Abstract
Purpose
To present a patient with acute idiopathic blind spot enlargement syndrome who had abnormal changes in the outer retinal microstructure limited to areas with reduced responses on multifocal electroretinograms as well as to the area involving a scotoma.
Methods and Results
We report the case of a 44-year-old man who developed an arcuate scotoma which was associated with a physiological blind spot in the left eye. The ophthalmoscopic, fluorescein angiographic, and full-field electroretinogram findings were normal. The amplitudes of the multifocal electroretinograms were reduced in the area of the scotoma. Optical coherence tomography showed that both the external limiting membrane and the inner and outer segment (IS/OS) line were intact, but that the middle cone outer segment tip line between the IS/OS line and the retinal pigment epithelium was absent in the nasal macular area of the left eye.
Conclusions
These findings indicate that the integrity of not only the external limiting membrane and IS/OS line but also the cone outer segment tip line is important for the function of the retina.
Key Words: Acute idiopathic blind spot enlargement syndrome, Optical coherence tomography, External limiting membrane, Inner and outer segment line, Cone outer segment tips
Introduction
The acute idiopathic blind spot enlargement (AIBSE) syndrome was first reported in 1988 by Fletcher et al. [1] as a clinical entity with sudden scintillations and a temporal scotoma centered on the blind spot in an otherwise normal fundus. Later, AIBSE was reported to belong to a family of pathologically and etiologically related retinal diseases, including acute zonal occult outer retinopathy (AZOOR), acute macular neuroretinopathy, multiple evanescent white dot syndrome, multifocal choroiditis, and panuveitis, called the AZOOR complex [2, 3]. Optical coherence tomography (OCT) studies of eyes with AZOOR or AZOOR complex disorders revealed abnormalities in the microstructures of the outer retina, e.g. the disruption or loss of the external limiting membrane (ELM) or the loss of the inner and outer segment (IS/OS) line in the areas of the visual field defects [4, 5, 6, 7, 8, 9].
Spectral domain OCT (SD-OCT) can acquire higher-resolution images of the retina, and the IS/OS line, the ELM, and the Verhoeff's membrane can thus be easily differentiated [10, 11, 12]. Srinivasan et al. [10] used a high-speed, ultrahigh-resolution OCT to show that the Verhoeff's membrane was located at the cone outer segment tips (COSTs). Here, we present a patient with AIBSE at the convalescent stage whose SD-OCT images showed an intact ELM and a continuous IS/OS line but an absence of the COST line. These findings were found only in the retinal areas with reduced responses on multifocal electroretinograms (mfERGs) and in the area of the scotoma.
Case Report
A 44-year-old Japanese man presented with a blind area in his left visual field which he had discovered 10 years earlier. At that time, he visited a local eye clinic but was not diagnosed with any specific retinal disease. In August 2009, he noticed that the blind area had worsened and visited another eye clinic where he was diagnosed with left optic neuritis. A brain MRI showed no abnormalities, and the patient received systemic steroid therapy that was, however, not effective.
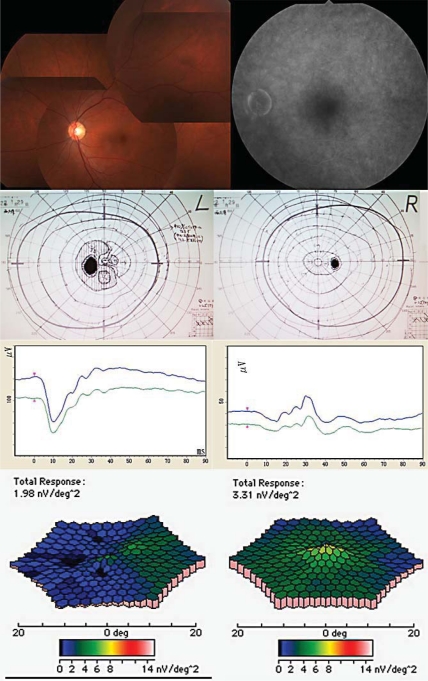
His symptom did not improve and he thus visited the Inoue Eye Clinic in March 2010. At the initial examination, his best-corrected visual acuity was 1.2 with a refractive error of −3.0 diopters in both eyes. The intraocular pressure was 11 mm Hg in the right and 10 mm Hg in the left eye. The anterior segment was normal. Ophthalmoscopy and fluorescein angiography revealed that the retina and choroid were normal (fig. 1). Goldmann visual field tests showed a relative arcuate scotoma associated with the physiological blind spot in the left eye. Single-flash, full-field mixed rod-cone electroretinograms (ERGs), photopic cone ERGs, and oscillatory potentials were normal in both eyes. However, the amplitudes of the mfERGs were reduced both in the area corresponding to the visual field defects in the left eye and in the central areas in both eyes.
