Abstract
Piroxicam is a non-steroidal anti-inflammatory drug widely used in rheumatic diseases. The aim of this study was to investigate Piroxicam-induced histopathological changes in livers and kidneys of male albino mice.
Methods
Animals were classified into a control group and 4 treated groups. Piroxicam was injected intraperitoneally using 0.3 mg/kg every day for four weeks. Each week a group of mice was sacrificed. Liver and kidneys were obtained for histological and histochemical examination. Animals were classified into a control group and 4 treated groups. Piroxicam was injected intraperitoneally using 0.3 mg/kg every day for four weeks. Each week a group of mice was sacrificed. Liver and kidneys were obtained for histological and histochemical examination.
Results
Liver sections appeared with inflammatory cellular infiltration, vacuolated hepatocytes, dilated sinusoids, and increased number of Kupffer cells. Kidney sections appeared with some cellular inflammations. The glomeruli were shrunk resulting in widening of the urinary space. Oedema and vacuolations were noticed in the tubular cells. There was a positive correlation between these pathological changes and the increased treatment periods. Histochemical staining revealed that glycogen and protein contents had decreased in the hepatocytes. This depletion worsened gradually in liver cells after two, three, and four weeks. Similar depletion of the glycogen content was observed in kidney tissue. However, protein content appeared to be slightly decreased in the kidney tubules and glomeruli. Incensement of coarse chromatin in the nuclei of hepatocytes, Kupffer cells and most inflammatory cells were detected by Fuelgen method. Kidney tissues appeared with a severe decrease in coarse chromatin in the nuclei. Liver sections appeared with inflammatory cellular infiltration, vacuolated hepatocytes, dilated sinusoids, and increased number of Kupffer cells. Kidney sections appeared with some cellular inflammations. The glomeruli were shrunk resulting in widening of the urinary space. Oedema and vacuolations were noticed in the tubular cells. There was a positive correlation between these pathological changes and the increased treatment periods. Histochemical staining revealed that glycogen and protein contents had decreased in the hepatocytes. This depletion worsened gradually in liver cells after two, three, and four weeks. Similar depletion of the glycogen content was observed in kidney tissue. However, protein content appeared to be slightly decreased in the kidney tubules and glomeruli. Incensement of coarse chromatin in the nuclei of hepatocytes, Kupffer cells and most inflammatory cells were detected by Fuelgen method. Kidney tissues appeared with a severe decrease in coarse chromatin in the nuclei.
Conclusion
Piroxicam has a time-dependent toxic effect on both liver and kidney tissues.
Keywords: Histology, piroxicam, liver, kidney, mice
Introduction
Rheumatic diseases are encountered around the world [1], leading to a series of social and medical problems. The prevention and treatment of rheumatic diseases now ranks as one of the foremost challenges facing those concerned with public health problems. For this reason, a variety of the non-steroidal anti-inflammatory drugs (NSAIDs), belonging to different chemical classes, have been introduced for clinical use as anti-rheumatics. NSAIDs comprise one of several families of chemical agents with clinically useful anti-inflammatory and analgesic properties [1, 2].
Piroxicam is one of the most popular non-steroidal anti-inflammatory drugs used for the treatment of inflammatory conditions and rheumatic disorders. It has analgesic and antipyretic activity, and is one of the drugs being introduced in clinical practice [3]. Recently, Knottenbelt et al. [4] found that a significant inhibition of proliferation and induction of apoptosis occur when piroxicam is used in concentrations that exceed maximum recommended doses. They suggested that it is possible that local or topical treatment or altered dosing regimens may offer alternative approaches to the use of these drugs as antineoplastic agents.
On the other hand, several non-steroidal anti-inflammatory drugs have been associated with liver damage [5]. Many NSAIDs have been withdrawn from the market because of adverse hepatic drug reactions. Some cases of liver disease have been reported in patients taking piroxicam. In contrast, other studies performed in hospitalized patients did not find any association [6]. Therefore, the present study was designed to investigate the histological and histochemical effects of piroxicam on the liver and kidney of male laboratory mice treated with the non-steroidal anti-inflammatory drug piroxicam.
Materials and methods
Experimental animals: Healthy adult male mice (Mus musculus) approximately 3–5 months old ranging in weight from 20–25 g, purchased from the Research Institute of Ophthalmology, Giza, Egypt were used in the present investigation. Animals were housed in specially designed cages and were kept in the laboratory under constant conditions for at least one week before use. They were fed a standard commercial diet. The experiments were approved by the state authorities and followed Egyptian law on animal protection.
Healthy adult male mice (Mus musculus) approximately 3–5 months old ranging in weight from 20–25 g, purchased from the Research Institute of Ophthalmology, Giza, Egypt were used in the present investigation. Animals were housed in specially designed cages and were kept in the laboratory under constant conditions for at least one week before use. They were fed a standard commercial diet. The experiments were approved by the state authorities and followed Egyptian law on animal protection.
