Abstract
Objective:
The purpose of this pilot study was to evaluate the feasibility and tolerability of weekly intratumoral TNFerade™ injections combined with concurrent capecitabine and radiotherapy in the treatment of patients with locally advanced rectal cancer.
Methods
Patients with T3, T4, or N+ rectal cancer received radiotherapy to a total dose of 50.4–54 Gy in combination with capecitabine 937.5 mg/m2 p.o. b.i.d. TNFerade™ at a dose of 4 × 1010 particle units was injected into the rectal tumor on the first day of radiotherapy and weekly for a total of 5 injections. Surgery was performed 5–10 weeks after the completion of chemoradiation.
Results
Nine patients were enrolled in this pilot trial. The stage was cT2 in 2 patients, cT3 in 6 patients, cT4 in 1 patient, N– in 7 patients and N+ in 2 patients. Eight patients completed all treatments. Grade 3 hematologic toxicity was observed in 2 patients. There was no toxicity directly attributable to the injection procedure. A complete pathologic response was observed in 2 of 9 patients.
Conclusions
This study demonstrates the feasibility of weekly intratumoral TNFerade™ injections during chemoradiotherapy for locally advanced rectal cancer. Pathologic responses with this combination compare favorably to published rates.
Key Words: Rectal carcinoma, Radiation, Neoadjuvant, TNFerade™
Introduction
Over 40,000 cases of adenocarcinoma of the rectum occurred in the United States in 2008 [1]. Patients with locoregionally advanced disease (T3, T4, or N+) have a high risk of local recurrence if treated with surgical therapy alone [2, 3, 4]. Concurrent 5-fluorouracil-based chemoradiotherapy improves survival and local control after surgical resection of locally advanced rectal cancer [2, 5, 6]. Treatment of rectal cancer with chemotherapy and radiation in a neoadjuvant setting further improves local control and reduces toxicity compared to postoperative chemoradiation [7]. Because local recurrences are morbid and difficult to manage effectively, radiation is being evaluated with new agents delivered alone or with additional adjuvant therapy to improve outcomes and reduce toxicity. Neoadjuvant chemoradiation may also increase the number of patients eligible for sphincter-preserving surgery [7]. Patients in whom sphincter-preserving surgery is not possible due to technical factors may also benefit from neoadjuvant therapy [7].
Tumor necrosis factor-α (TNF-α) is a soluble cytokine that mediates cellular immune response and is cytotoxic to tumor cell lines [8]. Intratumoral delivery of TNF-α in combination with radiation has been shown to provide additive or greater than additive effects in human tumor xenografts [9, 10, 11]. A phase I clinical trial of systemic TNF-α in combination with radiation in patients with locally advanced and metastatic tumors has shown promising response rates; however, severe idiosyncratic toxicity required discontinuation of therapy in a large proportion of patients [12]. Major toxicities with this approach are independent of the dose of TNF-α delivered and include angina, respiratory distress, arrhythmias, allergic reactions, and leukopenia.
Efforts to deliver TNF-α locally to tumors to minimize systemic effects have included the generation of TNFerade™ biologic, and E1-, E4-, and partial E3-deleted replication-deficient adenovirus type 5 vector. TNFerade™ contains the human TNF-α cDNA with a portion of the EGR-1 chemoradiation-inducible promoter ligated upstream. Preclinical studies have shown that radiation combined with TNFerade™ vector administration was associated with a 5-fold increase in TNF-α levels and a delay in tumor growth compared with tumors treated with vector alone. Similar results have been obtained in studies combining TNFerade™ with chemotherapeutic agents [13, 14]. TNFerade™ has been combined with radiotherapy in patients with advanced solid tumors and sarcoma and with chemoradiotherapy in patients with locally advanced pancreatic cancer [15, 16, 17].
