically significant.
Cardiac Disease
The mean PedsQL™ subscale and summary scores self-reported by the LT patients were similar to those self-reported by patients who had cardiac disease. However, parents of the LT patients reported significantly lower overall HRQOL, physical health, psychosocial health, emotional functioning, social functioning, and school functioning for their children compared to parents of patients with cardiac disease (overall HRQOL effect size = −0.27; physical health effect size=−0.32; psychosocial health effect size = −0.23; emotional functioning effect size = −0.19; social functioning effect size = −0.33; school functioning effect size = −0.34).
Renal Transplant
The mean PedsQL™ subscale and summary scores reported by the LT patients and their parents were similar to those reported for renal transplant patients. None of the child self-report or parent proxy-report PedsQL™ comparisons between the liver transplant and renal transplant samples were statistically significant.
Renal Dialysis
The LT patients self-reported significantly higher overall HRQOL and physical health compared to pediatric patients on renal dialysis (overall HRQOL effect size = 0.52; physical health effect size = 0.79). Parents of LT patients reported higher overall HRQOL, physical health, psychosocial health, emotional functioning, and social functioning for their children compared to parents of patients on renal dialysis (overall HRQOL effect size = 0.74; physical health effect size = 0.66; psychosocial health effect size = 0.67; emotional functioning effect size = 0.56; social functioning effect size = 0.49).
Inflammatory Bowel Disease
The mean PedsQL™ subscale and summary scores self-reported by the LT patients were similar to those self-reported by pediatric patients with inflammatory bowel disease, with the exception of social functioning in which LT patients self-reported a significantly lower score (effect size=−0.30). Parents of the LT patients reported higher overall HRQOL, physical health, psychosocial health, and emotional functioning for their children compared to parents of patients with inflammatory bowel disease (overall HRQOL effect size = 0.38; physical health effect size = 0.35; psychosocial health effect size = 0.34; emotional functioning effect size = 0.38).
Discussion
The objective of this study was to compare the HRQOL of pediatric LT recipients to children with other chronic health conditions that share some similar characteristics to LT recipients. Examples of shared characteristics include a history of surgical intervention, the need to take daily medications and the history of having had a life threatening illness. This comparison begins to define how the health state of the LT recipient fits within the spectrum of more common and thus familiar chronic health conditions. Defining HRQOL within this context will help clinicians to better understand the burden of chronic illness for the pediatric LT recipient.
We found pediatric LT patients evidenced significantly better physical health than children with JRA from the perspective of both patients and parents. This was not unexpected, since few LT recipients report pain or physical disabilities, the hallmark symptoms of JRA. Also as expected, parents and patients agreed that HRQOL across all domains was similar for liver and renal transplant recipients and that most aspects of health of the LT recipients were better than that of children on renal dialysis.
Of interest, both LT patients and their parents reported their level of school functioning was lower than children with JRA and diabetes, but similar to other chronic illnesses including patients on dialysis. As noted above, when we compared HRQOL of this cohort to an age, gender and ethnicity matched sample of healthy children, the LT patients and parents reported significantly lower functioning across all domains with the largest effect sizes being in the PedsQL™ School Functioning Scale (8). This finding is consistent with recent data from a longitudinal study of 823 pediatric LT patients from the SPLIT research consortium which reported 34% of patients were receiving special educational services in school and 20% had repeated a grade, with older participants more likely to have been held back; missing more than 10 days of school per year was reported by one-third of the LT sample (31). In the current LT sample, questions related to missing days of school had the largest impact on the PedsQL™ School Functioning Scale Score. Since this aspect of HRQOL appears to be a significant issue for patients within all of the six disease categories we studied, treatment strategies that minimize school absences and resources that support academic progress, despite absences, would likely improve HRQOL for children with a wide array of chronic illnesses.
Parents and children in the LT sample and the other chronic disease samples did not always agree on the level of HRQOL experienced by the child. For the most part, parents reported outcomes that were slightly lower than what patients reported for themselves, consistent with findings from other pediatric chronic conditions (3). The notable exception was the cardiac disease group. The low agreement between the mean subscale values for the child self-report and parent proxy-report for the cardiac sample may in part be attributed to the fact that the parent proxy-report sample (n = 222) included 65 parents of children who did not provide self-report (23). Nonetheless, these parent-child discrepancies are consistent with both the adult and pediatric literature suggesting information provided by proxy-respondents is not equivalent to reports by the patient (32, 33). For the LT sample, the intraclass correlations (ICCs) for all sub-scales were in the moderate range, suggesting that parents’ perceptions were reasonably aligned with their children. Yet, it may be difficult for parents to assess their children’s function in domains with less observable or more internal symptoms, such as pain, fatigue and emotional distress. Parents may however, be more aware of age-appropriate functional expectations and better able to assess when their children are struggling as compared to their peers. These observed differences demonstrate the need to evaluate both children’s and parents’ perspectives regarding HRQOL in clinical practice and clinical trials since their different perspectives potentially provide unique information.