Experimental design: Twenty-eight animals were used for histological studies; the animals were divided into 5 groups (one control group of eight animals and four treated groups (T1, T2, T3, T4) of five animals each. Each treated group was injected intraperitoneally daily with piroxicam (0.3 mg/kg/day) for one, two, three, and four weeks respectively. The negative control group was injected with the same volume of distilled water used in the treated animals. After one, two, three, and four weeks a group of mice (2 control and 5 treated) was sacrificed 24 hours after the last injection.
Twenty-eight animals were used for histological studies; the animals were divided into 5 groups (one control group of eight animals and four treated groups (T1, T2, T3, T4) of five animals each. Each treated group was injected intraperitoneally daily with piroxicam (0.3 mg/kg/day) for one, two, three, and four weeks respectively. The negative control group was injected with the same volume of distilled water used in the treated animals. After one, two, three, and four weeks a group of mice (2 control and 5 treated) was sacrificed 24 hours after the last injection.
Histological and histochemical preparations: Animals from control and treated groups were sacrificed; dissected and small pieces of the liver and kidney were quickly removed, then fixed in Carnoy's fixative fluid. Following fixation, specimens were dehydrated, embedded, and then sectioned to 5 microns thickness. For histological examinations, sections were stained with Ehrlich Haematoxylin and Eosin [7]. In the histochemical study, sections were stained with periodic acid-Schiff's method to demonstrate carbohydrates [8], with bromophenol blue method to demonstrate total proteins [9], and with Fuelgen method to demonstrate DNA [10]. Many slides have been carefully examined for each animal (each group contained 5 animals and for each animal at least 3 slides from different areas of the organ were examined.
Animals from control and treated groups were sacrificed; dissected and small pieces of the liver and kidney were quickly removed, then fixed in Carnoy's fixative fluid. Following fixation, specimens were dehydrated, embedded, and then sectioned to 5 microns thickness. For histological examinations, sections were stained with Ehrlich Haematoxylin and Eosin [7]. In the histochemical study, sections were stained with periodic acid-Schiff's method to demonstrate carbohydrates [8], with bromophenol blue method to demonstrate total proteins [9], and with Fuelgen method to demonstrate DNA [10]. Many slides have been carefully examined for each animal (each group contained 5 animals and for each animal at least 3 slides from different areas of the organ were examined.
Results
Histological changes in liver tissue
Examination of liver sections of mice 24 hours following the last therapeutic dose injection for one week (T1) showed that the liver had lost its characteristic architecture compared with the control group (Fig. 1 A, B).
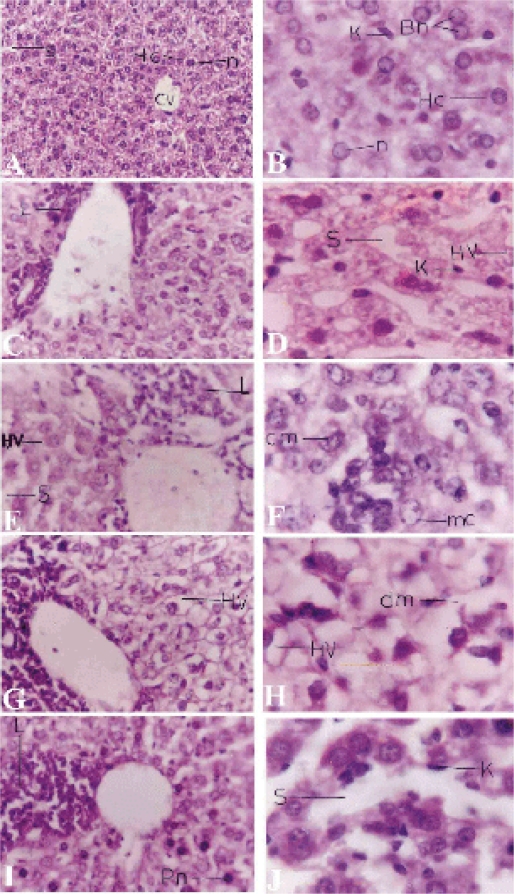
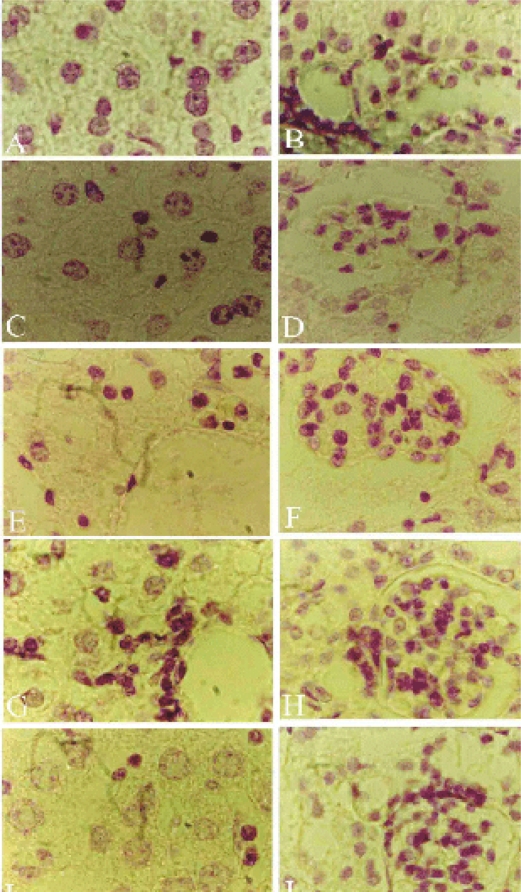
Figure 1.