This clinical trial was designed to assess the feasibility and safety of delivering TNFerade™ biologic in combination with capecitabine and radiation in the neoadjuvant setting in patients with locally advanced or recurrent rectal cancer. The delivery of intratumoral injections concurrent with chemotherapy and radiation raises technical and logistical challenges, and a pilot trial was felt to be the most effective way to test if this approach was technically and logistically feasible while also allowing a preliminary estimate of safety in a small patient subset. This trial was undertaken with the goal of an eventual development of a randomized registration trial to evaluate this combination compared to the current standard of care. Pathologic response and tumor regression were included as endpoints in this trial to determine if they may be suitable endpoints for a future randomized trial with this combination.
Patients and Methods
Patients
Patients older than 18 years, with histologically confirmed, nonmetastatic T3, T4, or locally recurrent adenocarcinoma of the rectum, were eligible for this study. Patients with regional lymph node involvement were also eligible for this study. All study participants were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, adequate hematologic (absolute neutrophil count ≥1,500/mm3; platelets of ≥100,000/mm3), renal (serum creatinine of ≤2.0 mg/dl), hepatic (total bilirubin ≤2.0 mg/dl, aspartate aminotransferase/alanine aminotransferase <2.5 × upper limit of normal), and coagulopathic [international normalized ratio ≤1.5 and partial thromboplastin time (PTT) <upper limit of normal] function. Patients who had received prior radiotherapy for rectal cancer or who had a history of coagulopathy, cerebrovascular disease, thrombotic or embolic disorders were excluded. They were required to be suitable candidates for surgery and could not be receiving chronic corticosteroids. The study protocol was approved by the Institutional Review Board of the National Cancer Institute (NCI) and thus meets the standards of the Declaration of Helsinki in its revised version of 1975 and its amendments of 1983, 1989, and 1996. All patients provided informed consent before enrollment.
Treatment Plan
All patients underwent computed tomography (CT) scan of the chest, abdomen, and pelvis and MRI or CT of the brain prior to enrollment. Transrectal ultrasound or pelvic MRI with an endorectal coil and colonoscopy were obtained within 4 weeks of study entry. Duplex ultrasound of the lower extremities was obtained to rule out deep venous thrombosis in all patients at the time of enrollment. Diverting colostomy was performed if required for symptoms of obstruction prior to initiation of therapy. The anticipated feasibility of a sphincter-preserving resection was scored for each patient prior to initiation of neoadjuvant therapy by an experienced colorectal surgeon.
Patients were treated with oral capecitabine at a dose of 937.50 mg/m2 b.i.d. (Monday through Friday) delivered concurrently with external beam irradiation. Capecitabine was initiated on the first day of irradiation and continued until the final day of radiation. Radiotherapy was delivered in daily fractions of 1.8 Gy (Monday through Friday). It was delivered to the tumor, sacral hollow, and pelvic lymph nodes at a dose of 45 Gy followed by a 5.4- to 9-Gy boost to the sacral hollow and gross tumor with margin for a total tumor dose of 50.4–54 Gy.
TNFerade™ at a dose of 4 × 1010 particle units in 2 ml of injectate was delivered locally into the rectal tumor via endoscopy or with transrectal ultrasound guidance with an 18-gauge deployable injection needle array with machined submillimeter side holes in each array tip (Quadrafuse™ or Quadra-fuse ST Multi-Pronged Injection Needle; Rex Medical, Radnor, Pa., USA) [18]. For tumors smaller than the needle array, a single 20-gauge custom order needle with 3 side holes and no end hole was used for injections (Bernardino; Cook Medical, Indianapolis, Ind., USA). Injections were performed under direct ultrasound visualization with multiple injections and rotations and retractions of the needles in order to obtain a broad coverage of the tumor with the 2-ml volume. Injections were performed on the first day of radiotherapy and at weekly intervals thereafter for a total of 5 injections. Patients received local anesthesia and/or intravenous conscious sedation or deep sedation for the injections.