Across all domains but emotional functioning, pediatric LT recipients self-reported significantly lower HRQOL than children with type 1 diabetes. This finding stands in contrast to Taylor et al. (2009) who compared the HRQOL of 55 adolescent LT recipients to young people with asthma and diabetes and found that LT recipients scored similarly to these chronic illness groups (17). Similar to pediatric LT patients, children with type 1 diabetes and their families are required to perform a number of daily self-management behaviors, such as regular blood glucose monitoring, multiple insulin doses, regulation of carbohydrate intake, and frequent exercise (34). There are well organized support programs for children with type 1 diabetes that promote clinical management and adherence. In fact, a number of behavioral interventions have been developed for children with type 1 diabetes that have shown improvements in treatment adherence (35), coping skills (36, 37), and parent-child communication (38, 39). Given the importance of self-management in both of these populations, and our findings that pediatric LT recipients in the present study self-reported significantly lower HRQOL than children with type 1 diabetes across multiple domains, it may be useful for future research to investigate how components of behavioral interventions developed for children with type 1 diabetes can be extended to the care of pediatric LT patients.
In the present study, pediatric LT patients reported comparable HRQOL to children who had undergone renal transplantation from the perspective of both patients and parents. These data, combined with findings from a separate study in which pediatric LT patients from the present sample evidenced significantly lower generic HRQOL than a matched healthy sample (8), suggest that the HRQOL of pediatric LT patients is more similar to a child with a chronic health condition than a healthy child. Given these findings, it is recommended that the measurement of HRQOL in pediatric LT patients should be considered for routine assessment in clinical practice as this would aid clinicians in addressing both the physical and psychosocial problems that accompany liver transplantation.
There are a number of limitations to the present study. First, the non-response rates across the chronic disease comparison samples are not known, which may limit the generalizability of our findings. For the LT sample, race/ethnicity was the only measurable difference found in our sample compared to the non-participants (8). Specifically, non-participants were more likely to be Hispanic. Although the PedsQL™ is available in Spanish, some data collection centers did not have Spanish speaking staff available for recruiting patients. However, it should be noted the percentage of Hispanic patients in this sample was similar to that of pediatric liver recipients listed in the United Network for Organ Sharing (UNOS) registry for the calendar year of 2006, 15% versus 21%, respectively (www.unos.org). Given that the chronic disease samples came from an existing database, some of the samples were small which may have impacted our ability to detect statistically significant group differences. It should be noted, however, that we presented effect sizes for each comparison and effect sizes are relatively independent of sample size. Working from an existing database, we were not able to match the chronic disease samples to the pediatric LT sample on demographic characteristics; within each chronic disease group, it is also likely that there were patients with varying disease severities that would be expected to impact HRQOL. Finally, we did not have data on time since transplant for the renal transplant sample.
In conclusion, pediatric LT patients manifested impaired HRQOL similar to children with chronic diseases and were more significantly impaired across a number of dimensions for several conditions. These data suggest pediatric LT patients are facing ongoing daily challenges that warrant continuous monitoring and indicate the need for innovative interventions to improve the HRQOL of these patients.
Acknowledgments
This project was supported by grant number R01 HD045694 of the National Institute of Child Health and Human Development and grant number U01 DK061693 of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health. The sponsoring agency was not involved in the collection, analysis, or interpretation of data or the generation of the report.
Studies of Pediatric Liver Transplantation Functional Outcomes Group
University of California, Los Angeles (Sue McDiarmid, MD)
Cincinnati Children’s Hospital Medical Center (John Bucuvalas, MD)
The Children’s Hospital, Denver (Ronald Sokol, MD)
Children’s Medical Center, Dallas (Jami Gross, MD)
Hospital for Sick Children, Toronto (Vicky Ng, MD)
University of Nebraska (Alan Langnas, DO)
Mount Sinai Medical Center (Nanda Kerkar, MD)
University of Alberta, Edmonton (Susan Gilmour, MD)
Children’s Memorial Hospital (Estella Alonso, MD)
Children’s Hospital of Philadelphia (Barbara Haber, MD)
University of Miami/Jackson Memorial (Andreas Tzakis, MD)
University of California, San Francisco (Philip Rosenthal, MD)
Johns Hopkins University (Wikrom Karnsakul, MD)
Children’s Mercy Hospital, Kansas City (James F. Daniel, MD)
St. Louis Children’s Hospital (Yumirle Turmelle, MD)
Texas Children’s Hospital (Saul Karpen, MD, PhD)
University of Minnesota (Abhi Humar, MD)
Children’s Hospital of Pittsburgh (George Mazariegos, MD)
University of North Carolina, Chapel Hill (Jeffrey Fair, MD)
University of California, San Diego (Joel E. Lavine, MD)
Alfred I. DuPont Hospital for Children (Stephen Dunn, MD)
Boston Children’s Hospital (Maureen Jonas, MD)
University of Michigan (Emily Fredericks, PhD)
Footnotes
The data presented in this manuscript is original data, the authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing Interests: Dr. Varni holds the copyright and the trademark for the PedsQL™ and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory™.
