Abstract
Background
Neo-angiogenesis is an essential process in physiological and pathological conditions. However, it is a complex process. Several studies demonstrated that intra-tumoural microvessel number is a significant predictor of metastasis and clinical outcome in many tumours, including oral malignancies. The immuno-surveillance cells, mast cells and eosinophils are implicated in the biological behaviour of tumours. Nevertheless, their function in tissues is uncertain. Mast cells are involved in homeostatic regulation of blood vessels as well as host defence. In some malignancies, high mast cell density has been found to correlate with favourable prognosis. However, others reported unfavourable associations. Tumour associated tissue eosinophilia is a well-known phenomena. It has been associated with good and poor prognosis. However, the role of eosinophils in tumours remains controversial. Therefore, this study was designed to investigate the prognostic value of microvessel, mast cell and eosinophil densities in the context of clinico-pathological parameters and survival in squamous cell carcinoma of the tongue.
Materials and Methods
Anti-CD105 and anti-tryptase monoclonal antibodies were utilized to highlight and count microvessels and mast cells respectively in 81 cases of tongue squamous cell carcinoma. Eosinophils were demonstrated using carbol chromotrope histochemical stain. The densities were counted per mm2 and correlated with patients’ outcome and other clinico-pathological parameters using non-parametric tests and student's t-test. Clinically, the cases were divided into 4 main groups depending on survival time, lymph-node or distant metastasis.
Results
The 5 year survival was significantly lower in patients with a low mast cell density than those with a high density (p=0.006, Kruskal-Wallis test). The survival group-A demonstrated significantly higher mast cell and microvessel numbers than group-D (p=0.007, student's t-test) respectively. Patients with well- differentiated squamous cell carcinoma had significantly higher numbers of mast cells when compared to patients with poorly differentiated squamous cell carcinoma (p<0.05, student's t-test). The lymph node involvement correlation between the survival group-A and survival group-D was also significant (p=0.001, Mann-Whitney U test).
Conclusion
Data from this study indicates that accumulating mast cells in tumours play a part in inhibiting tumour progression and is potentially angiogenic in tumours.
Keywords: Tongue squamous cell carcinoma, Angiogenesis, Mast cells, Eosinophils, Survival
Introduction
Neo-angiogenesis is an essential step for many physiological processes, such as growth, wound healing, organ regeneration and reproductive functions. Abnormal blood vessel growth occurs in several pathological conditions, including tumour growth and metastasis [1]. Angiogenesis is however a complex multistep process, and one that is not fully understood. A cascade of events involving endothelial migration and proliferation, microvessel differentiation and anastomosis, and extracellular remodelling has been suggested [2, 3]. One of the main differences between normal and pathological angiogenesis is that in the latter, the vessels are highly disorganised and their walls have many openings, leading to ‘leaky vessels’ [4].
Tumour-associated angiogenesis is important in maintaining tumour growth and facilitates its metastatic spread through connections with the existing vasculature [5, 6]. Several studies demonstrated that intra-tumoural neovascularisation is a significant predictor of metastasis and clinical outcome in oral malignancies [7–9]. The association between microvessel density (MVD), clinicopathological parameters and prognosis in oral squamous cell carcinoma (SCC) has been investigated. Data shows significant correlation between MVD, tumour behaviour and survival [10–13].
Immuno-surveillance cells, namely mast cells (MCs) and eosinophils (Eos) have been implicated in the biological behaviour of tumours. Although MC function in tissues is still largely unknown, their activation occurs through both immune and non-immune mechanisms. They are involved in homeostatic regulation of nerves and blood vessels as well as host defence. MC mediators are known to affect endothelial cells by inducing vasodilatation and recruitment of inflammatory cells. It has been postulated that MCs play a role in promoting angiogenesis in some malignant tumours and their association with various tumours has been described [14–17].
In some malignancies, high mast cell density (MCD) has been found to correlate with favourable prognosis. However, others reported unfavourable associations [18–20]. Eosinophils are rare granulocytes that are normally associated with allergic diseases or responses to various parasitic infections. Tumour associated tissue eosinophilia (TATE) has been observed for many decades in tumours involving larynx, oesophagus, pharynx, skin, breast, cervix, lung and gastrointestinal system [21–29]. Nevertheless, the genuine role of Eos in tumour stroma remained a controversial topic. Both, favourable and unfavourable prognoses have been reported in TATE [30–35].
It was not until the early 1980's, when a study of TATE in head and neck cancer gained attention. In the majority of the reports, TATE was associated with favourable outcomes [36, 37]. Nevertheless, unfavourable association has also been reported [38].
TATE may represent a local inflammatory reaction leading to tumour cell damage [39], but products of tumour necrosis may itself induce tissue eosinophilia [40]. Abundant Eo infiltration has been noticed near hemosiderin deposits in solid tumours. This raised the assumption that accumulation of Eo could be due to presence of intracellular erythrocytic pathogens [41]. Furthermore, attachment of activated eosinophil to tumour cells and loss of its proteins, in addition to detection of IL-5 suggested that Eos might play a role in the host defence mechanism [42].
Currently, the exact functional relevance of MC and Eo association with various tumours remains indefinite. The correlation of MVD, MCD and eosinophil density (ED) with prognosis in tongue carcinoma is also uncertain. Therefore, this study was designed to investigate the prognostic value of these cells and MVD in the context of clinicopathological parameters and survival in SCC of the tongue.
Material and Methods
Patient selection
Eighty one patients, treated and followed at Leeds Dental Institute between 1994 and 1998 who were diagnosed as having SCC of the tongue and who had enough follow-up information, were enrolled in the study. None of the patients had received chemotherapy or radiotherapy before surgery. Informed consent for research and approval by local research ethics committees was obtained (04/Q1107/27). Tissue sections were reviewed and representative paraffin embedded tissue blocks selected.