Toxicities were graded using the NCI Common Toxicity Criteria version 3. Symptomatic treatment and appropriate medical management of all toxicities were performed (i.e. antidiarrheal therapy with loperamide). Capecitabine dosing was modified as follows with the exception that dose modifications were not made for lymphopenia alone. Capecitabine was interrupted for all grade 2 or greater toxicity until resolved to grade 0–1. Dose modifications upon resumption of capecitabine for grade 2 toxicity were as follows: first occurrence no dose reduction, second occurrence 25% dose reduction, third occurrence 50% dose reduction, and fourth occurrence discontinue permanently. Percent dose reductions were based on the starting dose of capecitabine. For grade 3 toxicities, a 25% dose reduction was used following the first occurrence and a 50% dose reduction was used for a second occurrence. Capecitabine was discontinued for a third occurrence of grade 3 toxicity. For grade 4 toxicity, capecitabine was discontinued or the dose reduced by 50% at the judgment of the treating physician. A second occurrence of grade 4 toxicity required permanent discontinuation of capecitabine.
Weekly evaluations during chemoradiotherapy included clinical evaluations, assessment of toxicity, and laboratory testing [complete blood count, serum electrolyte panel, BUN, creatinine, prothrombin time (PT)/PTT]. Seven weeks after completion of chemoradiotherapy, patients underwent restaging with a CT of the chest, abdomen, and pelvis, complete blood count, serum electrolytes, BUN, creatinine, PT/PTT, and carcinoembryonic antigen. Surgical resection of the rectal tumor was accomplished with a sphincter-preserving low anterior resection if possible. An abdominoperineal resection (APR) was performed if necessary to achieve clear margins. Surgery was delayed in patients with grade 3 or greater radiation toxicity or grade 4 or greater hematologic toxicity until toxicity resolved to grade 2 or less.
Following at least a 4-week recovery period after surgery (range 4.3–8 weeks), patients underwent staging evaluation including a CT of the chest, abdomen, and pelvis, complete blood count, serum chemistries (electrolytes, renal function, hepatic function), PT/PTT, and carcinoembryonic antigen level. Patients with no evidence of metastatic or recurrent disease began 5-fluorouracil-based adjuvant chemotherapy off protocol.
Pharmacokinetics
Plasma samples were collected just prior to the first administration of TNFerade™ and 15 min, 60 min, 3 h, and 18–24 h after the first administration. Plasma samples were also collected at 2 and 4 weeks following completion of therapy. They were processed immediately and stored at −80°C until further use.
A TaqMan™-based assay was used for pharmacokinetic studies. The assay detects a specific sequence of the adenovirus vector DNA sequence. The 5′-PCR primer, the 3′-PCR primer, and the fluorescently labeled probe are located in the transcriptionally inert spacer region of the vector sequence. The lower limit of detection of the assay is 10 copies of vector/1 μg of human genomic DNA and the lower limit of quantification is 50 copies of vector/ 1 μg of human genomic DNA. A standard curve was prepared with vector diluted in human genomic DNA with concentrations ranging from 10 to 1 × 106 vector copies/μg of human DNA.
DNA was extracted from 100 μl of serum and eluted into 100 μl of resuspension buffer using the MagAttract Virus Mini Kit with the infectious disease package application (Qiagen, Valencia, Calif., USA). Genomic DNA (1 μg) was added to serum samples as a carrier. Normal control serum was purified with each batch to rule out cross-contamination. PCR amplification was performed with the ABI PRISM 7700 Sequence Detection System using PCR master mix (Applied Biosystems, Foster City, Calif., USA). Samples were run in triplicate with one replicate spiked with vector to rule out the presence of PCR inhibitors. Each plate was run with standards and additional controls including genomic DNA control, no template control, and the extraction control. A standard curve was generated by plotting the CT value versus copy number per standard sample. The mean copy number for each sample was calculated from the standard curve.
Assessment of Pathologic Response
Characteristics of tumor regression at the time of resection are described in table 1. Following surgery, the tumor was fixed and processed per standard institutional guidelines. Pathologic response was determined by a single pathologist with expertise in gastrointestinal pathology for all cases. The entire gross tumor and fibrotic area of the specimen were evaluated microscopically for the percent of viable tumor remaining (fig. 1). Pathologic response was evaluated with the rectal cancer regression grade (RCRG) [19]. In brief, RCRG1 responses had only microscopic foci of tumor with marked fibrosis, RCRG2 responses exhibited marked fibrosis with residual gross disease, and RCRG3 responses exhibited little or no fibrosis with abundant macroscopic disease. Complete pathologic response was defined as the absence of viable tumor cells in all evaluated sections.