Light micrograph of the liver sections. A,B Control liver with central vein (CV) and surrounding hepatocytes (HC), sinusoids (S) lined with Kupffer cells (K). C,D T1 group liver sections treated with piroxicam for one week with inflammatory cellular infiltrations (L) around the hepatic vein, dilated blood sinusoids (S), hepatocytic vacuolations (HV) and prominent Kupffer cells. E,F T2 group liver sections treated for 2 weeks appearing with hepatocytic vacuolations, inflammatory cellular infiltration, dilated sinusoids. In the higher magnification marginal chromatin (mc) pattern appear in some hepatocytes and clumped chromatin (cm) in others. G,H T3 group liver sections treated for 3 weeks with increased inflammation and vacuolation. I,J T4 group treated sections for 4 weeks appearing with more inflammatory cells, some pyknotic cells (Pn), widened sinusoids and numerous Kupffer cells. Sections were stained with hematoxylin-eosin. Magnifications, X400 (A,C,E,G&I) and X1000 (B,D,F,H&J).
Examination of liver sections of mice 24 hours following the last therapeutic dose injection for one week (T1) showed that the liver had lost its characteristic architecture compared with the control group (Fig. 1 A, B).
The cytoplasm of the hepatocytes was characterized by having coarse, pink, darkly stained granules and few vacuoles in the T1 group (Fig. 1 C, D) and an increased number of vacuoles in the T2 group (Fig. 1 E,F). Inflammatory cellular infiltration was abundant around the central vein in both T1 and T2 groups. In the T2 group the nuclei appeared larger and more irregular in shape than the T1 group (Fig. 1 D, F), with very little peripheral condensed chromatin. Meanwhile, clumped chromatin was also observed in some hepatocytes (Fig. 1 F). In the case of the T3 group, hepatocytes were swollen and their cytoplasm appeared to be highly vacuolated with irregular, darkly stained nuclei. The sinusoidal spaces were highly obliterated. The cellular infiltration was more intense than the previous groups. In the case of T4 group, the hepatocytes appeared more or less normal in size and shape, but their cytoplasm stained darker with eosin than the previous treatments. In addition, the cytoplasmic vacuoles were less in number, and the nuclei of most of the hepatocytes appeared with numerous dense clumps of chromatin and some nuclei appeared pyknotic. The blood sinusoids were wider than the T1 group. Kupffer cells nuclei appeared pyknotic and increased in number compared with previous treatments (Fig. 1 I, J).
Histological changes in renal tissue
The normal histological structure of the kidney is shown in figure 2 A, B. T1 group kidney sections appeared with a slight shrinkage of the glomeruli. (Fig. 2 C, D).
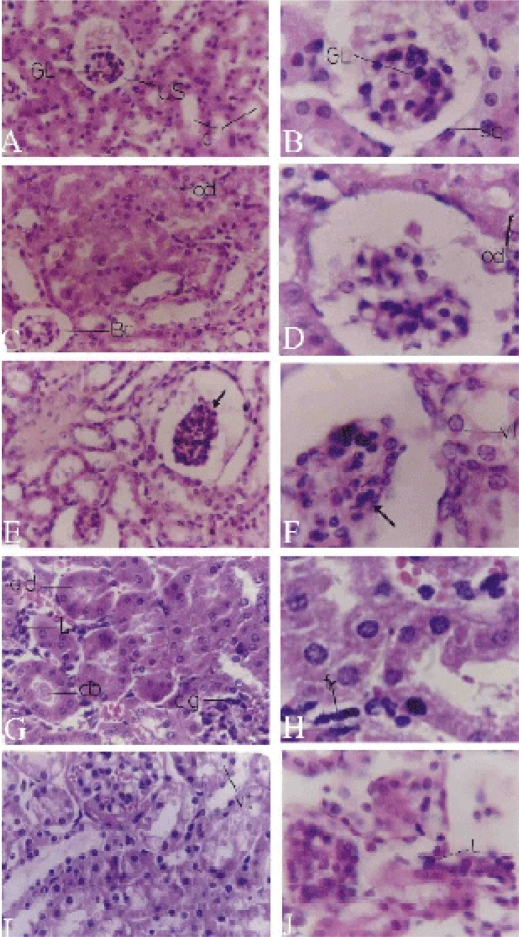
Figure 2.