Immunohistochemical staining procedure
The standard streptavidin-peroxidase conjugated method was used throughout the research. For microvessel staining, 4µm sections from each tumour block were dewaxed. Endogenous peroxidase was blocked using 2% hydrogen peroxide in methanol for 20 min. Each step of incubation was followed by a thorough washing of the sections with tris buffered solution (TBS). Sections were primarily incubated with casein 1/10 v/v for 5 min to suppress non-specific binding of subsequent reagents. After incubation with primary antibody against CD105 (Endoglin) receptor (clone SNGH, NeoMarkers, Fremont, CA USA), mouse monoclonal; 1/40 v/v dilution for overnight incubation, the sections were then treated with secondary biotinylated antibodies for 30 min followed by streptavidin for 30 min. Finally, all slides were treated with 3, 3‘ Diaminebenzidine (DAB, Sigma D-5637) and counterstained with Mayer's haematoxylin.
For MCs demonstration, serial sections from the same blocks used to evaluate MVD were cut and stained for mast cell tryptase. After dewaxing the sections, antigen retrieval using proteolytic enzyme (0.1% chymotrypsin) for 15 min in an incubator at 37C° was performed. Sections were given a thorough wash with TBS then incubated with 1/10 v/v casein for 10 min, followed by monoclonal anti-mast cell tryptase antibody (B25487, Calbiochem), 1/250 dilution for 60 min. The rest of the procedure was continued as described previously.
Eos was demonstrated using carbol chromotrpe (CC) stain; well known in demonstrating eosinophils [43]. After dewaxing and washing in running tap water, the sections were stained with Harris' haematoxylin solution to stain nuclei for 5 min.
The sections were then washed in water, differentiated in 1% acid alcohol, blued in Scott's tap water substitute and washed in tap water. The sections were then stained with carbol chromotrope solution for 30 min followed by a water wash for two min. The sections were then dehydrated cleared and mounted in DPX.
For evaluation of the inter/intra-examiner consistency, 22 sections were selected randomly from the sample and the re-counting procedure for the three variables was performed by the same examiner.
Another colleague, who was not involved in the research but familiar with the procedure, was asked to carry out the same re-counting procedure.
Estimation of MVD, MCD and ED
The MVD was assessed in tumour and peritumoural areas following similar principles described by Weidner et al [44]. Sections were scanned at ×100 magnification and areas of high vascular density identified (hot spots). Individual vessels were then counted in at least three fields with the aid of a grid in 0.23 mm--------2 using a ×25 objective. Any discrete, brown stained endothelial cells were considered as a countable microvessel, although some of the vessel lumens were unclear. The highest figure in the counted hot spot fields was considered as the MVD for a given case. By using the same counting method as for MVD evaluation, MCs were counted in hot spots, in an area of 0.09 mm2 using a ×40 objective. The highest obtained value was considered as the MCD for a given case.
ED counts were determined using the same method used for counting MCs and the highest eosinophil count achieved was recorded as the ED.
Survival classification
Clinically, the cases were divided into 4 main survival groups; A, B, C and D depending on survival time, lymph-node or distant metastasis (Table 1). Only one case fell under survival group C and on statistical advice, it was moved into group D, thereforemaking the survival groups to go down to 3. The MVD, MCD and ED counts were each divided into two groups depending on the median values, high= ≥ 29, low= < 29 for MVD; high= ≥ 17, Low= < 17 for MCD and high= ≥ 48, low= < 48 for ED.
Table 1.
Classification and description of the survival groups
| Group | Description |
|---|---|
| A | 60 m+ with no recurrence or metastasis 30-59 m with recurrence or metastasis |
| B | 60 m+ with recurrence or metastasis |
| C | 30-59 m with recurrence or metastasis |
| D | <30 m regardless of recurrence or metastasis |
m = months
Statistical analysis
For statistical analysis, Scientific Package for Social Sciences for Windows (version 14.0) (SPSS, Inc, Chicago, IL) was used to analyse variables and produce graphical representation. The 5 year survival rate was correlated with the high and low MVD, MCD and ED groups using Cox's Regression analysis. The nonparametric Kruskal-Wallis test was used to test possible significance. Assessment of the MVD, MCD and ED in relation to the survival groups was also performed by the non-parametric Kruskal-Wallis test and student's t-test. The histological grades were correlated with MVD, MCD and ED using the Mann-Whitney U test.
Results
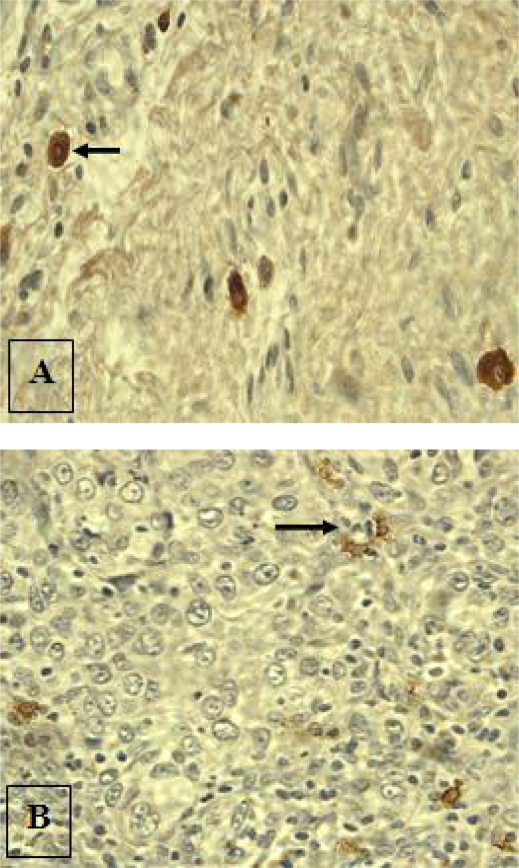
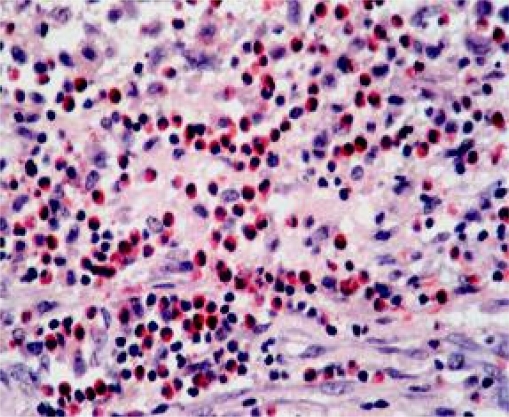
The MCs that were distant from the tumour edges appeared large and darkly stained, while the intra-tumoural MCs were smaller in size, lightly stained and showed signs of degranulation (Fig. 1). CC stain demonstrated eosinophils quite nicely (Fig. 2).