Table 1.
Operative characteristics following TNFerade™ during chemoradiation for rectal cancer in 9 subjects
| n | |
|---|---|
| Sphincter-sparing operation predicted before treatment | 5 |
| Sphincter-sparing operation feasible at surgery | 8 |
| Complete pathologic response | 2 |
| Rectal cancer regression grade | |
| RCRG1 | 7 |
| RCRG2 | 2 |
| RCRG3 | 0 |
| Margin-positive resection | 0 |
Fig. 1.
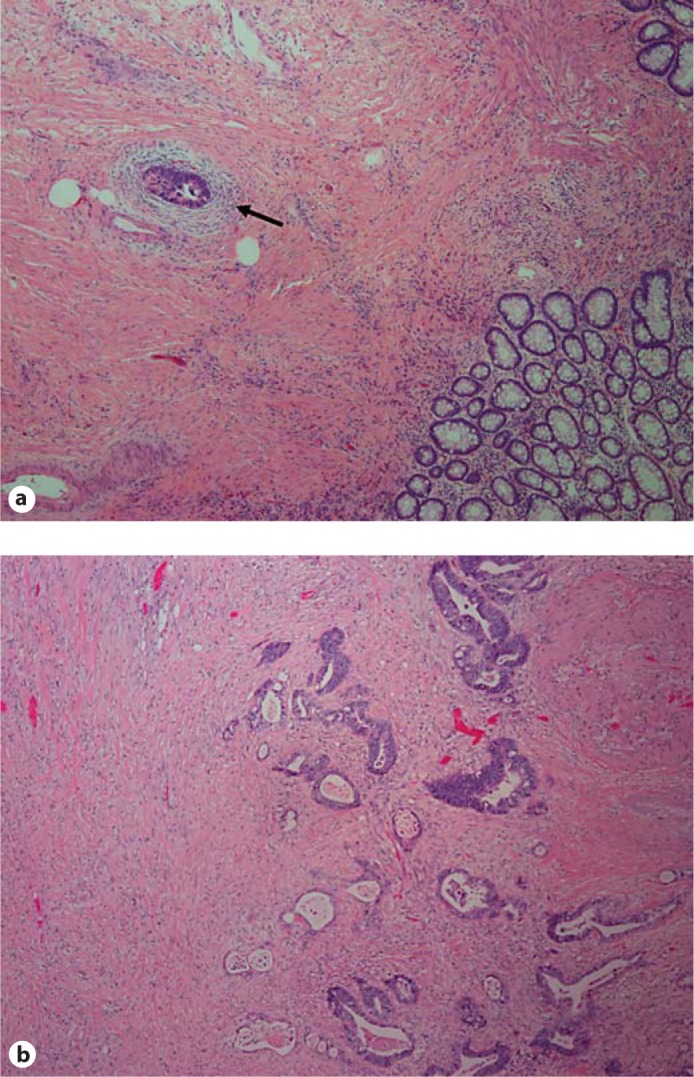
Pathologic response after treatment. Pathologic response was evaluated in all patients. Two representative hematoxylin and eosin-stained sections are shown (40× magnification). a At the time of surgical resection and pathologic evaluation, no gross tumor was identified. Microscopically, there was marked fibrosis with small foci of residual tumor (arrow). This response was classified as RCRG1. b Gross inspection revealed an ulcerated, firm mass consistent with tumor. Microscopically, there was fibrosis with residual tumor. This response was classified as RCRG2.
Assessment of Recurrence
Staging assessments consisted of history and physical exam as well as CT scans of the chest, abdomen, and pelvis every 12 weeks for 36 months and thereafter as per best clinical practice. Patient evaluations are scheduled to continue until 15 years following therapy. Recurrences were assessed by follow-up radiographic evaluation ≥4 weeks after the initial response criteria were met. Additional imaging of other sites was obtained in follow-up only if clinically indicated. Surveillance colonoscopy was performed at yearly intervals.
