Abstract
Objective
To determine predictors for outcomes of traumatic brain injury (TBI) in infants and children younger than twelve years admitted to our pediatric intensive care units (PICU).
Methods
This is a retrospective cohort study from 2004-5, done at the PICU of King Fahad Hofuf Hospital, Eastern Province, Saudi Arabia. One hundred and six patients with TBI; 65 boys and 41 girls ages 12 or under, with a mean age of 5.7 years, were included. Of them, 11.3% died (Deaths group), 11% survived with neurological deficits (NDgroup), and 77% survived with no neurological deficits (NND-group). The potential predictors for death or neurological deficits were examined.
Results
83% of deaths had initial Glascow coma scale (GCS) of ≤ 4/15, 50% of ND had initial GCS ≤8 and 27% of NND had GCS < 12. The initial brain CT was abnormal in 92% of deaths and ND groups, but in only 37% of NND. Combined brain pathologies were found in 92% of deaths, 63% of ND and only in 5% of NND. Hypotension was seen in 67% of deaths, 17% ND and only in 1% of NND. Mechanical ventilation was required in all deaths and more than half of ND. Liver enzymes were high in 50% of deaths and 66% of ND but in only 20% of NND. Serum albumin was low in 33% of deaths, 42% of ND and only 1% NND.
Conclusion
Glasgow coma score, brain CT findings, combined brain pathologies, hypotension, high liver enzymes and low serum albumin predict outcome after TBI in pediatric age group.
Keywords: brain injury, pediatric trauma, children, head trauma
Introduction
Traumatic brain injury (TBI) is a common condition in pediatric intensive care units (PICU). It is an important cause of morbidity and mortality in children. Comprehensive studies on outcomes after TBI are scarce [1]. Hospital admission for observation is currently a standard practice for patients with TBI, despite the performance of diagnostic studies to exclude the presence of an intracranial injury. There are many reasons for this practice; one of them is the medicolegal consideration about the risk of early discharge [2].
The aim of this study was to determine clinical, laboratory, and radiological variables which might be predictors for PICU outcomes of TBI in children under twelve years of age.
Methods
This is a retrospective cohort study which reviews children with TBI who had been admitted to the PICU of the King Fahad Hofuf Hospital, which is the main surgical referral centre of the Al-Hasaa area (Eastern Province, Arabia). The period of the study extended from January 2004 to December 2005. The main criteria for hospital admissions were: reduced Glasgow Coma Score (GCS), presence of abnormal findings on computerized tomography scans (CT) of brain, patient's age below one year, multiple traumas, post-traumatic seizures, vomiting, headache or amnesia, signs of trauma above the clavicles, circulatory or respiratory instability, and presence of a significant underlying medical disorder. On admission, patients with TBI had been classified according to the GCS into mild (13–15), moderate (9–12) and severe (≤8). After initial basic life support; all patients were subjected to clinical examination and radiological and laboratory investigations, depending on the severity of TBI. CT scan of the brain was done in all cases upon admission and repeated within 12 hours if there was intracranial bleeding or clinical deterioration. Stable cases were usually observed for no less than 12 hours and no more than 24 hours. Patients were then discharged from the PICU to the intermediate care unit or the ward. Criteria for discharge were: stability of vital signs for at least twelve hours, stable venous/arterial blood gases, oxygen saturation >95% on room air, and the ability to tolerate oral or nasogastric feedings.
Every patient was carefully observed for neurological deficits which were defined as muscle paresis, persistent impairment of consciousness, persistent or intermittent seizures, and persistent cranial nerve palsies beyond the PICU stay period. Exclusion criteria included patients who died before arrival to the PICU, those with spinal cord injuries, those discharged against medical advice, or those with previous neurological problems such as cerebral palsy or other serious medical disorders. Outcomes (from PICU) were classified into deaths and survivors with neurological deficits (ND) and survivors with no neurological deficits (NND).