Light micrographs of kidney. A,B Control kidney with Bowman's capsule with peripheral squamous epithelium(sq), glomerulus (GL), urinary space (US) and normal convoluted tubules (c). C,D T1 group kidney treated with piroxicam for one week with shrinked glomeruli, widened urinary space of the Bowman's capsule and oedema (od). E,F T2 group kidney treated for 2 weeks appearing with shrunken glomeruli (arrow), vacuolated tubules and darkly stained nuclei of the mesangial cells and vacuolations (vt) of the kidney tubules. G,H T3 group kidney treated for 3 weeks appearing with congested glomeruli (cg), odema of kidney tubules (od), inflammation between tubules (L), cell debris inside the tubules (db) and fibroblasts (fr). I,J T4 group treated for 4 weeks appearing with congested glomeruli, vacuolated kidney tubules (vt) and inflammatory cellular infiltration (L). Sections were stained with H&E. Magnifications, X400 (A,C,E,G&I) and X1000 (B,D,F,H&J).
This shrinkage increased in the T2 group (Fig. 2 E, F). However, in T3 and T4 groups, some glomeruli increased in size obliterating the urinary spaces (Fig. 2 G,I). The urinary space appeared wider in the T1 and T2 groups than in the control group (Fig. 2 A–F). Slight oedema of the tubular cells appeared in the T1 group (Fig. 2 D) and became more pronounced in the T3 group (Fig. 2 G). The tubular cells appeared swollen with coarse pink cytoplasmic granules especially in the cells of the proximal convoluted tubules. Some blood sinusoids appeared to be filled with erythrocytes (Fig. 2 G).
The capillaries of most glomeruli of the T2 and T3 groups appeared more or less congested (Fig. 2 E, G). A few proximal convoluted tubule cells were vacuolated and swollen with pink acidophilic granules and the nuclei appeared to be slightly swollen than normal. Lumens of these convoluted tubules were obliterated or highly reduced; containing some debris of hyaline casts (Fig. 2 E–H). Inflammatory cells were observed in the intertubular spaces of the T3 group (Fig. 2 G). The erythrocytes had a deformed structure. In addition, fibrocytes increased in the intertubular tissue (Fig. 2 H). The kidney of the T4 group had severe inflammatory cellular infiltration (Fig. 2 J). Also, the mesangial cells increased in both number and size, and their nuclei appeared pyknotic (Fig. 2 I, J). Most of the cells of the convoluted tubules were highly swollen and their lumens were nearly obliterated. The lumens of some tubules appeared to contain some remains of the glomerular filtrate.
Histochemical changes in liver and kidney tissue
Glycogen content: Control liver sections stained with PAS method are shown in figure 3 A. The T1 group had a slight decrease in glycogen in some hepatocytes. Glycogen appeared around the cell membrane (Fig. 3 C).
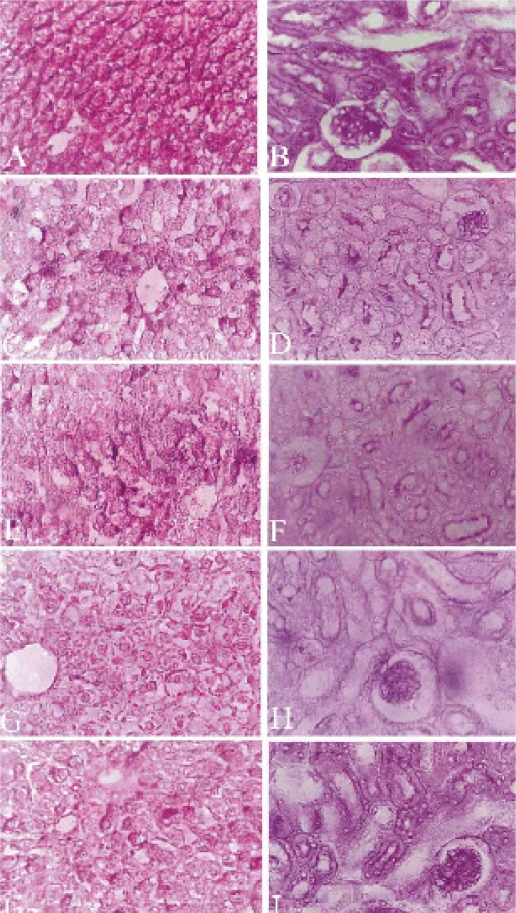
Figure 3.
Light micrograph of the liver (A,C,E,G&I) and kidney (B,D,F,H&J) sections showing the distribution of glycogen. A Control liver, glycogen in hepatocytes with intense red color, nuclei have no stains. C T1 group liver section with a slight decrease in glycogen. E T2 group with apparent decrease of glycogen. G T3 group with a severe decrease in glycogen. I T4 group with a marked decrease in glycogen. B Control kidney section with moderate PAS positive material in the cytoplasm and brush borders of the proximal convoluted tubules. Glomeruli were intensely positive to PAS reaction. D,F,H&J are T1,T2,T3&T4 groups respectively with a decreased amount of PAS positive material in tubules and glomeruli. Sections were stained with periodic Schiff's method. Magnifications, X 400.