Results
Patient Characteristics
Nine patients (6 men and 3 women) were enrolled in this pilot study. Characteristics of the patients are described in table 2. Two patients had T2 disease, 6 had T3 disease and 1 had T4 disease, and 2 patients were node positive. Four patients were predicted to require an APR by the treating surgeon prior to chemoradiotherapy. No patient had detectable anal sphincter involvement prior to treatment.
Table 2.
Patient characteristics
| Sex | |
| Male | 6 |
| Female | 3 |
| Age, years | mean 42 (range 31–61) |
| Pretreatment stage | |
| T2 | 2 |
| T3 | 6 |
| T4 | 1 |
| Recurrent | 1 |
| Nl | 1 |
| N2 | 1 |
Pharmacokinetics
Complete pharmacokinetic data was available for 8 patients. In 1 patient, the vector was not detected at any time point. In 2 of 8 patients, the plasma vector was detectable in quantifiable levels. For these 2 patients, the corresponding values detected at 15 min after the first injection were 93 and 129 copies of TNFerade/μl of plasma. In these same 2 patients, the level of detectable vector at 60 min after the injection on week 1, day 1 was 6 and 3 copies of TNFerade/μl of plasma, respectively. In 5 of 8 patients, the vector was detectable but below the limits of quantification. In all instances in which the vector was detectable, it was only found in the 1st hour after injection on week 1, day 1. No vector was detected at week 3, week 5, and week 2 and 4 posttreatment plasma samples in any patient.
Feasibility
The primary endpoint of this study was to evaluate the feasibility of intratumoral delivery TNFerade™ biologic with concurrent capecitabine and radiotherapy. This approach required coordination and cooperation between multiple services including medical oncology, surgical oncology, radiation oncology, and interventional radiology. Anatomic location of tumor in some cases altered the approach for delivery of injectate (transrectal ultrasound versus endoscopy), but with these two approaches injection was accomplished successfully in all patients. Location of tumor, stage of tumor, and other patient and tumor characteristics did not interfere with the ability to deliver intratumoral injections successfully. No treatment or injection was postponed or cancelled for logistical reasons.
Safety
Eight patients completed all treatments. CTC V3.0 toxicity is described in table 3. Two patients experienced capecitabine-related grade 3 hematologic toxicity. One of these grade 3 hematologic toxicities occurred concurrent with ileitis requiring discontinuation of capecitabine and radiotherapy at 4,320 cGy. Capecitabine dose reductions occurred in 3 other patients for hematologic or gastrointestinal toxicity. Radiotherapy was completed without delay in 8 patients (5,040–5,400 cGy).
Table 3.
Toxicity
| Grade |
||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Hematologic | ||||
| Thrombocytopenia | − | − | − | − |
| Anemia | − | 1 | 2 | − |
| Leukopenia | − | − | 2 | 1 |
| Lymphopenia | − | − | 6 | 2 |
| Neutropenia | − | − | 2 | 1 |
| Constitutional | ||||
| Fatigue | 2 | 3 | − | − |
| Rigors | 2 | − | − | − |
| Fever | 5 | 1 | − | − |
| Anorexia | 3 | 1 | − | − |
| Gastrointestinal | ||||
| Nausea | 3 | 3 | − | − |
| Diarrhea | 1 | 2 | 2 | − |
| Enteritis | − | − | 1 | − |
| Small bowel obstruction | 1 | − | 2 | − |
| Vomiting | 2 | 1 | − | − |
| Abdominal pain | − | 4 | 1 | |
| Anorectal pain | 1 | − | − | − |
| Metabolic | ||||
| Liver function abnormalities | 1 | − | 2 | − |
| Hypophosphatemia | − | − | 4 | − |
| Hyponatremia | − | − | 4 | − |
| Vascular | ||||
| Epistaxis | 1 | − | − | − |
| Thrombosis | − | Ia | − | − |
| Dermatologie – rash | 3 | 2 | − | − |
Numbers represent the total number of patients experiencing each grade of toxicity.