Author Contributions:
Study concept and design: James W. Varni, Estella Alonso, John C. Bucuvalas
Acquisition of data: Katie Neighbors, James W. Varni, Estella M. Alonso, John C. Bucuvalas
Analysis and interpretation of data: Christine A Limbers, James W. Varni, Estella M. Alonso, John C. Bucuvalas, Thomas Webb, Karen Martz
Drafting of the manuscript: Christine A Limbers, James W. Varni, Estella M. Alonso
Critical revision of the manuscript for important intellectual content: Katie Neighbors, James W. Varni, Estella M. Alonso, John C. Bucuvalas, Thomas Webb, Karen Martz
Statistical expertise: Christine A Limbers, James W. Varni,Karen Martz
Obtaining funding: Katie Neighbors, Estella M. Alonso, John C. Bucuvalas
Administrative, technical, or material support: Katie Neighbors, Estella M. Alonso, John C. Bucuvalas
Supervision: James W. Varni, Estella M. Alonso
References
- 1.FDA. Patient-reported outcome measures: Use in medical product development to support labeling claims. Food and Drug Administration, U.S. Department of Health and Human Services; Rockville, MD: 2009. Guidance for Industry. [Google Scholar]
- 2.Kushner RF, Foster GD. Obesity and quality of life. Nutrition. 2000;16(10):947–52. doi: 10.1016/s0899-9007(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 3.Palmer SN, Meeske KA, Katz ER, Burwinkle TM, Varni JW. The PedsQL™ Brain Tumor Module: Initial reliability and validity. Pediatric Blood and Cancer. 2007;49:287–93. doi: 10.1002/pbc.21026. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Constitution of the World Health Organization: Basic Document. Geneva, Switzerland: World Health Organization; 1948. [Google Scholar]
- 5.Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Medical Care. 1989;27:S217–S33. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 6.Varni JW, Seid M, Rode C. The PedsQL™: measurement model for the Pediatric Quality of Life Inventory. Medical Care. 1999;37:126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Weissberg-Benchell J, Zielinski TE, Rodgers S, Greenley RN, Askenazi D, Goldstein SL, Fredericks EM, McDiarmid S, Williams L, Limbers CA, Tuzinkiewicz K, Lerret S, Alonso EM, Varni JW. Pediatric health-related quality of life: Feasibility, reliability and validity of the PedsQL™ Transplant Module. American Journal of Transplantation. 2010;10:1677–1685. doi: 10.1111/j.1600-6143.2010.03149.x. [DOI] [PubMed] [Google Scholar]
- 8.Alonso EM, Limbers C, Neighbors K, Martz K, Bucuvalas JC, Webb T, et al. Cross-sectional analysis of health-related quality of life in pediatric liver transplant recipients. J of Pediatr. 2010;156:270–6. doi: 10.1016/j.jpeds.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso EM, Neighbors K, Barton FB, McDiarmid SV, Dunn SP, Mazariegos GV, et al. Health-related quality of life and family function following pediatric liver transplantation.[see comment] Liver Transplantation. 2008;14(4):460–8. doi: 10.1002/lt.21352. [DOI] [PubMed] [Google Scholar]
- 10.Alonso EM, Neighbors K, Mattson C, Sweet E, Ruch-Ross H, Berry C, et al. Functional outcomes of pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 2003 Aug;37(2):155–60. doi: 10.1097/00005176-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Apajasalo M, Rautonen J, Sintonen H, Holmberg C. Health-related quality of life after organ transplantation in childhood. Pediatr Transplant. 1997;1:130–7. [PubMed] [Google Scholar]
- 12.Asonuma K, Inomata Y, Uemoto S, Egawa H, Kiuchi T, Okajima H, et al. Growth and quality of life after living-related liver transplantation in children. Pediatr Transplant. 1998 Feb;2(1):64–9. [PubMed] [Google Scholar]
- 13.Bucuvalas JC, Britto M, Krug S, Ryckman FC, Atherton H, Alonso MP, et al. Health-related quality of life in pediatric liver transplant recipients: A single-center study. Liver Transpl. 2003 Jan;9(1):62–71. doi: 10.1053/jlts.2003.50012. [DOI] [PubMed] [Google Scholar]