Glycogen depletion became more prominent in liver cells of mice treated for two and three weeks. In addition, the glycogen was not homogenously distributed (Fig. 3 E, G). Liver sections examined after four weeks of treatment showed decreased glycogen content (Fig. 3 I) as compared to the control liver sections (Fig. 3 A).
Glycogen content in the T1 group kidney sections appeared similar to liver sections. Tissue revealed a reduced amount of cytoplasmic glycogen, with diminished density of basement membranes, and brush borders of proximal convoluted tubules (Fig. 3 D). Also, the glomeruli were less positive than those of the control group. However, glomeruli of the T2 group revealed more positive PAS reaction and intense brush border of the proximal convoluted tubules (Fig. 3 F). The T3 and T4 group kidney sections appeared intensely positive to PAS reaction with moderate dense positive material inside the lumen of the kidney tubules (Fig. 3 H, J).
Total proteins: Examination of liver and kidney sections from the control group, stained by bromophenol blue method, showed normal protein content (Fig. 4 A, B).
Figure 4.
Light micrograph of the Liver (A,C,E,G&I) and kidney (B,D,F,H,&J) sections showing the protein content. A Control liver with a considerable amount of protein elements in the cytoplasm of hepatocytes. C T1 group liver section with a weak response towards bromophenol blue reaction. E T2 group with a decreased protein content. G T3 group with a very low amount of protein content, although the stainability of plasma membrane increased. I T4 group with a decreased amount of protein. B Control kidney section with homogenous dense blue colored granules as their positive affinity to bromophenol blue. D,F,H&J are T1,T2,T3&T4 groups respectively with increased protein content. Sections were stained with bromophenol blue method. Magnifications, X 400.
Liver sections of the T1 group had a slight decrease in the protein content (Fig. 4 C). Sections of the T2 group showed that the cytoplasm contained numerous dark blue stained protein granules (Fig. 4 E). For the T3 group the protein content was significantly decreased and the cytoplasm appeared more vacuolated (Fig. 4 G). In the liver sections of the T4 group, protein content was moderately decreased in the hepatocytes (Fig. 4 I). Proteins of the control kidney tubules and glomeruli showed homogeneously dense blue-colored granules due to a positive affinity to bromophenol blue staining (Fig. 4 B). In all treated groups, the protein content was slightly decreased in the kidney tubules and glomeruli (Fig. 4 D, F, H, and J).
Deoxyribonucleic acid (DNA): The T1 group liver sections, stained by Fuelgen method, showed high content of coarse chromatin in the nuclei of the hepatocytes as well as in the nuclei of Kupffer cells (Fig. 5 C).
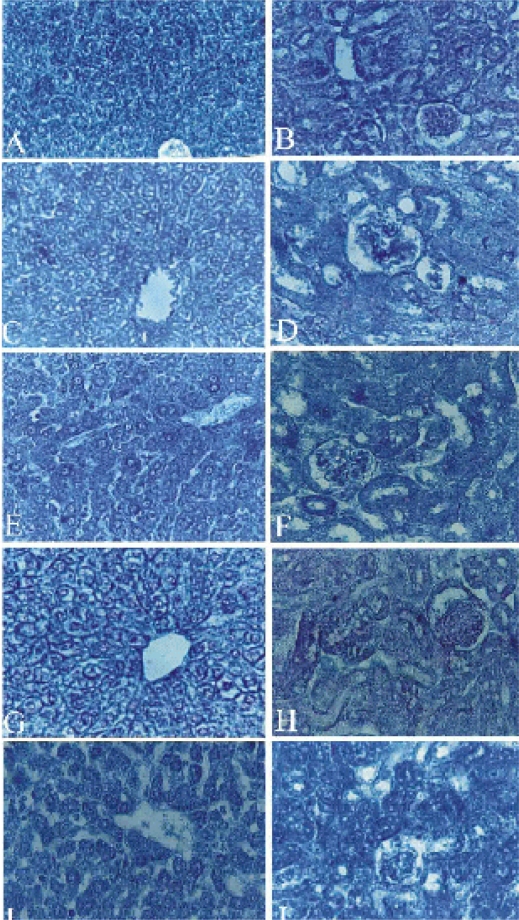
Figure 5.
