Abstract
Background
Diabetic nephropathy (DN) is a leading cause of mortality and morbidity in patients with type 1 and type 2 diabetes. The multicenter FIND consortium aims to identify genes for DN and its associated quantitative traits, e.g. the urine albumin:creatinine ratio (ACR). Herein, the results of whole-genome linkage analysis and a sparse association scan for ACR and a dichotomous DN phenotype are reported in diabetic individuals.
Methods
A genomewide scan comprising more than 5,500 autosomal single nucleotide polymorphism markers (average spacing of 0.6 cM) was performed on 1,235 nuclear and extended pedigrees (3,972 diabetic participants) ascertained for DN from African-American (AA), American-Indian (AI), European-American (EA) and Mexican-American (MA) populations.
Results
Strong evidence for linkage to DN was detected on chromosome 6p (p = 8.0 × 10−5, LOD = 3.09) in EA families as well as suggestive evidence for linkage to chromosome 7p in AI families. Regions on chromosomes 3p in AA, 7q in EA, 16q in AA and 22q in MA displayed suggestive evidence of linkage for urine ACR. The linkage peak on chromosome 22q overlaps the MYH9/APOL1 gene region, previously implicated in AA diabetic and nondiabetic nephropathies.
Conclusion
These results strengthen the evidence for previously identified genomic regions and implicate several novel loci potentially involved in the pathogenesis of DN.
Key Words: Albuminuria, Diabetes mellitus, Renal failure, End-stage renal disease, Linkage, Allelic association
Introduction
Diabetic nephropathy (DN) associated with type 1 and type 2 diabetes mellitus (DM) remains the leading cause of chronic kidney disease in the US, contributing to approximately 50% of incident cases of end-stage renal disease (ESRD) [1]. DN imposes a significant personal and socioeconomic burden on patients and their families, society, and healthcare systems due, in part, to its contribution to cardiovascular disease [2, 3].
Genetic factors contribute to risk of DN in all ethnic groups [4, 5]. Genomewide linkage scans of kidney function in the presence of DM implicated regions on chromosomes 3q, 7p, 7q, 9, 10q and 18q (online suppl. table S1; see www.karger.com/doi/10.1159/000326763 for all online suppl. material), supporting the view that genetic factors contribute to DN and albuminuria. To date, four genomewide association analyses have been reported for DN [6, 7, 8, 9]. These and other studies have repeatedly implicated a number of genes in DN, including ELMO1, PVT1, ACACB and SLC12A3, as well as specific candidate genes ACE, CNDP1, NOS3, SOD2, APOE and PRKCB [4, 10]. The estimated sibling risk ratio for DN is about 2.3 [11], but only a modest proportion of that risk is explained by the above loci. Herein, the Family Investigation of Nephropathy and Diabetes (FIND) reports the largest genomewide linkage study for DN and urine albumin:creatinine ratio (ACR) in African Americans (AA), Southwest American Indians (AI), European Americans (EA) and Mexican Americans (MA) [12].
Materials and Methods
Study Populations
The FIND study design has been reported [12]. Families of probands with DN with a diabetic sibling with or without nephropathy were recruited from eleven participating investigative centers. Living parents and other relatives (i.e. avuncular, cousin, half-sibling and grandparental affected pairs) were recruited when available. Recruitment was performed according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects with approval of the institutional review board at each center and the FIND Genetic Analysis and Data Coordinating Center.
Phenotypes
Measurement of DN-related phenotypes in FIND has been described [12, 13] (online suppl. material: Methods). The total available sample, with DN scored as affected or unaffected, included 2,616 individuals in 1,235 pedigrees across all ethnic groups (table 1), comprising 1,435 full-sib pairs and 188 half-sib pairs. Whether an individual was considered affected or unaffected with DN in linkage analysis depended on the participant category [13]. Probands met the FIND criteria for DM and had either biopsy-proven DN, ESRD attributable to DN or chronic kidney disease attributable to DN. To be considered ‘unaffected’, individuals were required to have DM for at least 10 years without evidence of kidney disease, as ascertained through history, estimated glomerular filtration rate and urine ACR.
Table 1.
