Abstract
Inflammatory fibroid polyps (Vanek's tumor) are rare benign localized lesions originating in the submucosa of the gastrointestinal tract. Intussusceptions due to inflammatory fibroid polyps are uncommon; moreover, ileo-ileal intussusception with small bowel necrosis and perforation has rarely been reported. We report a 56-year-old woman who was admitted two days after complaints of nausea and vomiting. Abdominal examination revealed distension, signs of gastrointestinal perforation and clanging intestinal sounds. The patient underwent a emergency laparotomy which found a 17-cm invaginated mid-ileal segment with necrosis, perforation and fecal peritonitis. The ileal segment was resected and single-layer end-to-end anastomosis was performed. Histopathological analysis showed an ulcerative lesion with variable cellularity, formed by spindle cells with small number of mitosis and an abundant inflammatory infiltrate comprising mainly eosinophils. Immunohistochemistry confirmed the diagnosis of ileal Vanek's tumor. Although inflammatory fibroid polyps are seen very rarely in adults, they are among the probable diagnoses that should be considered in obstructive tumors of the small bowel causing intussusception with intestinal necrosis and perforation.
Key Words: Intestinal polyps, Polyps, Ileal neoplasm, Intussusception, Intestinal obstruction, Intestinal perforation, Immunohistochemistry
Introduction
Inflammatory fibroid polyps (IFPs) are rare, localized, non-neoplastic lesions originating in the submucosa of the gastrointestinal tract. The disease was first described in the stomach by Vanek in 1949 [1] and is a benign, non-encapsulated lesion, composed mainly of loose connective tissues, vessels and with an eosinophilic inflammatory component [2]. The involvement of the small intestine and colon by IFPs is rare. Patients with IFPs located on the small bowel usually present with episodes of relapsing abdominal colics and intermittent episodes of intestinal bowel intussusceptions [3, 4, 5, 6, 7, 8]. Although malignant diseases represent the major causes of intussusceptions in adults, there are few reports of intestinal obstruction and perforation caused by IFPs [3, 4, 7, 8].
We report an unusual case of intestinal obstruction complicated by necrosis and perforation due to ileo-ileal intussusception caused by an IFP, whose diagnosis was confirmed by immunohistochemistry.
Case Report
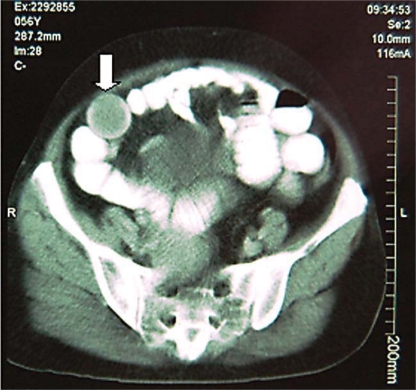
A 56-year-old lady was admitted with a 45-day history of post-alimentary intermittent and generalized abdominal colics. A CT scan of the abdomen showed the presence of a soft tissue largest intraluminal mass located in the distal ileum with signs of intermittent ileo-ileal intussusception (fig. 1). Two days after the CT scan the patient was admitted to the emergency department with stasis vomiting, abdominal distension and no elimination of flatus or feces. Abdominal examination revealed intense and diffuse abdominal pain with distension and clanging intestinal sounds. Digital rectal examination did not show the presence of feces, mucus or blood. Laboratory investigation showed leukocytosis (15,600/mm3), Hb 15.6 g/dl, Htc 48%, creatinine 2.8 mg/dl, urea 82 mg/dl, sodium 145 mmol/l and potassium 3.2 mmol/l, and arterial gasometry showed metabolic acidosis. Plain films of the abdomen disclosed multiple intestinal air-fluid levels and pneumoperitoneum. A provisional diagnosis of intestinal obstruction with perforation due to intestinal intussusception was made.
Fig. 1.
CT scan showing the IFPs inside the lumen of the distal ileum (arrow).
Upon passage of a gastric and vesical tube, appropriate fluid resuscitation and correction of metabolic acidosis, the patient underwent emergency laparatomy via a midline incision. On abdominal exploration, was found an ileo-ileal intussusceptions 50 cm proximal to the ileocecal valve. The ileal intussuscepted segment was obstructing the intestinal lumen, causing dilatation in the proximal bowel. We noted inside the intussuscepted ileum a nodular pedunculated mass, 4 cm in maximal diameter, which extended to the intestinal serosal surface in the perimeter of the lesion. Near the invaginated segment we found a necrotic area approximately 5 cm in length with a central perforation by where intestinal contents leaked with generalized fecal peritonitis. The intussuscepted ileal segment was resected and the fecal stream was re-established by a single-layer end-to-end anastomosis. The peritoneal cavity was thoroughly washed with warm saline solution and closed by layers without drainage. The patient received parenteral antibiotic therapy with ciprofloxacin and metronidazole, had an uneventful postoperative course and was discharged on the sixth postoperative day.