Light micrograph of the Liver (A,C,E,G&I) and kidney (B,D,F,H&J) sections showing the DNA content. A Control liver with a faint red purple colored particles in the nucleoplasm of hepatocytes and Kupffer cells. C T1 group liver section with increased coarse chromatin. E T2 group with a moderate reduction of coarse chromatin, densely stained nuclei of the Kupffer clls and inflammatory cells. G T3 group with a decreased amount of DNA content. I T4 group with a very low amount of DNA in hepatocytes and darkly stained nuclei of the inflammatory cells. B Control kidney section with DNA particles (chromatin) appeared as faint red purple color. D,F,H&J are T1,T2,T3&T4 groups respectively with decreased DNA content in tubules while increased stainablity of mesangial cells. Sections were stained with Feulgen method. Magnifications, X 400.DISCUSSION
The T2 group liver sections had a moderate reduction in fine chromatin (Fig. 5 E) than did the control group (Fig. 5 A), but more than the T1 group (Fig. 5 C). In addition, Kupffer cells appeared densely stained while the nuclei of most of the inflammatory cells appeared darkly stained (Fig. 5 E, G). The T4 group liver sections showed an increase in colored DNA particles and vacuolated nuclei. The nuclei of the inflammatory cells that appeared near the central vein were densely stained (Fig. 5 J).
Kidney sections showed that some particles containing DNA material appeared in the nuclei. In some cells, these particles were abundant, densely stained and scattered in the nucleoplasm, while in the other cells they appeared fewer in number, faintly stained, and were noted mainly in the nuclear periphery. In other cells, the granules of DNA were attached to the nucleoli.
The T1, T2 and T3 groups were observed to have a severly decreased coarse chromatin in the nuclei (Fig. 5 D, F, H) while the T4 group showed a slight increase in the chromatin granules (Fig. 5 J). The nuclei of the mesangial cells had a more positive reaction in all treated groups than those of the control group (Fig. 5 B).
Discussion
In this study, the effect of piroxicam on liver and kidney was time dependant. There were increased effects with prolonged time of dose administration. Retention of water inside hepatocytes resulted in oedema, which may have occurred due to a reduction of energy necessary for the regulation of ion concentration in the cells as suggested by Yukiko et al., [11]. In general, NSAIDs are well known to induce hepatic injury [5, 12]. Also the pathological changes may lead to impaired liver function which interferes with the secretion of plasma proteins [5, 6]. This leads to decreased blood osmotic pressure, with subsequent decreased drainage of tissue fluids, which explains the oedema and congestion observed in the different tissue.
Zhang and Wang [13] suggested that the cytoplasmic vacuolation is mainly a consequence of considerable disturbance in lipid inclusions and fat metabolism occurring during pathological changes. Also, vacuolar degeneration has been regarded by Durham et al. [14] to be an alteration produced to collect the injurious substances in the cells. In this study, the vacuolation of the cytoplasm of the liver cells appeared at first in the hepatocytes of the peripheral zone of the hepatic lobules, extending gradually toward the center. This may be due to the direction of the lobular blood supply. Vacuolation and damage of liver cells were noted by other investigators following treatment with different agents [15–17].
Results also showed a remarkable cellular infiltration in the hepatic tissue. This supports El-Banhawy et al. [15] whose studies suggested that abundance of leucocytes, in general, and lymphocytes, in particular, are a prominent response of body tissues facing any injurious impacts. Leukocyte elevations and adherence to the vascular endothelium have been suggested by Miura et al. [18] and McCafferty et al. [19] to play an important role in the pathogenesis of NSAID-associated injury.
Drug concentration in the blood is affected by capillary constriction leading to a decrease in glomerular filtration of that drug which minimizes its effect and protects the tubular cells [20]. This may affect the shrinkage and atrophy of the glomeruli. At the same time, the mesangial cell processes may be retracted due to the contraction of their filaments, which may be stimulated by angiotensin II present in these cells. However, the glomerular hypertrophy after 4 weeks may be due to the proliferation of mesangial cells, which secrete more matrixes. Furthermore, the blood capillaries appeared engorged with red blood cells and the urinary space was completely obliterated. This finding may agree with the effect of endomethacin injection for three weeks [21].
The tubular lesions observed in the present study were accompanied by invasion of inflammatory cells to the intertubular tissues in a trial to minimize the injury. Some of these external stressors apparently caused the tubular lesions. In the present study, the different segments of the loop of Henl were less affected with piroxicam dose, which may suggest that the main target of this drug is the convoluted and collecting tubules. Similar findings had been presented by El-Banhawy, et al. [21].
The cells of the proximal convoluted tubules became edematous leading to distention and retraction of most microvilli and destruction of others. These may be due to a decreased reabsorption of the glomerular filtrate which counteracts the toxicity of the drug. In agreement with the present results are those of Jackson and Lawrence [22] who found that treatment with either indomethacin or phenylbutazone caused papillary necrosis, tubular degradation as well as inflammatory cellular infiltration. Moreover, Abrahams and Levinson [23] reported damage in the cell lining of the collecting tubules in the kidney of rats when administered excessive quantities of analgesic mixture of asprin, phenacetin and caffine. Furthermore, striking lesions in the kidney cells and tissues of rats treated with the analgesic narcotic drug flunitrazepam were observed by El-Banhawy et al. [24].