Description of the FIND study sample
| Probands | DN + Rel. | DN- Rel. | p1 | p2 | |
|---|---|---|---|---|---|
| Participants | |||||
| Total | 1,277 | 731 | 608 | ||
| AA | 348 (27.3) | 183 (25.0) | 146 (24.0) | ||
| AI | 254 (19.9) | 200 (27.4) | 95 (15.6) | ||
| EA | 196 (15.3) | 58 (7.9) | 116 (19.1) | ||
| MA | 479 (37.5) | 290 (39.7) | 251 (41.3) | ||
| Female | 692 (54.2) | 425 (58.1) | 431 (70.9) | 0.087 | <0.0001 |
| Age, years | 58 ± 11 | 58 ± 12 | 59 ± 11 | 0.7 | 0.073 |
| ESRD | 509 (40.0) | 53 (7.3) | 0 (0.0) | <0.0001 | - |
| DM diagnosis age, years | 35 ± 12 | 39 ± 13 | 41 ± 12 | <0.0001 | 0.0076 |
| DM duration, years | 23 ± 9 | 17 ± 11 | 18 ± 8 | <0.0001 | 0.3 |
| BMI | 30.2 ± 7.2 | 31.6 ± 8.3 | 33.0 ± 7.9 | 0.0002 | 0.0014 |
| Participants without ESRD | |||||
| HbAlc, % | 7.2 ± 1.7 | 8.3 ± 2.3 | 7.8 ± 1.8 | <0.0001 | <0.0001 |
| Serum creatinine, mg/dl | 2.9 ± 2.5 | 1.8 + 1.8 | 0.87 ± 0.23 | <0.0001 | <0.0001 |
| BUN, mg/dl | 41 ± 21 | 26 ± 18 | 16 ± 7 | <0.0001 | <0.0001 |
| Urine ACR, g/g | 2.9 ± 1.0 | 2.3 ± 2.0 | 0.03 ± 0.40 | <0.0001 | <0.0001 |
| Urine PCR, g/g | 3.5 ± 1.4 | 3.2 ± 3.0 | 0.13 ± 0.64 | 0.0033 | <0.0001 |
| eGFR, ml/min/1.73m2 | 11 ± 17 | 49 ± 43 | 88 ± 24 | <0.0001 | <0.0001 |
Data are means ± SD or n (% of total). DN+ Rel. = Relatives affected with overt DN; DN- Rel. = relatives unaffected with DN after having DM for at least 10 years; HbAlc = hemoglobin Ale; BUN = blood urea nitrogen; PCR = urine protein/creatinine ratio; eGFR = estimated glomerular filtration rate; pj = comparison of probands with affected relatives; p2 = comparison of affected relatives with unaffected relatives.
Urine was not collected from ESRD patients and a urine ACR of 3 g/g was assigned to these subjects. ACR values greater than 3 g/g in non-ESRD cases with DN were Winsorized to 3 g/g.
The binary DN trait was adjusted for sex, and the quantitative ACR trait for sex and age at DM diagnosis in all analyses. We did not adjust for use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, as information on these medications was not available for all participants.
Analytical Methods
We conducted the linkage and association analyses using the Illumina IV panel of approximately 5,500 autosomal single nucleotide polymorphisms (SNPs) that passed initial quality control criteria (online suppl. table S2). The Illumina IV markers were selected for uniform spacing across the genome [14] and high information content across multiple ethnic groups [15]. The Haseman-Elston regression approach [16], implemented in SIBPAL (part of the S.A.G.E. software package), was the primary analysis method. The Haseman-Elston linkage test was performed using multipoint IBD sharing estimates, which combine genetic information across multiple linked SNPs, obtained from reduced sets of SNPs designed to maximize information in the absence of linkage disequilibrium. The W4 weighting scheme was used to adjust for dependence of sib-pairs within a sibship and of squared trait sums and differences as previously described [13], except that half-sib pairs were included in the analysis where possible. Linkage analysis was first attempted for each study sample including half-sib pairs. The analysis was repeated without half-sibs if model instability was encountered.
Linkage analyses were computed separately in each ethnic group. In addition to asymptotic p values, statistical significance was estimated via permutation testing, constructing an approximate empirical distribution of the test statistic. Because SIBPAL estimates empirical p values on full-sib pairs only, we did not estimate empirical p values for analyses including half-sib pairs. Fisher's method was used to combine p values across ethnic groups. Following the linkage scans, we performed association testing using all SNPs that passed quality control criteria and the approach implemented in ASSOC, adjusting for familial relationships within a linear mixed model framework (online suppl. material: Methods). Because of differences in allele frequencies and linkage disequilibrium structure, the sets of SNPs contributing to linkage and association analyses differed among ethnic groups (online suppl. table S2).