- 14.Cole CR, Bucuvalas JC, Hornung RW, Krug S, Ryckman FC, Atherton H, et al. Impact of liver transplantation on HRQOL in children less than 5 years old. Pediatric Transplantation. 2004;8(3):222–7. doi: 10.1111/j.1399-3046.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 15.Fredericks EM, Lopez MJ, Magee JC, Shieck V, Opipari-Arrigan L. Psychological functioning, nonadherence and health outcomes after pediatric liver transplantation. American Journal of Transplantation. 2007;7(8):1974–83. doi: 10.1111/j.1600-6143.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- 16.Midgley DE, Bradlee TA, Donohoe C, Kent KP, Alonso EM. Health-related quality of life in long-term survivors of pediatric liver transplantation. Liver Transpl. 2000 May;6(3):333–9. doi: 10.1053/lv.2000.6139. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RM, Franck LS, Gibson F, Donaldson N, Dhawan A. Study of the factors affecting health-related quality of life in adolescents after liver transplantation. American Journal of Transplantation. 2009;9(5):1179–88. doi: 10.1111/j.1600-6143.2009.02604.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976 Aug;58(2):259–63. [PubMed] [Google Scholar]
- 19.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL™ in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory™ Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 20.Varni JW, Burwinkle TM, Szer IS. The PedsQL™ Multidimensional Fatigue Scale in pediatric rheumatology: reliability and validity. Journal of Rheumatology. 2004;31(12):2494–500. [PubMed] [Google Scholar]
- 21.Uzark K, Jones K, Burwinkle TM, Varni JW. The Pediatric Quality of Life Inventory in children with heart disease. Progress in Pediatric Cardiology. 2003;18:141–8. [Google Scholar]
- 22.Limbers CA, Newman DA, Varni JW. Factorial invariance of child self-report across age subgroups: A confirmatory factor analysis of ages 5 to 16 years utilizing the PedsQL™ 4.0 Generic Core Scales. Value in Health. 2008;11:659–68. doi: 10.1111/j.1524-4733.2007.00289.x. [DOI] [PubMed] [Google Scholar]
- 23.Uzark K, Jones K, Slusher J, Limbers CA, Burwinkle TM, Varni JW. Quality of life in children with heart disease as perceived by children and parents. Pediatrics. 2008;121:e1060–e7. doi: 10.1542/peds.2006-3778. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein SL, Graham N, Burwinkle T, Warady B, Farrah R, Varni JW. Health-related quality of life in pediatric patients with ESRD. Pediatric Nephrology. 2006;21:846–50. doi: 10.1007/s00467-006-0081-y. [DOI] [PubMed] [Google Scholar]
- 25.Marcus S, Strople J, Neighbors K, Weissberg-Benchell J, Nelson S, Limbers CA, et al. Fatigue and health-related quality of life in pediatric inflammatory bowel disease. Clinical Gastroenterology and Hepatology. 2009;7:554–61. doi: 10.1016/j.cgh.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Medical Care. 2001 Aug;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Statistics in Medicine. 1990;9(7):811–8. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 29.SAS. System for Windows v 8.02. Cary, NC: SAS Institute; [Google Scholar]
- 30.SPSS. SPSS 16.0 for Windows. Chicago: SPSS, Inc; 2008. [Google Scholar]
- 31.Gilmour S, Sorenson L, Anand R, Yin W, Alonso E. School outcomes in children registered in the Studies of Pediatric Liver Transplantation (SPLIT) consortium. Liver Transpl. 2010 doi: 10.1002/lt.22120. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross-informant correlations for situational specificity. Psychological Bulletin. 1987;101:213–32. [PubMed] [Google Scholar]
- 33.Sprangers MAG, Aaronson NK. The role of health care providers and significant others in evaluating the quality of life of patients with chronic disease: A review. Journal of Clinical Epidemiology. 1992;45:743–60. doi: 10.1016/0895-4356(92)90052-o. [DOI] [PubMed] [Google Scholar]
- 34.Anonymous. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group.[see comment] Journal of Pediatrics. 1994;125(2):177–88. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 35.Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1997;130(2):257–65. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- 36.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. Journal of Pediatrics. 2000;137(1):107–13. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- 37.Greco P, Pendley JS, McDonell K, Reeves G. A peer group intervention for adolescents with type 1 diabetes and their best friends. Journal of Pediatric Psychology. 2001;26(8):485–90. doi: 10.1093/jpepsy/26.8.485. [DOI] [PubMed] [Google Scholar]
- 38.Anderson BJ, Brackett J, Ho J, Laffel LM. An office-based intervention to maintain parent-adolescent teamwork in diabetes management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22(5):713–21. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- 39.Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, et al. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. Journal of Pediatric Psychology. 2000;25(1):23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]