Potential predictors for outcomes were monitored for all patients. These included GCS at admission, initial CT brain findings (done within the first 3 hours after injury), type of brain lesions, associated skull fractures, other body traumas, initial hypotension (mean arterial blood pressure lower than 5th percentile value for age), liver enzymes (Aspartate and Alanine transaminases) levels, and serum albumin.
This study was conducted according to the local regulations of the King Fahad Hofuf Hospital.
Statistical methods: Data were subjected to statistical analysis including Chi-square and validity tests. The last included specificity, sensitivity, positive and negative predictive values, and positive and negative likelihood ratios (LR+ and LR)
Results
106 children (65 boys and 41 girls) less than age twelve (mean age of 5.7) with TBI were included. All patients were Arabic, of which 92.5% were Saudi citizens. Automobile accidents represented the most common cause of TBI in our study (43.4%), followed by pedestrian accidents, then fall from a height (23.5% and 22.5% respectively). Much less common were bicycle related accidents. Rare causes reported, were physical abuse in a 3 months female baby. Mechanism of injury was not identified in two patients. Two thirds of deaths and one third of survivors with neurological deficit (ND) were exposed to automobile accidents. Fifty percent of ND was pedestrian accidents and falls.
Deaths
Twelve children died (7 girls, 5 boys) with mean age of 5.5 years. GCS was ≤4/15 in 10 patients. Two patients had GCS of 9 on presentation, which deteriorated rapidly to 3 in <12 hours. Other traumatic injuries including fractures, lung contusion, and peri-orbital hematoma were identified in 7 patients (58.3%). Eight cases presented with hypovolemic shock. Of those, 5 cases had subsequent multi organ system failure (41.7%). All patients received mechanical ventilation (MV). Ventilator settings were adjusted to keep partial pressure of carbon dioxide at the lower normal values. Liver enzymes were high in 6 patients (50%), serum albumin was low in 4 patients (33.3%). Brain CT findings were abnormal in all. Combined brain pathologies were evident in 11 patients (91.7%). The most frequently encountered pathology was intracranial edema followed by contusion, then subdural hematoma (SDH) (Table 1). Five patients (41.7%) had skull fractures of different patterns. Two had basal skull fractures. PICU stay ranged from <12 hours up to 240 hours with a mean of 76 hours.
Table 1.
Pathological types of brain injury on CT brain scan in the three groups of study
| Brain pathology | Death (12) | ND (12) | NND (82) | Total | X2 | P |
|---|---|---|---|---|---|---|
| edema | 8 | 8 | 4 | 20 | 6.3 | <0.05 |
| contusion | 4 | 4 | 9 | 17 | 9.1 | 0.01 |
| SDH | 4 | 4 | 1 | 9 | 5.6 | <0.025 |
| EDH | – | 3 | 7 | 10 | 4.7 | NS |
| ICH | 2 | 2 | – | 4 | 0.9 | NS |
| SAH | 1 | 1 | 2 | 4 | 0.07 | NS |
| IVH | 1 | 1 | – | 2 | 2.7 | NS |
NS: non-significant, SDH: subdural hematoma, EDH: extradural hematoma, SAH: subarachnoid hemorrhage, IVH: intraventricular hemorrhage.
Table 2.
Isolated versus combined brain pathologies in the three groups of the study.
| Death (12) | ND (12) | NND (82) | Total | X2 | P | |
|---|---|---|---|---|---|---|
| IP | 1 | 4 | 26 | 31 | 2.87 | NS |
| CP | 11 | 7 | 4 | 22 | 59.6 | <0.001 |
| Total | 12 | 11 | 30 | 53 | 26.2 | <0.001 |
IP: isolated pathology, CP: combined pathologies, NS: non-significant.