Venous access related.
No toxicity was attributable to the injection procedure. Specifically, there were no complications of bleeding or clinically evident injection site reactions. Of note, constitutional toxicities were higher than would be expected for capecitabine and radiotherapy; however, these toxicities were all grade 1–2. One grade 2 catheter-associated thrombosis was observed in a patient who had a port placed after surgery for the purpose of receiving additional chemotherapy. There were no other thrombotic events. No correlation was observed between quantifiable or detectable vector and any previously described TNF toxicity including constitutional symptoms.
Response and Recurrence
Surgery was performed between 5 and 9 weeks after completion of therapy. It was delayed in 1 patient until 10 weeks due to complications of cystic fibrosis thought to be unrelated to therapy. Four patients were deemed to require APR prior to therapy; however, after neoadjuvant therapy only 1 patient required an APR and the remaining 8 patients successfully underwent sphincter-sparing surgeries. Two of 9 patients achieved a complete pathologic response. Using RCRG, 7 of 9 patients were scored as RCRG1 and 2 of 9 patients were scored as RCRG2. All margins were negative in all patients.
With a median potential follow-up of 41.6 months, only 1 patient has died at 39 months after study entry from an epicardial metastasis. Two patients recurred at distant sites. One patient developed pulmonary metastases 15.5 months after completion of local therapy. These pulmonary recurrences were controlled with wedge resections. This patient subsequently had an additional local recurrence at 39.5 months. A 2nd patient developed disseminated distant failure without evidence of local failure. Two patients failed locally, 1 described above and the other at 18 months after completion of local therapy.
Discussion
The addition of more active agents may increase the efficacy of chemoradiotherapy for patients with locally advanced rectal cancer, leading to enhanced local control or an increased ability to obtain a margin-negative resection with sphincter preservation. This pilot study demonstrated the safety and feasibility of delivering intratumoral TNFerade™ injections weekly during the course of chemoradiotherapy in the neoadjuvant setting for locally advanced rectal adenocarcinoma.
The most common grade 3 or 4 toxicities observed in this cohort of patients were consistent with prior experience with pelvic radiation and capecitabine and consisted primarily of leukopenia, lymphopenia, and diarrhea. Fever, rigors, and fatigue occurred more frequently than would be expected with capecitabine and radiotherapy alone; however, these toxicities were grade 1 or 2 in all cases and self-limited. A single case of ileitis requiring discontinuation of therapy near the total dose of radiotherapy occurred. Although it is impossible to exclude TNFerade™ as an explanation for this toxicity, this is a known complication of chemoradiotherapy for rectal cancer [20].
TNFerade™ was developed as a mechanism to deliver a vector capable of providing high levels of local TNF-α after induction without the complications observed with systemic delivery of TNF-α. The ability to deliver the vector locally with minimal systemic leak is an important characteristic to obtain tumor-selective effects and to avoid the idiosyncratic toxic reactions associated with systemic delivery of TNF-α. In the 8 patients in whom complete pharmacokinetic data were available, the vector was detected in 7 of 8 patients, but was below the limit of quantification in all but 2 patients. The low level and rapid clearance of the vector from the bloodstream within 1 h suggest a small amount of leak of the vector at the time of injection. Importantly, the vector was not observed in the plasma at later time points after the first injection or at subsequent injections suggesting clearance or that treatment effect may have reduced systemic leak.
Although we were unable to evaluate vascular anatomy and function serially in this trial, TNF-α is known to alter tumor blood vessel integrity. It is possible that the effects of TNF-α on tumor vessels may reduce the likelihood of systemic leak of future injections. In regard to any clinical impact of systemic leak, an evaluation of toxicity attributable to TNF-α is of importance. The toxicity observed in this study that has previously been attributed to TNF-α was low grade and relatively infrequent suggesting that local delivery of the vector may be an effective means to minimize the systemic impact of TNF-α.