Many researches have reported marked alteration in the fine structure of the cellular component of the proximal convoluted tubulesas a result of the treatment with different toxic substances [25–28].
After four weeks of treatment, it was observed that hepatocytes seemed approximately normal. Other investigators have reported tissue adaptation to the injury produced by NSAIDs [29, 30]. However, no histochemical changes improvement, particularly DNA, was noted in the liver tissue after four weeks of injections. Actually, hepatocytes DNA content was very low. Depleted and disturbed DNA in the hepatocytes indicated that these cells remained impaired by the action of the drug. In a previous study with piroxicam we have reported time dependant chromosomal abnormalities in bone marrow cells [30].
Regarding the histochemical changes observed in this study under piroxicam administration, results clearly indicated reduction in the polysaccharides and total proteins in the liver and kidney tissues. These changes were consistent with those induced histopathologically for the T1, T2 and T3 groups. A similar decrease was induced by voltarine [30]. The decrease in carbohydrate content was attributed by some investigators to be due to increased stress on organs, leading to high energy consumption which allowed an equalized pressure to be exerted upon them. The decrease in protein could be attributed to the disruption of lysosomal membranes under the effect of various toxicants leading to the liberation of their hydrolytic enzymes in the cytoplasm resulting in marked lysis and dissolution of the target material. This result confirmed that of Awasthi et al. [32] who found elevated lysosomal enzymatic activity accompanied by a decrease in protein and nucleic acids content in response to organophosphate insecticide.
It is clear from this study that piroxicam has drastic toxic effects on liver and kidney tissues as represented by the observed histopathological changes. This would suggest that NSAIDs, especially piroxicam, should be used under strict medical control, and these serious side effects should be considered and taken into consideration when using prioxicam in treatment of patients.
References
- 1.Zvaifler NJ. New perspectives on the pathogenesis of rheumatoid arthritis. Am J Med. 1988;85:12. doi: 10.1016/0002-9343(88)90356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke JD, Scudamore RA. Studies on the pathogenesis of rheumatoid arithritis. Br J Rheumatol. 1989;28:243. doi: 10.1093/rheumatology/28.3.243. [DOI] [PubMed] [Google Scholar]
- 3.Insel PA. Analgesic antipyretics and anti-inflammatory agents: Drugs employed in the treatment of rheumatoid arthritis and gout. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. 8th ed. New York: Pergamon press; 1991. p. 638. [Google Scholar]
- 4.Knottenbelt C, Chambers G, Gault E, Argyle DJ. The in vitro effects of piroxicam and meloxicam on canine cell lines. J Small Anim Pract Jan. 2006;47(1):14–20. doi: 10.1111/j.1748-5827.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- 5.Lapeyre-Mestre M, de Castro AM, Bareille MP, Del Pozo JG, Requejo AA, Arias LM, Montastruc JL, Carvajal A. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol. 2006;20(4):391–395. doi: 10.1111/j.1472-8206.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 6.Lacroix I, Lapeyre-Mestre M, Bagheri H, Pathak A, Montastruc JL. Nonsteroidal anti-inflammatory drug-induced liver injury: a case-control study in primary care. Fundam Clin Pharmacol. 2004;18(2):201–206. doi: 10.1111/j.1472-8206.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- 7.Adam H, Caihak G. Fischer Verlag Stuttgart; 1964. Grosses zoologisches parktikum tell. Arbeitsmethoden der makroskopischen und mikroskopischen anatomic Mit 283 Abbildungen Gustav. [Google Scholar]
- 8.Hotchkiss RD. A microchemical reaction resulting in the staining of polysaccharide structures in fixed tissue‘preparations. Arch Biochem. 1948;16:131. [PubMed] [Google Scholar]
- 9.Maize D, Brewer PA, Affert M. The cytochemical staining and measurements of protein with mercuric bromophenol blue. Bid Bull. 1953;104:57–67. [Google Scholar]
- 10.Garvin AJ, Hall BJ, Brissie RA, Spicer SS. Cytochemical differentiation of nucleic acid with sciff-methylene blue sequence. J histochem. Cytochem. 1976;24:587–590. doi: 10.1177/24.4.58023. [DOI] [PubMed] [Google Scholar]