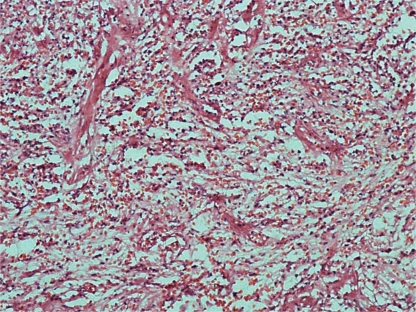
Macroscopic examination of the resected ileal segment revealed a dumbbell-shaped, circumscribed fleshy white mass measuring 4.5 cm in diameter. This lesion was distant from the lower 6.5 cm surgical margins, extended into the mucosa forming an ulcerated polyp, and nearby there was a blackened area in the intestinal wall with a small central perforation. Microscopic examination revealed an ulcerative lesion with variable cellularity, formed by spindle cells having bland nuclei and clear cytoplasm with a small number of mitosis. These cells were surrounded by florid connective tissue proliferation arising from the submucosa and inner muscularis with distortion and reaction of the overlying serous mambrane (fig. 2). There was an abundant inflammatory infiltrate comprising plasma cells, lymphocytes and eosinophils. The lesion involved the entire thickness of the bowel wall with extensive ulceration of the overlying mucosa. Among the spindle cells we observed blood vessels with thickened walls and vessel congestions at the submucosa and mucosa.
Fig. 2.
Photomicroscopy of IFP showing spindle cells with bland nuclei and clear cytoplasm with connective tissue proliferation arising from the submucosa. Abundant inflammatory infiltrate comprising plasma cells, lymphocytes and eosinophils. HE. ×200.
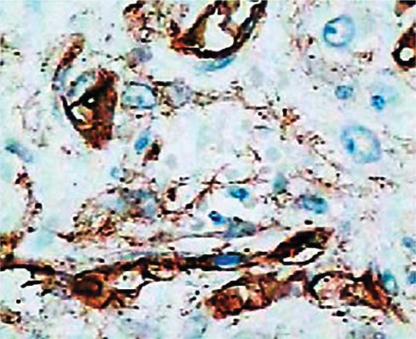
On immunohistochemistry, the spindle cells were negative for cytokeratins, CD117 (c-kit), S100 protein, actin, desmin, and focally positive for CD34 (antigen of hematopoietic cells and pericytes – QBEnd10) (fig. 3). The morphological features were typical of IFP (Vanek's polyp) and the immunoprofile was consistent with this diagnosis. Cellular proliferation was studied using Ki-67 immunohistochemical assay and showed rare mitotic figures. The diagnosis of IFP with small cellular proliferation could be made. The patient recovered without complications and remained well and symptom-free 14 months after discharge.
Fig. 3.
Focal positive immunostaining for monoclonal CD34 primary antibody of hematopoietic cells and pericytes. IH. ×200.
Discussion
IFPs are rare benign non-neoplastic lesions of the gastrointestinal tract [8]. The first six cases of the disease were described in 1949 as a submucosal granuloma of the stomach with eosinophilic infiltration [1]. They later became recognized under a variety of different names: inflammatory pseudotumor, granuloma with eosinophils, eosinophilic granuloma, and polyp with eosinophilic granuloma [4]. Helwig and Ranier in 1953 [9] introduced the term IFP to define the disease. IFP affects both sexes and all age groups, with a peak of incidence in the fifth and sixth decades [4, 5]. At the time of diagnosis most IFPs, as in the present patient, have a diameter of 3 to 4 cm, however, there is also a report of a case with an IFP 12.5 cm in size [10]. The lesions have always been recorded as solitary polyps.
IFPs can develop anywhere in the gastrointestinal tract but occur most commonly in the stomach (about 70% of cases), mainly in the gastric antrum and less frequently in the intestine [8]. The occurrence of IFPs in the small bowel IS rare, accounting for only 18% of all cases, and less frequent in the ileum [11]. The ileum segment is the most common site where these polyps cause intussusception.