Figure 1.
Anti-MC tryptase immunohistochemical staining demonstrating intact MCs distant from tumours (A, arrow) and degranulating intra-tumoural MCs (B, arrow) (original magnification ×400 approx).
Figure 2.
Carbol chromotrope stain demonstrating eosinophils in the control section (central eosinophilic granuloma). (Mag. ×400 approx).
Sixty five percent (n=53) of the cases were males and 35 % (n=28) were females. The mean age was 54.9 yr and 65.6 yr for males and females respectively. Only one male case was of unknown age. 84% (n=68) of the cases were welldifferentiated, 11.1% (n=9) were moderately differentiated and 4.9% (n=4) were poorlydifferentiated SCC.
Node metastasis was present in 21.0% (n=17) of the cases and recurrence was present in 13.6% (n=11). The survival rate was calculated from the date of surgery to the date of death or date of the information obtained if patients were still alive. In cases where cause of death was unknown, patients dying within 2 years from the day of surgery were considered ‘dead of the disease’. Survival time was obtained for all cases except one case. The minimum survival time was 1 month, maximum was 115 months and the mean overall survival was 52.36 months (±37.4 SD). The mean survival, age, and the histopathological parameters of each survival group are given in tables 2&3. The median score values for ED, MCD, MVD and survival in months are shown in table 4.
Table 2.
Number of cases, overall survival mean and standard deviation, mean age and standard deviation in each survival group.
| Group | N | OS (mean±SD) | Age (mean±SD) |
|---|---|---|---|
| A | 36 | 79.4±21.1 | 58.9±12.4 |
| B | 13 | 78.3±9.9 | 60.9±8.4 |
| C | 1 | 48 | 49 |
| D | 30 | 8.8±6.9 | 66.6±17.5 |
N = Number of cases, OS = Overall survival in months
Table 3.
Number(n) of SCC cases with grade, proportion of MVD, proportion of MCD, proportion of ED and the number of positive node cases in each survival group.
| Group | Differentiation (n) | MVD | MCD | ED | NM | ||
|---|---|---|---|---|---|---|---|
| WD | MD | PD | |||||
| A | 33 | 2 | 1 | 24.3±12.3 | 32.1±15.1 | 60.6±50.5 | 0 |
| B | 10 | 3 | 0 | 20.8±6.7 | 27.3±16.6 | 57.9±55.8 | 8 |
| C | 1 | 0 | 0 | – | – | – | 0 |
| D | 24 | 4 | 3 | 16.6±10.4 | 22.9±15.2 | 58.6±63.6 | 9 |
WD= Well differentiated, MD= moderately differentiated, PD= MD= poorly differentiated, NM= node metastasis. Note; One case is of unknown survival.
Table 4.
The median values for ED, MCD, MVD and survival in moths according to tumor differentiation, lymph node metastasis and tumor size.
| ED | MCD | MVD | Survival | |
|---|---|---|---|---|
| WD SCC | 48 | 17 | 29 | 60 |
| MD SCC | 68 | 19 | 24 | 40 |
| PD SCC | 39 | 15 | 35 | 8 |
| LN +ve | 48 | 17 | 28 | 60 |
| LN −ve | 48 | 18 | 30 | 16 |
| T1 | 41 | 16 | 28 | 60 |
| T2 | 56 | 22 | 33 | 60 |
| T3 | 54 | 19 | 36 | 60 |
| T4 | 38 | 16 | 13 | 6 |
ED = Eosinophil density, MCD = mast cell density, MVD = microvascular density, WD = Well differentiated, MD = moderately differentiated, PD = MD = poorly differentiated, LN = lymph node, T1–T4 = tumor size.
The score for MVD ranged from 0 to 66 with a mean of 27.9 ± 15.7, MCs ranged from 3 to 58, with a mean of 20.69 ± 11.3 and 0 to 303 with a mean of 59.4 ± 55.8 for ED. The inter and intra-examiner consistency counts revealed insignificant differences.
Survival at 5 years was 68% in patients with high MCD count compared to 38% in those patients with a low count. The MVD groups and the overall 5 year survival correlation was insignificant. However, the correlation was significant when only survival group A and D were tested (p = 0.02, Mann Whitney U test). Furthermore, the survival group-A cases showed a higher number of blood vessels than group-D cases (p<0.05, student's t-test) (Table 2).