Results
Sample Characteristics
The FIND sample comprised 2,616 participants in 1,235 pedigrees qualifying as affected or unaffected for DN, and 3,089 subjects with measures of urine ACR. Although the entire FIND sample did not measure autoantibodies diagnostic for type 1 diabetes, roughly 90–95% of DN-affected individuals were believed to have type 2 diabetes based on clinical criteria and a type 1 diabetes prevalence of 4% observed in a subsample of 857 individuals [17]. Significant differences in BMI, age at DM diagnosis and DM duration prior to recruitment were present between probands and affected family members (p ≤ 2 × 10−4; table 1). Relatives of probands affected and unaffected for DN had significant differences in sex ratio, BMI and age at DM diagnosis. Significant differences in age were not detected between probands and DN-affected relatives, nor between DN-affected and -unaffected relatives (p > 0.05).
The FIND sample included 1,435 full-sib pairs and 113 half-sib pairs contributing to the genomewide linkage analysis for DN (online suppl. table S3), and 2,201 full-sib pairs and 268 half-sib pairs in the linkage analysis for urine ACR (online suppl. table S4). Subsets of these samples were included in preliminary scans for these traits [13].
Linkage and Association Analysis in DN
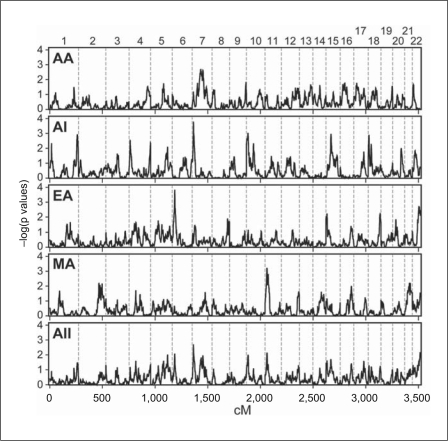
The major ethnic-group-specific linkage signals for the DN binary trait, adjusted for sex, occurred on chromosomes 6p, 7p, 7q and 11p (fig. 1; table 2). The EA sample provided the strongest overall evidence for linkage on chromosome 6p (asymptotic p = 0.00015 at 24.9 cM, equivalent to a LOD score of 2.84; fig. 2a). Permutation analysis yielded a slightly more significant empirical p value of 8.0 × 10−5, LOD = 3.09. The most significant peak on 7p in AI was nearly as strong (p = 0.00016 at 15.5 cM, LOD = 2.81; fig. 2b). Several other regions showed suggestive evidence for linkage in AI, including 1q, 15q and 18p (fig. 2c). Top results in AA and MA were less robust. No peak in AA had an asymptotic p value ≤0.001; whereas chromosome 11p showed suggestive evidence for linkage in MA (fig. 1). The most significant evidence for linkage across all four populations, on chromosome 7p (p = 0.0019, LOD = 1.81), was driven primarily by the AI group, with a smaller contribution from AA (fig. 2c).
Fig. 1.
Autosomal genomewide model-free linkage scan for DN. From top to bottom, panels display results for AA, AI, EA and MA samples, as well as pooled Fisher p values from all study samples (All).
Table 2.
Major linkage peaks for DN and urine ACR
| Chromosone | Group | cM | Cyto | Marker | Asymptotic p | LOD | Empirical p |
|---|---|---|---|---|---|---|---|
| DN | |||||||
| 1 | AI | 262.0 | 1q43 | - | 1.2 × 10−3 | 2.00 | - |
| 6 | EA | 24.9 | 6p24.3 | rs1087924 | 1.5 × 10−4 | 2.84 | 8.0 × 10−5 |
| 7 | AI | 15.5 | 7p21.3 | rs37995 | 1.6 × 10−4 | 2.81 | - |
| 7 | AA | 86.3 | 7q11.23 | rs3135677 | 1.9 × 10−3 | 1.82 | 5.4 × 10−4 |
| 10 | AI | 15.5 | 10p15.1 | rs2167703 | 9.3 × 10−4 | 2.10 | - |
| 11 | MA | 18.0 | 11p15.3 | - | 5.9 × 10−4 | 2.28 | - |
| 15 | AI | 46.3 | 15q21.1 | rs281265 | 1.1 × 10−3 | 2.04 | - |
| 18 | AI | 6.6 | 18p11.32 | rs770238 | 1.3 × 10−3 | 1.97 | - |
| ACR | |||||||
| 2 | AA | 157.2 | 2q22.3 | rs1370523 | 1.1 × 10−3 | 2.04 | - |
| 3 | AA | 96.4 | 3p13 | rs17108 | 1.8 × 10−4 | 2.76 | - |
| 7 | EA | 99.3 | 7q21.2 | rs9008 | 1.1 × 10−4 | 2.96 | 2.6 × 10−4 |
| 16 | AA | 73.1 | 16q13 | rs41383 | 5.5 × 10−4 | 2.31 | - |
| 21 | AA | 58.0 | 21q22.3 | rs220271 | 1.3 × 10−3 | 1.97 | - |
| 22 | MA | 40.0 | 22q12.3 | - | 5.8 × 10−4 | 2.29 | - |
cM = Kosambi centimorgans; Cyto = cytogenic location.