The symptoms are dependent on size and localization in the gastrointestinal tract. Patients with IFPS in the small bowel are most likely to present with chronic episodes of colicky abdominal pain, lower gastrointestinal bleeding, anemia and, more rarely, intestinal obstruction due to episodes of intestinal intussusception [11]. This case is one of the few reported in the literature where intestinal obstruction was caused by ileo-ileal IFP intussusceptions with necrosis and perforation [1, 2].
Benign tumors as the lead points of intussusceptions have been found to include lipomas, leiomyomas, neurofibromas, adenomas, and more rarely IFPS [3, 12]. Sixty-five percent of all adult intussusceptions occur due to a malignant or benign lesion usually appearing at the head of the invagination [3]. Malignant lesions are found in 43 to 80% of intussusceptions located in the colon compared to only about 14 to 47% of cases occurring in the small intestine. Recent review of cases of ileo-ileal intussusceptions due to IFPs published in English found only eight cases [4]. A review of eight cases of IFP found three cases located in the small bowel, two of which evolved with intestinal intussusceptions [13]. A preoperative diagnosis of intussusception, as done in the present patient, is rare but can be made on finding a palpable mass in the abdomen or with the use of imaging techniques [13]. The majority of intussusceptions are only diagnosed during surgery.
The sensitivities of the different radiological methods for diagnosis of intestinal intussusceptions show considerable variation. The primary imaging modality of choice is ultrasound scanning, which enables the diagnosis or exclusion of intussusception with a sensitivity of 98 and 100%, respectively, a specificity of 88%, and a negative predictive value of 100%. With CT scan the mean sensitivity, specificity, positive predictive value, and negative predictive value for diagnosing surgical enteroenteric lesions using a measured lesion length of >3.5 cm were 100, 57.3, 5.7, and 100%, respectively. Similarly, the figures for polyps with a measured axial diameter >3 cm were 100, 32.9, 3.7 and 100%, respectively [14]. Magnetic resonance imaging (MRI) findings may be useful to diagnose intestinal intussusceptions. A study comparing MRI and CT for the diagnosis of intestinal obstruction showed that the cause of obstruction was correctly diagnosed by CT in 71% and by MRI in 95% of cases. The sensitivity, specificity and accuracy for MRI imaging was 95, 100 and 96%, respectively as compared to 71, 71 and 71% for helical CT [15].
The etiology and pathogenesis of IFP remains unknown, but it could be a consequence of chronic irritation and inflammation or a consequence of extreme reaction of the body to an intestinal trauma or a localized variant of eosinophilic gastroenteritis, given that it has a marked eosinophilic infiltration [11]. Based on histological examination alone, some authors initially believed that IFP was a true neoplasm of either neural or vascular origin. However, immunohistochemical studies refuted this possibility when it showed negative staining for S100 protein and factor VII-related antigen in proliferating cells [16]. Other immunohistochemical markers such as desmin and actin strongly suggest that these cells are not smooth muscle cells. It was suggested that IFP with concentric stromal proliferation may originate from a population of dendritic interstitial cells. An immunohistochemical study of 9 cases of IFP showed a strong positivity for vimentin and the absence of staining for macrophages (HAM-56) expression in all cases evaluated suggests a major component of spindle cells best recognizable as fibroblasts [13]. According to these different patterns of immunostaining there is a possibility of the existence of a span of morphological and immunohistochemical patterns indicating different phases of inflammatory reaction [13]. We found negative immunohistochemical staining for cytokeratin, smooth muscle actin, vimentin, desmin, S100 protein, CD117 (KIT gene product) and focal positivity for the antigen CD34, suggesting that IFP may develop from primitive perivascular or vascular cells.
Exploratory laparotomy is frequently recommended as the best treatment for intussusceptions caused by IFP. The lesion seems to lack malignant potential and recurrence of the polyp has been reported only once [17]. The operation should be performed as early as possible in order to prevent the intussusceptions from leading to ischemia, necrosis and subsequent perforation of the invaginated bowel segment. When surgery is delayed and intestinal perforation with peritonitis occurs, there is a considerable increase in morbidity and mortality. Fortunately, the patient in this report, despite having been operated on an emergency basis in the presence of an intestinal perforation and fecal generalized peritonitis, recovered satisfactorily after surgery and was discharged after 6 days, showing no signs of recurrence 14 months after surgery.
Footnotes
This work was carried out within the Postgraduate Health Sciences Program of São Francisco University, Bragança Paulista, São Paulo, Brazil.