Survivors with neurological deficits (ND)
Twelve children (8 girls, 4 boys), mean age of 5.3 years, survived with neurological deficit. Six patients (50%) had cranial nerve palsies (6th and 7th cranial nerves). Six patients (50%) had GCS ≤8. Two patients (16.7%) had GCS of 9 and 10 respectively. Four patients (33.3%) had GCS ≥12; two of them had initial GCS of 14 which, dropped to <8 within 4 hours. Other traumatic injuries such as fractures and/or lung contusions were present in 5 patients (41.7%). Seizures, particularly focal, and myoclonic types were observed in 3 patients (25%). Bloody CSF rhinorrhea and/or otorrhea were reported in 3 patients (25%). Hypotension was initially present in 2 patients (16.7%). High liver enzymes were noted in 8 patients (66.7%) while low albumin was found in 5 patients (41.7%). Seven patients (58.3%) needed MV for one to seven days. Brain CT scan was abnormal in 11 patients (91.7%). Skull bone fractures were detected in 2 patients (16.7%). Three patients (25%) presented with no abnormality of brain or skull.
Survivors with no neurological deficits (NND)
Eighty-two patients; (56 boys, 26 girls) with a mean age of 5.8 years, survived with no neurological deficit. Twenty six patients (31.7%) had GCS of 15, and in these patients, TBI was suggested from history, scalp wounds, subgaleal hematoma and presence of fractures or abnormal brain CT. Thirty four patients (41.5%) had GCS>12. Twenty two patients (26.8%) had GCS <12; of this group, 8 patients had GCS ≤8. Other traumatic injuries were seen in 24 patients (29.3%), including limb fractures, lung contusion, liver contusion, renal trauma, and peri-orbital hematoma. Hypotension was reported in only one patient (1.2%). Seizures were reported in 5 patients (6.1%). Eleven patients (13.4%) had bloody CSF rhinorrhea and/or otorrhea. Elevated liver enzymes were found in 23 patients (28%) while serum albumin was low in only 1 patient (1.2%). Mechanical ventilation (MV) for no more than 24 hours was needed in 11 patients (13.4%). Initial brain CT was abnormal in 30 patients (36.6%). Combined brain pathologies were reported in four patients (13.3%). Twenty-three patients (28%) had skull fractures. Mean PICU stay ranged from 1 day to 1 week (mean of 95 hours).
Table 3.
Validity of GCSs of 15 and <12 in predicting associated brain lesions.
| Validity | GCS 15 For intact brain | GCS<12 For abnormal brain |
|---|---|---|
| Sensitivity | 41.5% | 90.9% |
| Specifity | 92.5% | 73.8% |
| +ve predictive value | 84.6% | 47.6% |
| −ve predictive value | 61.2% | 96.9% |
| LR+ | 5.5*** | 3.5** |
| LR− | 0.52* | 0.12*** |
LR+: likelihood ratio for positives, LR−: likelihood ratio for negatives,
poor,
fair,
good.
Discussion
Trauma is a common cause of mortality and morbidity in infants and children under twelve years of age in KSA. In this study, all injuries happened as a result of blunt trauma. No significant differences were found between the three groups with regard to the cause of accident. These findings differ from the study by Borczuk [3], who reported that icyclists and pedestrians struck by cars were more likely than others to sustain intracranial injury.
Our findings indicated that patients with GCS>12 may have potentially serious intracranial pathology and GCS <12 is an important predictor for death or ND. This differs from the findings of Moran et al., [4] who found that patients with no loss of consciousness and GCS of >13 had normal CT scans. On the other hand, Simon et al., [5] found that 14% of patients with GCS score of 14–15 had intracranial injury. They reported that a GCS score of 14 increases the likelihood of intracranial injury in patients with no loss of consciousness. Livingston et al., [2] reported a negative predictive value of cranial CT of 99.70%. Halley et al., [6] reviewed 98 subjects with TBI and GCS of 13–15. They found that normal examination plus a normal CT carry 90% probability of intact brain compared to 87% probability with normal CT only. They also reported that 10.5% of subjects with normal examination were noted to have evidence of intracranial injury. See et al., [7] reported that GCS set at 5 strongly correlated with a poor outcome in pediatric traumatic brain injury. On the other hand, Thakker et al., [8] found that although a low GCS is strongly associated with death or poor functional outcome, many patients with GCS of ≤5 (40.7%) survived with normal function. Lieh-Lai et al., [9] reported that 64% of children who presented with GCS of >3–5 survived with a satisfactory outcome.