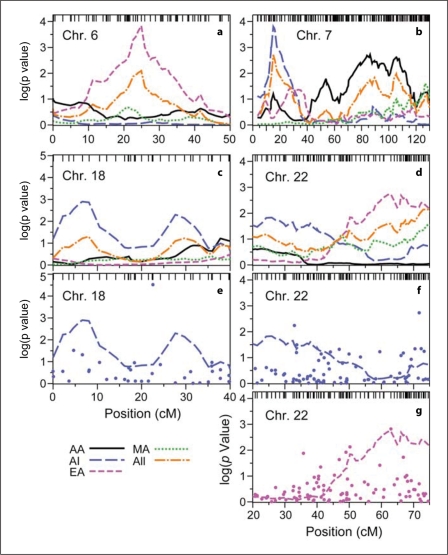
Fig. 2.
Detailed linkage and association results for DN for segments of chromosomes 6 (a), 7 (b), 18 (c, e) and 22 (d, f, g). Heavy lines trace linkage results for AA, AI, EA and MA samples, as well as combined Fisher p values (alternating dots and dashes). Tick marks at the top show the location of Illumina IV SNPs. In panel e, results from association analysis for AI (·) are shown along with the linkage profile from panel c. In panels f and g, association results for AI and EA are shown (·), respectively, with the linkage profiles from panel d.
The linkage analysis was followed by a sparse genomewide association scan. Association testing for DN detected SNPs with p < 0.0001 for at least one ethnic group in two locations on chromosome 18, and SNPs with p < 0.001 on several chromosomes (fig. 2; online suppl. fig. 3; online suppl. table S5). No SNP with p < 0.001 in the association analysis coincided with a region showing evidence for linkage in the same ethnic group. In the ethnicity-combined association analysis, Fisher p values below 0.001 were observed for SNPs on chromosomes 5, 17 and 18 (online suppl. fig. S3; online suppl. table S5). All of these results were driven by a single population.
The most significant association result for AI (rs1241893 on chromosome 18; 22.56 cM, 6.87 Mb, p = 3.0 × 10−5) lies between two regions with modest evidence for linkage in AI (fig. 2e). Two SNPs spaced 2.7 kb apart on chromosome 18 and exhibiting low p values for AA (rs1662910 and rs948438; 57.98 cM and 33.10 Mb) did not correspond to linkage peaks in any ethnic group. In contrast, chromosome 22 marker rs5769116 (62.92 cM, 45.44 Mb) revealed moderate evidence for both linkage (p = 0.0018, LOD = 1.83) and association (p = 0.0019) in EA (fig. 2g) some 10 Mb distal to MYH9/APOL1 – genes associated with several nondiabetic kidney diseases [9, 18, 19, 20].
Linkage and Association Analysis for Urine ACR
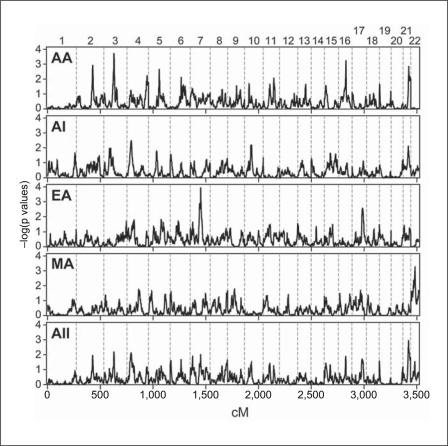
In the genomewide linkage analysis for the quantitative ACR trait, suggestive evidence for linkage was obtained on chromosomes 3, 7, 16 and 22 (fig. 3; table 2). As for DN, the strongest population-specific linkage peak for urine ACR was seen in EA – in this case on chromosome 7q (p = 0.00011 at 99.3 cM, LOD = 2.96; fig. 4b). This signal overlaps the broad peak of linkage for DN in AA, but evidence for linkage to DN in EA was minimal (fig. 2b). The AA sample yielded strong evidence for linkage on chromosome 3p (p = 0.00018 at 95.5 cM, LOD = 2.76; fig. 4a), and weaker evidence on chromosomes 16q and 21q (fig. 4c). The most significant evidence for linkage in the MA sample was found on chromosome 22 (40.00 cM; empirical p = 0.00058, LOD = 2.29). The peak occurred approximately 1 Mb proximal to MYH9 (fig. 4d); the asymptotic and empirical p values for linkage at rs735853, within the MYH9 gene, were 0.0031 and 0.0014 (LOD = 1.92), respectively. Nonetheless, no test of association for chromosome 22 SNPs for the MA sample yielded a p value less than 0.01 (online suppl. fig. 4; online suppl. table S6).