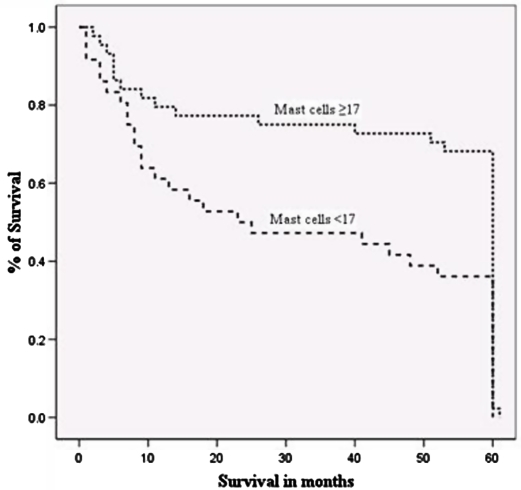
The 5 year survival was significantly shorter for patients with a low MCD than those with a high MCD count (Fig. 3, Kruskal-Wallis test, p = 0.006). The survival group-A had significantly higher mast cell numbers than group-D (p = 0.007, student's t-test) but not with survival group-B. The well-differentiated SCC cases had a significantly higher number of MCs than the poorly differentiated SCC cases (p < 0.05, student's t-test).
Figure 3.
Cox regression survival curve evaluating the high MCD (≥ 17) and low MCD (<17) groups in relation to overall 5 year survival (P = 0.006, Kruskal-Wallis test).
The high and low ED groups did not show any difference in 5 year survival. There were no differences in ED among the survival groups. The number of microvessels difference in the two ED groups was insignificant. Also, no correlation was found between ED and SCC differentiation. The spearman's correlation test revealed no linear association between the MCD and ED. The MVD and MCD showed no correlation with ED.
There were no differences in the percentages of MVD, MCD or ED in the positive and negative lymph node cases. When overall 5 year survival was correlated with nodes, negative node cases survived better than those cases with positive nodes, though the correlation was insignificant (Mann-Whitney U test, p = 0.091). Nevertheless, there was a significant correlation between node and the survival group-A and survival group-D (Mann-Whitney U test, p = 0.001).
Discussion
Neoangiogenesis is a complex process; multifactorialy regulated and thought to be fundamental in tumour development and progression. Although several studies have suggested that angiogenesis might be an independent prognostic factor, others have failed to show any correlation between tumour vascularisation and survival [7, 9, 13, 45–47]. Use of different antibodies to define endothelium, different methodology in assessment of MVD and inter-observer variation could contribute to these discordant results. Various endothelial markers including CD34, CD31, vascular endothelial growth factor (VEGF) and von Willebrand factor have been used for evaluation of neoangiogenesis in tumours. It is well known that these markers are not specific for neo-angiogenic vessels and can express on the pre-existing host vessels. Recently, use of anti-CD105 antibody has been widely accepted due to its relative specificity in identifying microvessels in various tumours [48, 49]. The counting method is critical in determining the number of vessels, MCs or Eos. Hot spots are invariably located in the stroma around tumour edges, therefore rendering random counting of fields meaningless. However, values obtained from averaging multiple hot spot fields would make little or insignificant difference from the highest hot spot count.
Furthermore, in certain tumours, the number of vessels or MCs is probably related to stromal volume, thus tumours with more stroma might exhibit more vessels and cells. These variations in the amount of stroma and tumour cells in various counting fields might influence the traditional counting procedure. Pazouki et al [50] counted the highest number of microvessels (MVD) per surface area of oral tumours and compared that with the conventional stereological method of measuring microvascular volume. The volume but not the MVD method showed significant and linear increase in vascularity with increasing severity of the disease. In a previous study, the density counting method for TATE was thought to be more appropriate than the classical counting method [51]. In fact, the suggested cut off value (50/mm2) that demarcates the low and high ED cases was very close to the median value (48/mm2) that was used in this study to delineate the low and high ED cases.
Tumour expansion is a progressive process and therefore continuously stimulates new vessel formation. Several studies of oral SCCs, including tongue, demonstrated a significant increase in vascularity during the tumour progress [52–55]. Moreover, the endoglin expression in oral SCC was significantly higher in comparison to the normal healthy mucosa and sequentially related to the tumour grades [50].
In this series of tongue SCC, there was no association between MVD and the overall 5 year survival or survival groups. In addition, the non-parametric tests failed to detect any correlation between MVD and tumour differentiation or nodal metastasis. These results are in agreement with Amar et al [46], who failed to demonstrate any association between MVD and patient prognosis or histological features in SCC of the tongue. A study of 33 cases of oral SCC revealed higher MVD with increasing tumour and node stages but not with survival [7].
In the current study, the high and low MVD groups showed no differences in survival. Interestingly, survival group-A showed higher numbers of microvessels and MCs in comparison to survival group-D. This pattern of vascularity between the two groups is probably related to the MCs and not to the tumour progression. However, this remains speculative. It has been postulated that growth of solid tumours is dependent upon adequate blood supply through stroma. Although the precise mechanism by which MCs are implicated in angiogenesis is not fully understood, several angiogenic factors, such as histamine, proteases (tryptase and chymase), cytokines (tumour necrosis factor-α, interleukin-8), VEGF and fibroblast growth factor (FGF-2) that are released by the accumulating MCs in and around tumours have been suggested [56, 57]. In animal studies, tumours of mast cell deficient mice revealed lower angiogenesis than normal mice [15]. A significant correlation between MCs and microvascular counts was shown in oral mucosa and our results are in line with those of the previous oral SCC studies [58, 59]. Furthermore, there had been linear increase of MCs from normal mucosa to the development of squamous carcinoma that suggested they may upregulate angiogenesis, possibly through their tryptase content [58].Oral tissues are highly vascular. Most studies that investigated MVD in the head and neck looked at samples from different locations, whilst in this study, the samples were exclusively from the tongue. Possibly, tumours of the tongue are not as reliant on neovascularisation.
Our results showed that the high mast cells group had longer survival (68%) than the low mast group (38%). Also, the mean MCD and MVD in survival group-A were significantly higher than those of survival group-D. On the other hand, the mean MCD difference between survival groups A and B were insignificant. The latter group comprised cases with recurrences or nodal metastasis but with survival time extending up to 60 months. This demonstrates that the MCD is a factor independent of metastasis or recurrence. Nevertheless, no correlation was found with other histopathological parameters.