- 11.Yukiko T, Sokpong L, Michio U. In vitro Effects of non-steroidal anti-inflammatory drugs on oxidative phosphorylation in rat liver mitochondria. Biochem Pharmacol. 1977;26:2101–2106. doi: 10.1016/0006-2952(77)90258-1. [DOI] [PubMed] [Google Scholar]
- 12.Hargus SJ, Martin BM, George JW, Pohl LR. Covalent modification of rat liver dipeptidyl peptidase IV (CD 26) by the non-steroidal anti-inflammatory drug diclofenac. Chem Res Toxicol. 1995;88:993–996. doi: 10.1021/tx00050a001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang LY, Wang CX. Histopathological and histochemical studies on toxic effect of brodifacoum in mouse liver. Acta Acad Med Sci. 1984;6(5):386–388. [PubMed] [Google Scholar]
- 14.Durham SK, Brouwer A, Barelds RJ. Comparative endotoxin-induced hepatic injury in young and aged rats. J Pathol. 1990;162:341–349. doi: 10.1002/path.1711620412. [DOI] [PubMed] [Google Scholar]
- 15.El-Banhawy MA, Sanad SM, Sakr SA, ElElaimy IA, Mahran HA. Histopathological studies on the effect of the anticoagulant rodenticide “Brodifacoum” on the liver of rat. J Egypt Ger Soc Zool. 1993;12(C):185–227. [Google Scholar]
- 16.Elewa FH. Histopathological alterations induced by antibiotics in the mouse hepatic and nervous tissues. J Egypt Ger Soc Zool. 1996;21(C):29–52. [Google Scholar]
- 17.Amer MA, Elewa FH, El-Shershaby AM, Abdel-Azia AM. Histopathological and histochemical effects of eimerian infection on the host tissues: liver of rabbit. Egypt J Zool. 1998;31:1–43. [Google Scholar]
- 18.Miura S, Suematsu M, Tanaka S, Nagata H, Houzawa Sl, Suzuki M, Kurose I, Serizawa H, Tsuchiya M. Microcirculatory distrubance in indomethacin- induced intestinal ulcer. Am J Physiol. 1991;24:213–309. doi: 10.1152/ajpgi.1991.261.2.G213. [DOI] [PubMed] [Google Scholar]
- 19.McCafferty D, Granger DN, Wallace JL. Indomethacin-induced gastric injury and leukocyte adherence in arthritic versus healthy rats. Gastroenterol. 1995;109:1173–1180. doi: 10.1016/0016-5085(95)90576-6. [DOI] [PubMed] [Google Scholar]
- 20.Stevens A, Lowe J. Histology Gower Medical Publishing. London. New York: 1997. [Google Scholar]
- 21.El-Banhawy MA, Ilham IS, Mohamed AS, Ramadan AR. The toxic impacts of the anti-inflammatory drug (Indomethacin) on the mice kidney tissues. J Egypt Ger Zool. 1994;14(C):177–201. [Google Scholar]
- 22.Jaekson B, Lawrence RJ. Renal papillary necrosis associated with indomethacin and phenylbutazone treated rheumatoid arthritis. Aus NZJ Med. 1978;8(2):165–167. doi: 10.1111/j.1445-5994.1978.tb04504.x. [DOI] [PubMed] [Google Scholar]
- 23.Abrahams C, Levinson E. Ultrastructure of the renal papilla in experimentally induced analgesic nephritis in rats. S Afr Med H. 1970;44(3):63–65. [PubMed] [Google Scholar]
- 24.El-Banhawy MA, Mohallal EM, Hamdy MH, Attia TNN. The toxic impacts of the narcotic drug (flunitrazepam) on the rat kidney tissues. Zag J Med Physiol. 1992;1(3):233–39. [Google Scholar]
- 25.Trump HF, Bulger RE. New York: Hoeber. Medical Division; 1968. The morphology of the kidney: the structure basis of renal disease, Harper and Row. [Google Scholar]
- 26.Corrire ED, Admas GL. Ultra-structural lesions in goats given a lethal dose of imidocarl diproprionate. Am J Vet Res. 1977;38(2):217–23. [PubMed] [Google Scholar]
- 27.El-Banhawy MA, Maguid HM, El-Akkad MM. Ultra-structural examination of the kidney of albino rats treated with ketamino hydrochloride (Ketalar) Zag Univ Med J. 1989;11(3):127–36. [Google Scholar]
- 28.Al-Thani AS. Faculty of Science. Cairo, Egypt: Ain Shams University; 1993. The side effects of chloramphenicol on some histological, histochemical and ultra-strucutral aspects of the liver and kidney of the white rat Ph.D. Thesis. [Google Scholar]
- 29.Skeljo MV, Cook GA, Elliott SL, Giraud AS, Yeomans ND. Gastric mucosal adaptation to diclofenac injury. Dig Dis Sci. 1996;41(1):32–39. doi: 10.1007/BF02208581. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim MA. M. Sc. Thesis. Fac Sci Helwan Univ; 1999. A study of the histochemical changes in some mammalian tissues induced by a NSAID (Diclofenac) [Google Scholar]
- 31.Mohamed AD. M.Sc. thesis. Egypt: Helwan University; 1999. Histological and cytogenetic effect of piroxicam induced in vivo mammalian system. [Google Scholar]
- 32.Awasthi M, Shah P, Dubale M, Gadhia P. Metabolic changes induced by organophosphates in the piscine organs. Environm Res. 1984;35:320–325. doi: 10.1016/0013-9351(84)90138-5. [DOI] [PubMed] [Google Scholar]