Fig. 3.
Genomewide linkage scan for ACR, adjusted for sex and age at DM diagnosis. See legend of fig. 1 for explanation of plots and symbols.
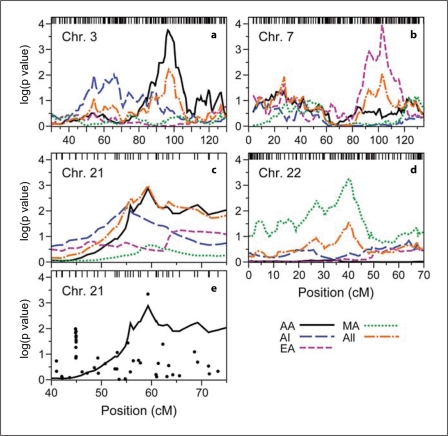
Fig. 4.
Detailed linkage and association results for ACR, on segments of chromosomes 3 (a), 7 (b), 21 (c, e) and 22 (d). Axes and lines are as in figure 2. In panel e, results from association analysis for the AA sample are shown (·) with the AA linkage profile from panel c.
Weak evidence of linkage for urine ACR in AI (p = 0.02) reinforced the AA chromosome 21q linkage peak (rs220271; 42.36 Mb), providing the strongest evidence for linkage in the ethnicity-combined sample (p = 0.0010 at 59.28 cM, LOD = 2.06; fig. 4c). This peak coincided with the second most significant association result in AA, but there were no other strong association results in the region (fig. 4e). The most significant population-specific p value for association with urine ACR, found on chromosome 4p in AI (p = 1.3 × 10−5 at 38.65 Mb, 58.04 cM), was of borderline genomewide significance (p = 0.06 after Bonferroni correction for 4,925 tests; online suppl. fig. 4; online suppl. table S6). Combined association results across all four populations revealed p values ≤0.001 on chromosomes 6, 8, 9, 11 and 21. All signals were primarily driven by a single ethnic group, except for chromosomes 11 and 21 (online suppl. table S6). On chromosome 11, both the EA and MA samples gave significant results at rs722317 (15.88 Mb, 24.27 cM; p = 0.00046 and 0.0026, respectively) to yield a Fisher p value of 7.3 × 10−5, the most significant combined association result for ACR. rs2250226 on chromosome 21 yielded p values of 0.0025 and 0.0013, respectively, in AA and MA (Fisher p = 0.00028).
Discussion
The FIND results from genomewide linkage and sparse association scans in four American ethnic groups with DN confirm previous linkage findings and suggest new genetic loci contributing to DN and related phenotypes. Suggestive evidence for linkage was observed on chromosomes 6p and 7p for DN, and on 3p, 7q, 16q and 22q for urine ACR in one or more ethnic groups. No region displayed consistent evidence for linkage across all 4 ethnic groups, although the modest urine ACR linkage and association peak on chromosome 21 in AA received weak support from AI. Significant evidence for linkage was detected in overlapping regions of chromosome 7q for both DN and urine ACR in AA and EA families, respectively.
Expanding the FIND family study samples from the partial cohort previously examined [13], altered the pattern of linkage across the autosomal genome. Newly detected linkage peaks with equivalent LOD scores ≥2.0 were observed on chromosomes 1q, 7p, 10p, 11p and 15q for DN, and on 2q, 3p, 16q and 22q for urine ACR. The full FIND sample provided greater power to detect genetic influences, as did modification of SIBPAL to include half-sib pairs in the Haseman-Elston regression. Moreover, use of a relatively dense set of 6,000 SNPs as a linkage panel, as opposed to a set of 400 microsatellite markers, ensures that a high level of information about linkage is available uniformly across each chromosome. The linkage peaks previously detected in a subset of these cases on chromosome 14q23 for DN and on 15q26 for urine ACR, now less prominent, were likely false positives, although genetic heterogeneity could also influence results. Similarly, there was no substantial overlap between the linkage findings reported here and in a linkage analysis of estimated glomerular filtration rate on the partial cohort [21].