Accumulation and degranulation of MCs around tumour areas has been observed in previous reports and in this study (Fig. 1). However, the functional significance of this phenomenon is contentious. MCs are conventionally divided into either tryptase positive or tryptase and chymase positive subtypes, and may undergo phenotypic changes. Significant increases of MC containing tryptase and chymase subpopulations were found at the tumour invasion areas, where its role is thought to degrade extracellular matrix. The tryptase containing MCs were found at the intratumoural stroma and play a role in angiogenesis [59]. The predominant MC type in tissues may be determined by environmental needs.
However, the coexistence of tryptase-expressing MCs and chymase and tryptase-expressing MCs in physiological conditions reflects a natural balance that contributes to tissue homeostasis [60]. Many reports believe that MCs play roles in angiogenesis and progression of tumours including oesophagus [15], pulmonary adenocarcinoma [16], colorectal carcinoma [17], and lip cancer [61]. On the contrary, high mast cell associated tumours revealed significantly different outcomes in ovarian cancer [14], colorectal cancer [62], breast cancer [63], and prostatic adenocarcinoma [64].
Based on the clinical observation, data from Tan et al [62] suggest that MCs have a cytotoxic effect on cancer cells; there was a direct correlation between the number of MCs and clinical outcome, which are in line with our findings.
The current view is that MCs are versatile in function and capable of regulating inflammation, host defence, and innate immunity by elaboration of several chemokines and cytokines. MC accumulation in tumours is probably part of a response to tumour derived chemoattractant [65]. In vitro generated MCs express natural cytotoxicity against tumour cells [66]. They are also cytotoxic for mammalian tumours when supplemented with iodide and H2O2. The latter is released from the tissue phagocytes and initiates MC degranulation and liberation of the cytotoxic endogenous peroxidase from its granules [67].
The anti-tumour activity by mast cells has also been attributed to MCs production of tumour necrosis factor (TNF-α), which is directly cytotoxic to certain tumour cells [68]. Cultured human MCs were found to induce apoptosis in Jurkat Tleukemia cells suggesting a possible role in immune surveillance against tumour cells that may extend beyond TNF-α sensitive tumour [69]. Significant increases in peri-tumoural MCs of lip carcinoma showed histochemical changes and signs of degranulation [70]. These features suggest that mast cells contribute to the hostdefence reaction of the tumour.
Recently, strong evidence that MCs can suppress the growth of tumour cells through an indirect mechanism involving heparin and fibroblasts adjacent to tumour cells has been demonstrated [71].
In addition, several studies showed an anti-tumour effect of low molecular weight heparin in animal models of malignancy, and the improvement of cancer outcome in humans [72, 73].
These data demonstrates the diversity of MC biological functions. Thus, MC could be detrimental to tumour via its components; the tryptase, chondroitin sulphate and cell mediators (e.g. IL-1, IL-4, IL6, TNF-α). Also, MC components, such as histamine, heparin and other growth factors may promote tumour progress and metastasis [74].
This study failed to find any association between the ED and survival, angiogenesis or the other histopathological parameters. In literature, poor and good prognosis has been associated with TATE. Many factors could contribute in the conflicting reports. Counting methodology, number of cases included in the study, biopsy or surgical samples, tumour differentiation and site are examples of the contributing factors. In the current study, and just for our interest, the cases were recounted using the classical method that was described by Lowe & Fletcher [26], and the cases were classified into low, moderate and high TATE. However, neither clinical nor histopathological association was detected.
Tumour-derived eosinophil chemotactic factors and anti-tumour activity of eosinophils have long been described in the literature [75–77]. However, the available data are insufficient. TATE could represent a response to local inflammatory reaction. In vivo studies revealed that MC chymase is a potent stimulus for inflammatory cells recruitment following mast cell activation [78].
In conclusion, from our studies and literature review, there is mounting evidence that accumulating MCs in tumours play a part in inhibiting tumour progression, probably via cytotoxic components and mediators. Also, MCs are potentially angiogenic in tumours. On the other hand, Eos did not show any correlation with the patients’ outcomes and their presence is probably due to potent chemotactic factors released by mast cells.
To the best of our knowledge, this is the first report on the significance of MCs in tongue carcinoma and strongly emphasizes the need for further studies to clarify more of these fascinating biological effects.