A secondary endpoint of this study was to evaluate pathologic response to the combination of capecitabine, radiation, and intratumoral injections of TNFerade™ with the intent of evaluating this measure for inclusion as an endpoint in a future randomized assignment trial with this combination as the experimental arm. Although the numbers of patients included in this trial are small and firm conclusions cannot be drawn, it is important in any study of a radiation or chemotherapy modifier to report the rate of response. In series evaluating the combination of capecitabine and pelvic radiotherapy for locally advanced rectal cancer, pathologic complete response rates of 8–16% and RCRG1 rates of 37–43% have been described [7, 21, 22, 23, 24, 25, 26, 27, 28]. In the 9 patients treated with the regimen of radiation, capecitabine, and intratumoral TNFerade™ injections, 2 met criteria for pathologic complete response and 7 of the 9 patients were scored as having a response compatible with RCRG1. Because the number of patients treated in this pilot trial is small, no firm conclusions can be drawn but these pathologic endpoints would seem to be appropriate endpoints in addition to local control and survival for a future randomized trial of this regimen.
All but 1 of the patients receiving intratumoral TNFerade™ injections was able to undergo sphincter-preserving surgery. Despite the high rate of local response, 2 patients failed locally after treatment including 1 patient who developed a pathologic complete response, reinforcing the importance of long-term follow-up and local control as an endpoint in future trials of this combination. Prior studies have suggested that the most common site of failure after therapy for rectal cancer is the sacral hollow, presumably due to lymph node involvement and disease extension into the perirectal fat. Although the perirectal fat was not specifically targeted with the injection, in some cases extravasation was observed on occasion by ultrasound and was likely a common event based on the volume of injectate delivered. Although not directly targeted with the injection, it is also possible that lymphatic drainage provides delivery of vector to regional lymph nodes. With future trials of similar agents, a consideration of the benefit of deep injections to target disease in the perirectal fat will need to be balanced with the concern for systemic leak.
A limitation of this pilot study is the relatively small number of patients treated. Although our patient population is small, these data suggest that treatment with intratumoral TNFerade™ injections in combination with capecitabine and pelvic radiotherapy results in promising pathologic response rates with minimal additional toxicity compared to capecitabine and radiotherapy alone. Intratumoral delivery of TNFerade™ was accomplished via weekly endoscopy or with transrectal ultrasound guidance and was logistically and technically feasible. This procedure is minimally invasive and well tolerated, with no toxicity directly attributable to the injection procedure.
Conclusion
The addition of weekly intratumoral injections of TNFerade™ to capecitabine 937.50 mg/m2 b.i.d. (Monday through Friday) delivered concurrently with 50.4 Gy of external beam irradiation was feasible and in general well tolerated.
Disclosure Statement
None declared.
Acknowledgements
This research was supported in part by the Intramural Research Program of NCI and Clinical Center, NIH.
This project was funded in part with federal funds from the National Cancer Institutes of Health under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465–1472. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 4.Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Medical Research Council Rectal Cancer Working Party. Lancet. 1996;348:1605–1610. [PubMed] [Google Scholar]
- 5.Thomas PR, Lindblad AS. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: a review of the Gastrointestinal Tumor Study Group experience. Radiother Oncol. 1988;13:245–252. doi: 10.1016/0167-8140(88)90219-8. [DOI] [PubMed] [Google Scholar]
- 6.Wolmark N, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388–396. doi: 10.1093/jnci/92.5.388. [DOI] [PubMed] [Google Scholar]