In general, these results did not closely overlap genomic regions implicated in previous non-FIND linkage studies for DN [5] (online suppl. table S1). Our linkage signals on chromosome 7p for DN in AI and 22q12.3 for urine ACR in MA, coincided with those for glomerular filtration rate and ACR, respectively, in type 2 diabetes from 63 mostly Caucasian extended pedigrees [22, 23]. The strongest evidence for linkage in this study occurred on chromosome 6p, with an empirical p value for linkage of 8.0 × 10−5 (LOD = 3.09) in EA for the binary DN trait.
The current report is the first of a linkage signal of such magnitude near the p terminus of chromosome 6 in a genomewide linkage study of DN or nondiabetic kidney disease. However, a genomewide scan for insulin resistance reported modest evidence for linkage in this location for acute insulin response to glucose in Hispanic families (LOD = 1.72 at 15 cM) [24]. Suggestive evidence for linkage to urine ACR was found on chromosome 22 in MA (p = 5.9 × 10−4, LOD = 2.29), with peak linkage near the nonmuscle myosin heavy chain 9/apolipoprotein L1 (MYH9/APOL1) gene region implicated in diabetic and nondiabetic ESRD and focal segmental glomerulosclerosis in AA [9, 18, 19, 25, 26]. Additional supporting evidence was observed in EA using the binary DN trait. However, no association signal was detected, which is reasonable since none of the reported MYH9/APOL1 kidney disease risk variants were included in our genotyping panels.
Several results from the FIND analyses occur in regions previously associated with DN phenotypes. Two SNPs near the candidate gene carnosinase 1 (CNDP1) showed evidence for association in at least one FIND cohort. The first SNP, rs999647, is 49 kb distal to CNDP1, with association specific to DN in AI families (p = 0.0057) and weak association with urine ACR in EA (p = 0.031; Fisher combined p = 0.028 over all cohorts). A neighboring SNP, rs872994, 919 kb distal to CNDP1 (at position 73,171,838, human genome build 18), displayed weak evidence for association in three ethnic groups, AI (p = 0.053), EA (p = 0.017) and MA (p = 0.033), with a combined Fisher p of 0.0043.
The chromosome 3p DN association in the AI population occurred at rs892605, approximately 400 kb proximal to the ghrelin (GHRL) gene, for which association with kidney function in DM, but not DM itself, has been reported [27, 28, 29].
Genomewide linkage scans for DN and related traits differ in many ways, including pedigree structures recruited, ascertainment criteria, definitions of DN and measurement of kidney function, diabetes type and duration. In contrast to other publications, FIND is a multiethnic sample of DN-affected and -unaffected subjects with extremes of phenotypic severity [12]. This genomewide linkage and sparse association scan in the full FIND study sample provides evidence for both novel (e.g. on chromosomes 3p and 6p) and previously detected (chromosomes 7p and 22) genetic loci predisposing to renal impairment and DN. Genomewide association studies have the most power to detect genetic variants with relatively modest effect, but require that causal alleles be well captured (tagged) by the marker panel, which is true in practice mostly for common variants. The linkage study presented here only has power to detect variants with relatively large effect, but can do so regardless of the population allele frequency and number of tightly linked variants involved. These results should provide guidance for further exploration of the genetic basis for diabetic kidney disease using novel technologies.
Supplementary Material
Supplementary data
Acknowledgements
We thank all FIND participants. This study was supported by grants U01DK57292, U01DK57329, U01DK057300, U01DK057298, U01DK057249, U01DK57295, U01DK070657, U01DK057303, U01DK070657, U01DK57304 and DK57292-05 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and, in part, by the Intramural Research Program of the NIDDK. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health (NIH), under contract N01-CO-12400 and the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This work was also supported by the National Center for Research Resources for the General Clinical Research Center grants: Case Western Reserve University, M01-RR-000080; Wake Forest University, M01-RR-07122; Harbor-University of California, Los Angeles Medical Center, M01-RR-00425; College of Medicine, University of California, Irvine, M01-RR-00827–29; University of New Mexico, HSC M01-RR-00997; and Frederic C. Bartter, M01-RR-01346. Genotyping was performed by the Center for Inherited Disease Research, which is fully funded through a federal contract from the NIH to Johns Hopkins University (N01-HG-65403). The results of this analysis were obtained using the S.A.G.E. package of genetic epidemiology software, which is supported by a U.S. Public Health Service Resource Grant (RR03655) from the National Center for Research Resources.
Supplementary information is available at the American Journal of Nephrology web site.