References
- 1.Balza E, Castellani P, Zijlstra A, Neri D, Zardi L, Siri A. Lack of specificity of endoglin expression for tumor blood vessels. Int J Cancer. 2001;94:579–85. doi: 10.1002/ijc.1505. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Ellis LM, Fidler IJ. Angiogenesis and metastasis. Eur J Cancer. 1996;32:2451–60. doi: 10.1016/s0959-8049(96)00389-9. [DOI] [PubMed] [Google Scholar]
- 4.Bruce IT, Konstantin VS. VEGF and Tumor Angiogenesis. Einstein Quart. J. Biol. and Med. 2001;18:59–66. [Google Scholar]
- 5.Folkman J. What is the evidence that tumours are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Weidner N. Tumor angiogenesis: review of current applications in tumor prognostication. Semin Diagn Pathol. 1993;10:302–13. [PubMed] [Google Scholar]
- 7.Lopez-Graniel CM, Tamez de Leon D, Meneses-Garcia A, Gomez-Ruiz C, Frias-Mendivil M, Granados-Garcia M, Barrera-Franco JL. Tumor angiogenesis as a prognostic factor in oral cavity carcinomas. J Exp Clin Cancer Res. 2001;20:463–8. [PubMed] [Google Scholar]
- 8.Gleich LL, Biddinger PW, Duperier FD, Gluckman JL. Tumor angiogenesis as a prognostic indicator in T2-T4 oral cavity squamous cell carcinoma: a clinical-pathologic correlation. Head Neck. 1997;19:276–80. doi: 10.1002/(sici)1097-0347(199707)19:4<276::aid-hed5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Williams JK, Carlson GW, Cohen C, Derose PB, Hunter S, Jurkiewicz MJ. Tumor angiogenesis as a prognostic factor in oral cavity tumors. Am J Surg. 1994;168:373–80. doi: 10.1016/s0002-9610(05)80079-0. [DOI] [PubMed] [Google Scholar]
- 10.Artese L, Rubini C, Ferrero G, Fioroni M, Santinelli A, Piattelli A. Microvessel density (MVD) and vascular endothelial growth factor expression (VEGF) in human oral squamous cell carcinoma. Anticancer Res. 2001;21:689–95. [PubMed] [Google Scholar]
- 11.Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: comparison with an anti-CD31 monoclonal antibody. Neuropathology. 2005;25:201–6. doi: 10.1111/j.1440-1789.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 12.Penfold CN, Partridge M, Rojas R, Langdon JD. The role of angiogenesis in the spread of oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 1996;34:37–41. doi: 10.1016/s0266-4356(96)90133-3. [DOI] [PubMed] [Google Scholar]
- 13.Forootan SS, Jones AS, Helliwell TR. Neoangiogenesis and squamous cell carcinoma of the tongue. Cancer Detect Prev. 1999;23:137–46. doi: 10.1046/j.1525-1500.1999.09911.x. [DOI] [PubMed] [Google Scholar]
- 14.Chan JK, Magistris A, Loizzi V, Lin F, Rutgers J, Osann K, DiSaia PJ, Samoszuk M. Mast cell density, angiogenesis, blood clotting, and prognosis in women with advanced ovarian cancer. Gynecol Oncol. 2005;99:20–5. doi: 10.1016/j.ygyno.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 15.Elpek GO, Gelen T, Aksoy NH, Erdogan A, Dertsiz L, Demircan A, Keles N. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54:940–4. doi: 10.1136/jcp.54.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takanami I, Takeuchi K, Naruke M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer. 2000;88:2686–92. [PubMed] [Google Scholar]
- 17.Acikalin MF, Oner U, Topcu I, Yasar B, Kiper H, Colak E. Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig Liver Dis. 2005;37:162–9. doi: 10.1016/j.dld.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Bajdik C, Gelmon K, Chia S, Hayes M. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favourable prognosis. Mod Pathol. 2004;17:690–5. doi: 10.1038/modpathol.3800094. [DOI] [PubMed] [Google Scholar]
- 19.Nagata M, Shijubo N, Walls AF, Ichimiya S, Abe S, Sato N. Chymase-positive mast cells in small sized adenocarcinoma of the lung. Virchows Arch. 2003;443:565–73. doi: 10.1007/s00428-003-0842-y. [DOI] [PubMed] [Google Scholar]
- 20.Tuna B, Yorukoglu K, Unlu M, Mungan MU, Kirkali Z. Association of Mast Cells with Microvessel Density in Renal Cell Carcinomas. Eur Urol. 2006;50:530–4. doi: 10.1016/j.eururo.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 21.Ercan I, Cakir B, Basak T, Ozdemir T, Sayin I, Turgut S. Prognostic significance of stromal eosinophilic infiltration in cancer of the larynx. Otolaryngol Head Neck Surg. 2005;132:869–73. doi: 10.1016/j.otohns.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 22.Ohashi Y, Ishibashi S, Suzuki T, Shineha R, Moriya T, Satomi S, Sasano H. Significance of tumor associated tissue eosinophilia and other inflammatory cell infiltrate in early esophageal squamous cell carcinoma. Anticancer Res. 2000;20:3025–30. [PubMed] [Google Scholar]
- 23.Goldsmith MM, Belchis DA, Cresson DH, Merritt WD, 3rd, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg. 1992;106:27–33. doi: 10.1177/019459989210600124. [DOI] [PubMed] [Google Scholar]
- 24.van Driel WJ, Kievit-Tyson P, van den Broek LC, Zwinderman AH, Trimbos BJ, Fleuren GJ. Presence of an eosinophilic infiltrate in cervical squamous carcinoma results from a type 2 immune response. Gynecol Oncol. 1999;74:188–95. doi: 10.1006/gyno.1999.5431. [DOI] [PubMed] [Google Scholar]
- 25.Takanami I, Takeuchi K, Gika M. Immunohistochemical detection of eosinophilic infiltration in pulmonary adenocarcinoma. Anticancer Res. 2002;22:2391–6. [PubMed] [Google Scholar]