References
- 1.Vanek J. Gastric submucosal granuloma with eosinophilic infiltration. Am J Pathol. 1949;25:397–411. [PMC free article] [PubMed] [Google Scholar]
- 2.Ng C, Lam KY, Gupta TS, Ho YH. Inflammatory fibroid polyp of the caecum in a patient with neurofibromatosis. Ann Acad Med. 2004;33:797–799. [PubMed] [Google Scholar]
- 3.Nkanza NK, King M, Hutt MSR. Intussusception due to inflammatory fibroid polyps of the ileum: a report of 12 cases from Africa. Br J Surg. 1980;67:271–274. doi: 10.1002/bjs.1800670414. [DOI] [PubMed] [Google Scholar]
- 4.Mohamud SO, Motorwala AS, Daniel AMR, Tworek JA, Shehab TM. Giant ileal inflammatory fibroid polyp causing small bowel obstruction: a case report and review of the literature. Cases J. 2008;1:341. doi: 10.1186/1757-1626-1-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costamagna D, Erra S, Zullo A, Servente G, Durando R. Small bowel intussusception secondary to inflammatory fibroid polyp of the ileum: report of a case. Chir Ital. 2008;60:323–327. [PubMed] [Google Scholar]
- 6.El Hajj II, Sharara AI. Jejunojejunal intussusception caused by an inflammatory fibroid polyp. Case report and review of the literature. J Med Liban. 2007;55:108–111. [PubMed] [Google Scholar]
- 7.O'Kane AM, O'Donnell ME, McCavert M, Taylos K, Lee J, Wilkinson AL. Inflammatory fibroid polyp of the ileum causing recurrent intussusception and chronic ischaemia: a case report. Cases J. 2008;1:244. doi: 10.1186/1757-1626-1-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehman S, Gamie Z, Wilson TR, Coup A, Kaur G. Inflammatory fibroid polyp (Vanek's tumour), an unusual large polyp of the jejunum: a case report. Cases J. 2009;2:7152. doi: 10.1186/1757-1626-2-7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helwig EB, Ranier A. Inflammatory fibroid polyps of the stomach. Surg Gynecol Obstet. 1953;96:335–367. [PubMed] [Google Scholar]
- 10.Harned RK, Buck JL, Shekitha KM. Inflammatory fibroid polyps of the gastrointestinal tract: radiologic evaluation. Radiology. 1992;182:863–866. doi: 10.1148/radiology.182.3.1535909. [DOI] [PubMed] [Google Scholar]
- 11.Barussaud M, Regenet N, Briennon X, de Kerviler B, Pessaux P, Kohneh-Sharhi N, Lehur PA, Hamy A, Leborgne J, le Neel JC, Mirallie E. Clinical spectrum and surgical approach of adult intussusceptions: a multicentric study. Int J Colorectal Dis. 2006;21:834–839. doi: 10.1007/s00384-005-0789-3. [DOI] [PubMed] [Google Scholar]
- 12.Karamercan A, Kurukahvecioglu O, Yilmaz TU, Aygencel G, Aytaç B, Sare M. Adult ileal intussusception: an unusual emergency condition. Adv Ther. 2006;23:163–168. doi: 10.1007/BF02850357. [DOI] [PubMed] [Google Scholar]
- 13.Santos Gda C, Alves VAF, Wakamatsu A, Zucoloto S. Inflammatory fibroid polyp. An immunohistochemical study. Arq Gastroenterol. 2004;41:104–107. doi: 10.1590/s0004-28032004000200007. [DOI] [PubMed] [Google Scholar]
- 14.Sundaram B, Miller CN, Cohan RH, Schipper MJ, Francis IR. Can CT features be used to diagnose surgical adult bowel intussusceptions? AJR Am J Roentgenol. 2009;193:471–478. doi: 10.2214/AJR.08.1801. [DOI] [PubMed] [Google Scholar]
- 15.Beall DP, Fortman BJ, Lawler BC, Regan F. Imaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomography. Clin Radiol. 2002;57:719–724. doi: 10.1053/crad.2001.0735. [DOI] [PubMed] [Google Scholar]
- 16.Navas-Palacios JJ, Colina-Ruizdelgado F, Sanchez Larrea MD, Cortes Causino J. Inflammatory fibroid polyp of the gastrointestinal tract: an immunohistochemical and electron microscopic study. Cancer. 1983;51:1682–1690. doi: 10.1002/1097-0142(19830501)51:9<1682::aid-cncr2820510921>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Yakan S, Caliskan C, Makay O, Denecli AG, Korkut MA. Intussusception in adults: clinical characteristics, diagnosis and operative strategies. World J Gastroenterol. 2009;15:1985–1989. doi: 10.3748/wjg.15.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]