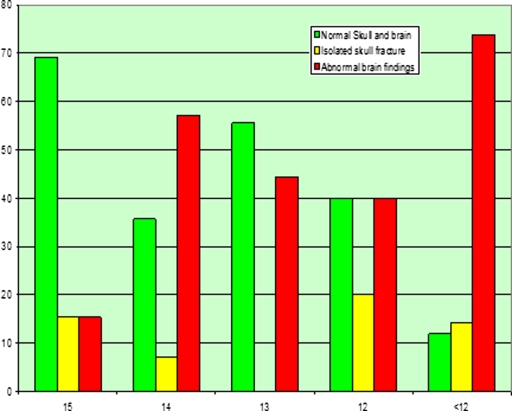
Figure 1.
Percentage of initial CT scan findings in corresponding to different figures of GCS
Table 4.
Validity of brain CT scan as a predictor for outcome.
| Sensitivity | 98.1 |
| Specifity | 63.4 |
| +ve predictive value | 58.5 |
| −ve predictive value | 98.1 |
| LR+ | 2.67* |
| LR− | 0.03**** |
fair,
excellent
Intracranial oedema represented the most common finding in deaths and survivors with ND with sensitivity of 66.7%, specificity of 95.5%, LR+ve of 14.8 and LR−ve of 0.3. Within the same groups, intracranial contusion and SDH were also significantly reported in 33% of patients. Our results are similar to those of See et al., [7] who reported, that SAH with intracranial oedema, SDH, ICH, and basal ganglion lesions were associated with severe injury and poor outcomes. As shown in our results (table 2), combined brain pathologies represented the great majority of the abnormal CT findings in deaths and survivors with neurological deficit (ND). This is in agreement with Borczuk [3] who reported that higher GCS scores have a greater chance of having a solitary CT abnormality.
Table 5.
Clinical, radiological and laboratory variables in the three groups of the study.
| predictor | Death (12) | ND (12) | NND (82) | Total(106) | X2 | P |
|---|---|---|---|---|---|---|
| GCS 15 | – | – | 26 | 26 | 10.1 | <0.01 |
| GCS<12 | 12 | 8 | 22 | 42 | 27.6 | <0.001 |
| Other injuries | 7 | 5 | 24 | 36 | 4.3 | NS |
| hypotension | 8 | 2 | 1 | 11 | 48.8 | <0.001 |
| MV | 12 | 7 | 11 | 30 | 44.7 | <0.001 |
| Skull fracture | 5 | 2 | 23 | 30 | 1.85 | NS |
| Abnormal CT brain | 12 | 11 | 30 | 53 | 26.2 | <0.001 |
| High liver enzymes | 6 | 8 | 23 | 37 | 8.22 | <0.05 |
| Low serum albumin | 4 | 5 | 1 | 10 | 29.1 | <0.001 |
MV: Mechanical ventilation, NS: non-significant.
Hypotension is a significant risk factor for death in these patients as it was noticed initially in 66% of deaths compared to 16% of ND and in only 1.2% of NND. The poor outcome was mostly associated with decreased cerebral perfusion pressure secondary to intracranial hypertension. Orliaguet et al., [10] stated that, initial hypotension in addition to GCS, and injury severity score are dependable predictors of outcome in children with traumatic brain injury.
Mechanical ventilation (MV) has been routinely used when GCS is <8, or if there is intracranial oedema. In this study, 27.3% of patients needed MV including all who eventually died, and more than half of those who sustained ND. According to our results, MV might be life-saving in 60% of patients who needed this type of respiratory support. However, the need for MV in itself may represent a risk factor for poor outcome (63.3% of patients on MV). Thakker et al, [8] reviewed 105 children who sustained head injuries, and who needed endotracheal intubation; the mortality was 18.1% and the neurological morbidity was 6.7%.