- 26.Lowe D, Fletcher CD, Shaw MP, McKee PH. Eosinophil infiltration in keratoacanthoma and squamous cell carcinoma of the skin. Histopathology. 1984;8:619–25. doi: 10.1111/j.1365-2559.1984.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 27.Samoszuk MK, Nguyen V, Gluzman I, Pham JH. Occult deposition of eosinophil peroxidase in a subset of human breast carcinomas. Am J Pathol. 1996;148:701–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Leighton SE, Teo JG, Leung SF, Cheung AY, Lee JC, van Hasselt CA. Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer. 1996;77:436–40. doi: 10.1002/(SICI)1097-0142(19960201)77:3<436::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986;58:1321–7. doi: 10.1002/1097-0142(19860915)58:6<1321::aid-cncr2820580623>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumorassociated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88:1544–8. [PubMed] [Google Scholar]
- 31.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J. Pathol. 1999;189:487–95. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Grigolato PG, Favret M, Benetti A, Berenzi A, Villanacci V. A case of gastric carcinoma with massive eosinophilia and squamous differentiation. Histochemical, immunohistochemical and ultrastructural aspects. Arch Anat Cytol Pathol. 1990;38:43–7. [PubMed] [Google Scholar]
- 33.Lowe DG. Carcinoma of the cervix with massive eosinophilia. Br J Obstet Gynaecol. 1988;95:393–401. doi: 10.1111/j.1471-0528.1988.tb06613.x. [DOI] [PubMed] [Google Scholar]
- 34.Goldsmith MM, Cresson DH, Askin FB. Theprognostic significance of stromal eosinophilia in head and neck cancer. Otolaryngol Head Neck Surg. 1987;96:319–24. doi: 10.1177/019459988709600403. [DOI] [PubMed] [Google Scholar]
- 35.Pretlow TP, Keith EF, Cryar AK, Bartolucci AA, Pitts AM, Pretlow TG, 2nd, Kimball PM, Boohaker EA. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. 1983;43:2997–3000. [PubMed] [Google Scholar]
- 36.Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002;41:152–7. doi: 10.1046/j.1365-2559.2002.01437.x. [DOI] [PubMed] [Google Scholar]
- 37.Gao J, He Y, Wu L. The clinical analysis of eosinophil infiltration in human squamous cell carcinoma of buccal mucosa. Hua. Xi. Kou. Qiang. Yi. Xue. Za. Zhi. 1997;5:228–9. [PubMed] [Google Scholar]
- 38.Horiuchi K, Mishima K, Ohsawa M, Sugimura M, Aozasa K. Prognostic factors for well-differentiated squamous cell carcinoma in the oral cavity with emphasis on immunohistochemical evaluation. J. Surg. Oncol. 1993;53:92–6. doi: 10.1002/jso.2930530209. [DOI] [PubMed] [Google Scholar]
- 39.Caruso RA, Giuffre G, Inferrera C. Minute and small early gastric carcinoma with special reference to eosinophil infiltration. Histol. Histopathol. 1993;8:155–66. [PubMed] [Google Scholar]
- 40.Lowe D, Fletcher CD. Eosinophilia in squamous cell carcinoma of the oral cavity, external genitalia and anus–clinical correlations. Histopathology. 1984;8:627–3. doi: 10.1111/j.1365-2559.1984.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 41.Srebro Z, Wilinski B, Dziobek K. Mast cell and eosinophil infiltration in various types of solid malignant tumors. Folia Med Cracov. 2004;45:73–8. [PubMed] [Google Scholar]
- 42.Tajima K, Yamakawa M, Inaba Y, Katagiri T, Sasaki H. Cellular localization of interleukin-5 expression in rectal carcinoma with eosinophilia. Hum. Pathol. 1998;29:1024–8. doi: 10.1016/s0046-8177(98)90212-x. [DOI] [PubMed] [Google Scholar]
- 43.Lendrum AC. The staining of eosinophil, polymorphs and enteromaffin cells in histological sections. J. Path. Bact. 1944;56:441. [Google Scholar]
- 44.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumours. Breast Cancer Res Treat. 1995;36:169–80. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 45.Martone T, Rosso P, Albera R, Migliaretti G, Fraire F, Pignataro L, Pruneri G, Bellone G, Cortesina G. Prognostic relevance of CD105+ microvessel density in HNSCC patient outcome. Oral Oncol. 2005;41:147–55. doi: 10.1016/j.oraloncology.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Amar A, Giovanini AF, Rosa MP, Yamassaki HO, Carvalho MB, Rapoport A. Microvascular density in carcinoma of the tongue] Rev Assoc Med Bras. 2002;48:204–8. doi: 10.1590/s0104-42302002000300031. [DOI] [PubMed] [Google Scholar]
- 47.Gleich LL, Biddinger PW, Pavelic ZP, Gluckman JL. Tumor angiogenesis in T1 oral cavity squamous cell carcinoma: role in predicting tumor aggressiveness. Head Neck. 1996;18:343–6. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<343::AID-HED5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 48.Salvesen HB, Gulluoglu MG, Stefansson I, Akslen LA. Significance of CD 105 expression for tumour angiogenesis and prognosis in endometrial carcinomas. APMIS. 2003;111:1011–8. doi: 10.1111/j.1600-0463.2003.apm1111103.x. [DOI] [PubMed] [Google Scholar]
- 49.Kyzas PA, Agnantis NJ, Stefanou D. Endoglin (CD105) as a prognostic factor in head and neck squamous cell carcinoma. Virchows Arch. 2006;448:768–75. doi: 10.1007/s00428-006-0195-4. [DOI] [PubMed] [Google Scholar]
- 50.Pazouki S, Chisholm DM, Adi MM, Carmichael G, Farquharson M, Ogden GR, Schor SL, Schor AM. The association between tumour progression and vascularity in the oral mucosa. J Pathol. 1997;183:39–43. doi: 10.1002/(SICI)1096-9896(199709)183:1<39::AID-PATH1088>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Alkhabuli JO, High AS. Significance of eosinophil counting in tumor associated tissue eosinophilia (TATE) Oral Oncol. 2006;42:849–50. doi: 10.1016/j.oraloncology.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 52.Schimming R, Marme D. Endoglin (CD105) expression in squamous cell carcinoma of the oral cavity. Head Neck. 2002;24:151–6. doi: 10.1002/hed.10040. [DOI] [PubMed] [Google Scholar]
- 53.Carlile J, Harada K, Baillie R, Macluskey M, Chisholm DM, Ogden GR, Schor SL, Schor AM. Vascular endothelial growth factor (VEGF) expression in oral tissues: possible relevance to angiogenesis, tumour progression and field cancerisation. J Oral Pathol Med. 2001;30:449–57. doi: 10.1034/j.1600-0714.2001.030008449.x. [DOI] [PubMed] [Google Scholar]