References
- 1.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Gustafson S, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen S-C, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. US Renal data system 2010 annual report. Am J Kidney Dis. 2011;57(suppl 1):e1–e526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Int Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 3.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 4.Freedman BI, Bostrom M, Daeihagh P, Bowden DW. Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol. 2007;2:1306–1316. doi: 10.2215/CJN.02560607. [DOI] [PubMed] [Google Scholar]
- 5.Maeda S. Genetics of diabetic nephropathy. Ther Adv Cardiovasc Dis. 2008;2:363–371. doi: 10.1177/1753944708094768. [DOI] [PubMed] [Google Scholar]
- 6.Hanson RL, Craig DW, Millis MP, Yeatts KA, Kobes S, Pearson JV, Lee AM, Knowler WC, Nelson RG, Wolford JK. Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes. 2007;56:975–983. doi: 10.2337/db06-1072. [DOI] [PubMed] [Google Scholar]
- 7.Shimazaki A, Kawamura Y, Kanazawa A, Sekine A, Saito S, Tsunoda T, Koya D, Babazono T, Tanaka Y, Matsuda M, Kawai K, Iizumi T, Imanishi M, Shinosaki T, Yanagimoto T, Ikeda M, Omachi S, Kashiwagi A, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakajima M, Nakamura Y, Maeda S. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes. 2005;54:1171–1178. doi: 10.2337/diabetes.54.4.1171. [DOI] [PubMed] [Google Scholar]
- 8.Pezzolesi MG, Poznik GD, Mychaleckyj JC, Paterson AD, Barati MT, Klein JB, Ng DPK, Placha G, Canani LH, Bochenski J, Waggott D, Merchant ML, Krolewski B, Mirea L, Wanic K, Katavetin P, Kure M, Wolkow P, Dunn JS, Smiles A, Walker WH, Boright AP, Bull SB, DCCT/EDIC Research Group. Doria A, Rogus JJ, Rich SS, Warram JH, Krolewski AS. Genome-wide association scan for diabetic nephropathy susceptibility genes in type I diabetes. Diabetes. 2009;58:1403–1410. doi: 10.2337/db08-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonough CW, Palmer ND, Hicks PJ, Roh BH, An SS, Cooke JN, Hester JM, Wing MR, Bostrom MA, Rudock ME, Lewis JP, Talbert ME, Blevins RA, Lu L, Ng MCY, Sale MM, Divers J, Langefeld CD, Freedman BI, Bowden DW. A genome-wide association study for diabetic nephropathy genes in African Americans. Kidney Int. 2011;79:563–572. doi: 10.1038/ki.2010.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman BI, Bowden DW, Murea M. Protein kinase C-β gene variants and type 2 diabetes-associated kidney failure: what can we learn from gene association studies in diabetic nephropathy? Am J Kidney Dis. 2011;57:194–197. doi: 10.1053/j.ajkd.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harjutsalo V, Katoh S, Sarti C, Tajima N, Tuomilehto J. Population-based assessment of familial clustering of diabetic nephropathy in type 1 diabetes. Diabetes. 2004;53:2449–2454. doi: 10.2337/diabetes.53.9.2449. [DOI] [PubMed] [Google Scholar]
- 12.Knowler WC, Coresh J, Elston RC, Freedman BI, Iyengar SK, Kimmel PL, Olson JM, Plaetke R, Sedor JR, Seldin MF, The Family Investigation of Nephropathy and Diabetes Research Group The Family Investigation of Nephropathy and Diabetes (FIND): design and methods. J Diabetes Complications. 2005;19:1–9. doi: 10.1016/j.jdiacomp.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Iyengar SK, Abboud HE, Goddard KAB, Saad MF, Adler SG, Arar NH, Bowden DW, Duggirala R, Elston RC, Hanson RL, Ipp E, Kao WHL, Kimmel PL, Klag MJ, Knowler WC, Meoni LA, Nelson RG, Nicholas SB, Pahl MV, Parekh RS, Quade SRE, Rich SS, Rotter JI, Scavini M, Schelling JR, Sedor JR, Sehgal AR, Shah VO, Smith MW, Taylor KD, Winkler CA, Zager PG, Freedman BI, Family Investigation of Nephropathy and Diabetes Research Group Genome-wide scans for diabetic nephropathy and albuminuria in multiethnic populations: the Family Investigation of Nephropathy and Diabetes. Diabetes. 2007;56:1577–1585. doi: 10.2337/db06-1154. [DOI] [PubMed] [Google Scholar]
- 14.Murray SS, Oliphant A, Shen R, McBride C, Steeke RJ, Shannon SG, Rubano T, Kermani BG, Fan J-B, Chee MS, Hansen MST. A highly informative SNP linkage panel for human genetic studies. Nat Methods. 2004;1:113–117. doi: 10.1038/nmeth712. [DOI] [PubMed] [Google Scholar]
- 15.Illumina Inc: SNP-Based Linkage IV Panel: discussion of design, validation and advantages. Document No. 370-2004-008, 2004.