- 7.Sauer R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 8.Old LJ. Tumor necrosis factor (TNF) Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 9.Gridley DS, Hammond SN, Liwnicz BH. Tumor necrosis factor-alpha augments radiation effects against human colon tumor xenografts. Anticancer Res. 1994;14:1107–1112. [PubMed] [Google Scholar]
- 10.Weichselbaum RR, et al. Gene therapy targeted by radiation preferentially radiosensitizes tumor cells. Cancer Res. 1994;54:4266–4269. [PubMed] [Google Scholar]
- 11.Hallahan DE, et al. Spatial and temporal control of gene therapy using ionizing radiation. Nat Med. 1995;1:786–791. doi: 10.1038/nm0895-786. [DOI] [PubMed] [Google Scholar]
- 12.Hallahan DE, et al. Phase I dose-escalation study of tumor necrosis factor-alpha and concomitant radiation therapy. Cancer J Sci Am. 1995;1:204–209. [PubMed] [Google Scholar]
- 13.Murugesan SR, et al. Combination of human tumor necrosis factor-alpha (hTNF-alpha) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009;16:841–847. doi: 10.1038/cgt.2009.32. [DOI] [PubMed] [Google Scholar]
- 14.Weichselbaum RR, Kufe D. Translation of the radio- and chemo-inducible TNFerade vector to the treatment of human cancers. Cancer Gene Ther. 2009;16:609–619. doi: 10.1038/cgt.2009.37. [DOI] [PubMed] [Google Scholar]
- 15.Mundt AJ, et al. A phase I trial of TNFerade biologic in patients with soft tissue sarcoma in the extremities. Clin Cancer Res. 2004;10:5747–5753. doi: 10.1158/1078-0432.CCR-04-0296. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A, et al. Clinical protocol. An open-label, phase I, dose-escalation study of tumor necrosis factor-alpha (TNFerade Biologic) gene transfer with radiation therapy for locally advanced, recurrent, or metastatic solid tumors. Hum Gene Ther. 2001;12:1109–1131. doi: 10.1089/104303401750214320. [DOI] [PubMed] [Google Scholar]
- 17.Chang KJ, et al. Endoscopic ultrasound delivery of an antitumor agent to treat a case of pancreatic cancer. Nat Clin Pract Gastroenterol Hepatol. 2008;5:107–111. doi: 10.1038/ncpgasthep1033. [DOI] [PubMed] [Google Scholar]
- 18.Sudheendra D, et al. Comparison of three different needles for percutaneous injections. Cardiovasc Intervent Radiol. 2007;30:151–152. doi: 10.1007/s00270-005-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler JM, et al. Quantification of histologic regression of rectal cancer after irradiation: a proposal for a modified staging system. Dis Colon Rectum. 2002;45:1051–1056. doi: 10.1007/s10350-004-6359-x. [DOI] [PubMed] [Google Scholar]
- 20.Wolff HA, et al. High-grade acute organ toxicity during preoperative radiochemotherapy as positive predictor for complete histopathologic tumor regression in multimodal treatment of locally advanced rectal cancer. Strahlenther Onkol. 2010;186:30–35. doi: 10.1007/s00066-009-2037-1. [DOI] [PubMed] [Google Scholar]
- 21.Bosset JF, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 22.Chan AK, et al. Posttreatment TNM staging is a prognostic indicator of survival and recurrence in tethered or fixed rectal carcinoma after preoperative chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:665–677. doi: 10.1016/j.ijrobp.2004.06.206. [DOI] [PubMed] [Google Scholar]
- 23.Habr-Gama A, et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9:90–99. doi: 10.1016/j.gassur.2004.10.010. discussion 99–101. [DOI] [PubMed] [Google Scholar]
- 24.Kuo LJ, et al. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol. 2007;14:2766–2772. doi: 10.1245/s10434-007-9471-z. [DOI] [PubMed] [Google Scholar]
- 25.Rodel C, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler JM, et al. Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced rectal cancer: correlation with rectal cancer regression grade. Dis Colon Rectum. 2004;47:2025–2031. doi: 10.1007/s10350-004-0713-x. [DOI] [PubMed] [Google Scholar]
- 27.Ballonoff A, et al. Preoperative capecitabine and accelerated intensity-modulated radiotherapy in locally advanced rectal cancer: a phase II trial. Am J Clin Oncol. 2008;31:264–270. doi: 10.1097/COC.0b013e318161dbd3. [DOI] [PubMed] [Google Scholar]
- 28.Desai SP, et al. A phase II study of preoperative capecitabine and radiation therapy in patients with rectal cancer. Am J Clin Oncol. 2007;30:340–345. doi: 10.1097/COC.0b013e318033ed63. [DOI] [PubMed] [Google Scholar]