A noticeable finding in this study was the abnormal rise in liver enzymes and low levels of serum albumin which were all observed within the first 24 hours after admission. These patients have no clinical evidence of obvious intra-abdominal injuries. This alteration in liver functions may be explained by possible hepatic ischemia resulting from minor hepatic sub-clinical trauma, hemodynamic instability or possible hypoxia associated with cardiopulmonary resuscitation, associated lung contusions or use of MV. These events were expected to be more pronounced in severe TBI and hence a higher incidence of death or neurological deficits. Rise in liver enzymes with or without low serum albumin can be added risk factors for increased risk of mortality and development of motor deficits.
The most important risk factors for death or neurological deficit are combined brain pathologies and GCS ≤8. This risk is increased when other risk factors are added. GCS≥12 predicts survival with no neurological damage.
Combination of GCS<12, combined brain pathologies, initial hypotension, the need for mechanical ventilation, and elevated liver enzymes were associated with 100% poor outcome (6 deaths and two survivors with neurological deficit).
Conclusion
Glasgow coma score, brain CT findings, combined brain pathologies, hypotension, high liver enzymes and low serum albumin significantly predict outcome after TBI in this pediatric age group. A large study is needed to devise a scoring system based on these factors.
References
- 1.Campbell CG, Kuehn SM, Richards PM, Ventureyra E, Hutchison JS. Medical and cognitive outcome in children with traumatic brain injury. Can J Neurol Sci. 2004 May;31(2):213–9. doi: 10.1017/s0317167100053853. [DOI] [PubMed] [Google Scholar]
- 2.Livingston DH, Lavery RF, Passannante MR, Skurnick JH, Baker S, Fabian TC, Fry DE, Malangoni MA. Emergency department discharge of patients with a negative cranial computed tomography scan after minimal TBI. Ann Surg. 2000 Jul;232(1):126–32. doi: 10.1097/00000658-200007000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borczuk P. Predictors of intracranial injury in patients with mild TBI. Ann Emerg Med. 1995 Jun;25(6):731–6. doi: 10.1016/s0196-0644(95)70199-0. [DOI] [PubMed] [Google Scholar]
- 4.Moran SG, McCarthy MC, Uddin DE, Poelstra RJ. Predictors of positive CT scans in the trauma patient with minor TBI. Am Surg. 1994 Jul;60(7):533–5. discussion 535–6. [PubMed] [Google Scholar]
- 5.Simon B, Letourneau P, Vitorino E, McCall J. Pediatric minor TBI: indications for computed tomographic scanning revisited. J Trauma. 2001 Aug;51(2):231–7. doi: 10.1097/00005373-200108000-00004. discussion 237–8. [DOI] [PubMed] [Google Scholar]
- 6.Halley MK, Silva PD, Foley J, Rodarte A. Loss of consciousness: when to perform computed tomography? Pediatr Crit Care Med. 2004 May;5(3):230–3. doi: 10.1097/01.pcc.0000123543.40224.73. [DOI] [PubMed] [Google Scholar]
- 7.See LC, Wong AM, Tang SF, Cheng PT, Chen CL, Chung CY. Critical score of Glasgow Coma Scale for pediatric traumatic brain injury. Pediatr Neurol. 2006 May;34(5):379–87. doi: 10.1016/j.pediatrneurol.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Thakker JC, Splaingard M, Zhu J, Babel K, Bresnahan J, Havens PL. Survival and functional outcome of children requiring endotracheal intubation during therapy for severe traumatic brain injury. Crit Care Med. 1997 Aug;25(8):1396–401. doi: 10.1097/00003246-199708000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Lieh-Lai MW, Theodorou AA, Sarnaik AP, Meert KL, Moylan PM, Canady AI. Limitations of the Glasgow Coma Scale in predicting outcome in children with traumatic brain injury. J Pediatr. 1992 Feb;120(2Pt 1):195–9. doi: 10.1016/s0022-3476(05)80426-3. [DOI] [PubMed] [Google Scholar]
- 10.Orliaguet GA, Blanot S, Laurent-Vannier A, Renier D, Carli PA. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: Experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006 Aug 1; doi: 10.1097/01.PCC.0000235245.49129.27. [DOI] [PubMed] [Google Scholar]