- 16.Shete S, Jacobs K, Elston R. Adding further power to the Haseman and Elston method for detecting linkage in larger sibships: weighting sums and differences. Hum Hered. 2003;55:79–85. doi: 10.1159/000072312. [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, Ipp E, FIND Research Group Diabetes types and autoimmunity in the Family Investigation of Nephropathy and Diabetes. J Am Soc Nephrol. 2005;16:149A. [Google Scholar]
- 18.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbert SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao WHL, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS, Family Investigation of Nephropathy and Diabetes Research Group MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito H, Kunishima S. Historical hematology: May-Hegglin anomaly. Am J Hematol. 2008;83:304–306. doi: 10.1002/ajh.21102. [DOI] [PubMed] [Google Scholar]
- 21.Schelling JR, Abboud HE, Nicholas SB, Pahl MV, Sedor JR, Adler SG, Arar NH, Bowden DW, Elston RC, Freedman BI, Goddard KAB, Guo X, Hanson RL, Ipp E, Iyengar SK, Jun G, Kao WHL, Kasinath BS, Kimmel PL, Klag MJ, Knowler WC, Nelson RG, Parekh RS, Quade SR, Rich SS, Saad MF, Scavini M, Smith MW, Taylor K, Winkler CA, Zager PG, Shah VO, Family Investigation of Nephropathy and Diabetes Research Group Genome-wide scan for estimated GFR in multi-ethnic diabetic populations: the Family Investigation of Nephropathy and Diabetes. Diabetes. 2008;57:235–243. doi: 10.2337/db07-0313. [DOI] [PubMed] [Google Scholar]
- 22.Placha G, Poznik GD, Dunn J, Smiles A, Krolewski B, Glew T, Puppala S, Schneider J, Rogus JJ, Rich SS, Duggirala R, Warram JH, Krolewski AS. A genome-wide linkage scan for genes controlling variation in renal function estimated by serum cystatin C levels in extended families with type 2 diabetes. Diabetes. 2006;55:3358–3365. doi: 10.2337/db06-0781. [DOI] [PubMed] [Google Scholar]
- 23.Krolewski AS, Poznik GD, Placha G, Canani L, Dunn J, Walker W, Smiles A, Krolewski B, Fogarty DG, Moczulski D, Araki S, Makita Y, Ng DPK, Rogus J, Duggirala R, Rich SS, Warram JH. A genome-wide linkage scan for genes controlling variation in urinary albumin excretion in type II diabetes. Kidney Int. 2006;69:129–136. doi: 10.1038/sj.ki.5000023. [DOI] [PubMed] [Google Scholar]
- 24.Rich SS, Bowden DW, Haffner SM, Norris JM, Saad MF, Mitchell BD, Rotter JI, Langefeld CD, Wagenknecht LE, Bergman RN. Identification of quantitative trait loci for glucose homeostasis: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2004;53:1866–1875. doi: 10.2337/diabetes.53.7.1866. [DOI] [PubMed] [Google Scholar]
- 25.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Knob AU, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African-Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman BI, Hicks PJ, Bostrom MA, Cunningham ME, Liu Y, Divers J, Kopp JB, Winkler CA, Nelson GW, Langefeld CD, Bowden DW. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75:736–745. doi: 10.1038/ki.2008.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SY, Jo DS, Hwang PH, Park JH, Park SK, Yi HK, Lee DY. Preproghrelin Leu72Met polymorphism is not associated with type 2 diabetes mellitus. Metabolism. 2006;55:366–370. doi: 10.1016/j.metabol.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Ukkola O, Kesäniemi A. Preproghrelin Leu72Met polymorphism in patients with type 2 diabetes mellitus. J Int Med. 2003;254:391–394. doi: 10.1046/j.1365-2796.2003.01208.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee DY, Kim SY, Jo DS, Hwang PH, Kang KP, Lee SJ, Kim W, Park SK. Preproghrelin Leu72Met polymorphism predicts a lower rate of developing renal dysfunction in type 2 diabetic nephropathy. Eur J Endocrinol. 2006;155:187–190. doi: 10.1530/eje.1.02171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data