- 54.Shieh YS, Lee HS, Shiah SG, Chu YW, Wu CW, Chang LC. Role of angiogenic and non-angiogenic mechanisms in oral squamous cell carcinoma: correlation with histologic differentiation and tumor progression. J Oral Pathol Med. 2004;33:601–6. doi: 10.1111/j.1600-0714.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 55.Hannen EJ, van der Laak JA, Manni JJ, Freihofer HP, Slootweg PJ, Koole R, de Wilde PC. Computer assisted analysis of the microvasculature in metastasized and nonmetastasized squamous cell carcinomas of the tongue. Head Neck. 2002;24:643–50. doi: 10.1002/hed.10100. [DOI] [PubMed] [Google Scholar]
- 56.Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang XN, Ansel JC, Butterfield JH, Planck SR, Rosenbaum JT. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995;147:564–73. [PMC free article] [PubMed] [Google Scholar]
- 57.Ribatti D, Crivellato E, Candussio L, Nico B, Vacca A, Roncali L, Dammacco F. Mast cells and their secretory granules are angiogenic in the chick embryo chorioallantoic membrane. Clin Exp Allergy. 2001;31:602–8. doi: 10.1046/j.1365-2222.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 58.Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:195–9. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 59.Ranieri G, Achille G, Florio G, Labriola A, Marzullo F, Paradiso A, Grammatica L. Biologicalclinical significance of angiogenesis and mast cell infiltration in squamous cell carcinoma of the oral cavity. Acta Otorhinolaryngol Ital. 2000;21:171–8. [PubMed] [Google Scholar]
- 60.Ozdemir O. Immunosurveillance function of human mast cell? World J Gastroenterol. 2005;11:7054–6. doi: 10.3748/wjg.v11.i44.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rojas IG, Spencer ML, Martinez A, Maurelia MA, Rudolph MI. Characterization of mast cell subpopulations in lip cancer. J Oral Pathol Med. 2005;34:268–73. doi: 10.1111/j.1600-0714.2004.00297.x. [DOI] [PubMed] [Google Scholar]
- 62.Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y, Zhao LJ. Prognostic significance of cell infiltrations of immunosurveillance in colorectal cancer. World J Gastroenterol. 2005;11:1210–4. doi: 10.3748/wjg.v11.i8.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aaltomaa S, Lipponen P, Papinaho S, Kosma VM. Mast cells in breast cancer. Anticancer Res. 1993;13:785–8. [PubMed] [Google Scholar]
- 64.Sari A, Serel TA, Candir O, Ozturk A, Kosar A. Mast cell variations in tumour tissue and with histopathological grading in specimens of prostatic adenocarcinoma. BJU Int. 1999;84:851–3. doi: 10.1046/j.1464-410x.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- 65.Yano H, Kinuta M, Tateishi H, Nakano Y, Matsui S, Monden T, Okamura J, Sakai M, Okamoto S. Mast cell infiltration around gastric cancer cells correlates with tumor angiogenesis and metastasis. Gastric Cancer. 1999;2:26–32. doi: 10.1007/s101200050017. [DOI] [PubMed] [Google Scholar]
- 66.Ghiara P, Boraschi D, Villa L, Scapigliati G, Taddei C, Tagliabue A. In vitro generated mast cells express natural cytotoxicity against tumour cells. Immunology. 1985;55:317–24. [PMC free article] [PubMed] [Google Scholar]
- 67.Henderson WR, Chi EY, Jong EC, Klebanoff SJ. Mast cell-mediated tumor-cell cytotoxicity. Role of the peroxidase system. J Exp Med. 1981;153:520–33. doi: 10.1084/jem.153.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benyon RC, Bissonnette EY, Befus AD. Tumor necrosis factor-alpha dependent cytotoxicity of human skin mast cells is enhanced by anti-IgE antibodies. J Immunol. 1991;147:2253–2258. [PubMed] [Google Scholar]
- 69.Gallagher SJ, Marshall JS, Hoskin DW. Human mast cells induce caspase-independent DNA fragmentation in leukemic T cells. Oncol Rep. 2003;10:1019–23. [PubMed] [Google Scholar]
- 70.Yang M, Zhang X, He A. Mast cells in the labial cancer: histochemical and electron microscopical study. Zhonghua Kou Qiang Yi Xue Za Zhi. 1997;32:13–5. [PubMed] [Google Scholar]
- 71.Samoszuk M, Kanakubo E, Chan JK. Degranulating mast cells in fibrotic regions of human tumors and evidence that mast cell heparin interferes with the growth of tumor cells through a mechanism involving fibroblasts. BMC Cancer. 2005;5:121. doi: 10.1186/1471-2407-5-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castelli R, Porro F, Tarsia P. The heparins and cancer: review of clinical trials and biological properties. Vasc Med. 2004;9:205–13. doi: 10.1191/1358863x04vm566ra. [DOI] [PubMed] [Google Scholar]
- 73.Zacharski1 Leo R, Ornstein1 Deborah L, Alexander C, Mamourian AC. Low-MolecularWeight Heparin and Cancer. Semin Thromb Hemost. 2000;26:069–078. doi: 10.1055/s-2000-9499. [DOI] [PubMed] [Google Scholar]
- 74.Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235–41. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Wasserman SI, Goetzl EJ, Ellman L, Austen KF. Tumor-associated eosinophilotactic factor. N. Engl. J. Med. 1974;290:420–4. doi: 10.1056/NEJM197402212900802. [DOI] [PubMed] [Google Scholar]
- 76.Slungaard A, Ascensao J, Zanjani E, Jacob H.S. Pulmonary carcinoma with eosinophilia. Demonstration of a tumor-derived eosinophilopoietic factor. N. Engl. J. Med. 1983;309:778–81. doi: 10.1056/NEJM198309293091307. [DOI] [PubMed] [Google Scholar]
- 77.Kataoka S, Konishi Y, Nishio Y, Fujikawa-Adachi K, Tominaga A. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. 2004;23:549–60. doi: 10.1089/dna.2004.23.549. [DOI] [PubMed] [Google Scholar]
- 78.He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br J Pharmacol. 1998;125:1491–500. doi: 10.1038/sj.bjp.0702223. [DOI] [PMC free article] [PubMed] [Google Scholar]