Fig. 1.
Fundus photographs, fluorescein angiogram, and full-field and multifocal ERGs from the left eye of the patient with AIBSE syndrome. Top: fundus photographs and fluorescein angiogram showing no abnormal findings. Second row: Goldmann visual field of the affected eye demonstrates an arcuate scotoma connected to the physiological blind spot. Third row: full-field ERGs revealing normal mixed rod-cone ERG and photopic cone ERG results in both eyes. Bottom: mfERGs showing a reduced response density in the area of the scotoma in the left eye. The response density in the central area is reduced in both eyes.
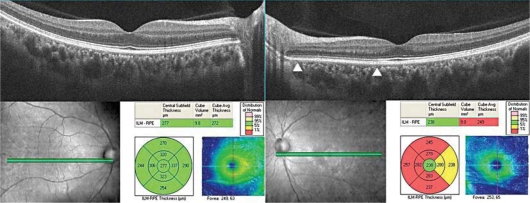
The entire macular area was scanned by SD-OCT (Cirrus OCT; Carl Zeiss Meditec, Inc.) with scan lengths of 9 mm. High-quality images were obtained using the 5-line raster mode. Both the ELM and the IS/OS line were continuous and distinct in the SD-OCT images. However, the COST line in the nasal macular area of the left eye was not detected (fig. 2). When the retinal thickness was measured by the volume scan mode of the SD-OCT, a decrease in the mean thickness in the nasal, superior, and temporal areas of the 9 sectors of the Early Treatment Diabetic Retinopathy Study (ETDRS) was found (fig. 2). The thinning appeared to be due to a thinning of the outer nuclear layer (ONL). These findings did not change on all examinations during the 8 months of follow-up.
Fig. 2.
SD-OCT images in our AIBSE patient. Upper left: image from a 9-mm horizontal scan of the right eye shows both an intact photoreceptor IS/OS line and an intact ELM. Upper right: image from a 9-mm horizontal scan of the left eye demonstrates an intact photoreceptor IS/OS line, an intact ELM and the absence of the COST line between the IS/OS line and the RPE in the nasal area of the macula (between arrowheads). Note that the COST line is intact on the temporal site with an intact visual field. Bottom: volume scans of the SD-OCT showing that the mean retinal thickness of the 9 areas of the Early Treatment Diabetic Retinopathy Study (ETDRS) decreased. The retinal thickness in the left eye is thinner than that of the right eye in the volume scan analyses.
Discussion
Since we did not examine our patient during the acute phase of the disease, our diagnosis of AIBSE syndrome was based only on our findings at the chronic stage. Because there was an arcuate segmental visual field defect connected to the blind spot, with no abnormalities in the ophthalmoscopic and fluorescein angiographic findings but with mfERG abnormalities in the areas of the visual field defect, we diagnosed our patient as having the AIBSE syndrome. Several authors [1, 2, 3, 4, 8] have suggested that the AIBSE syndrome belongs to a group of diseases including AZOOR, of which the primary pathological sites are photoreceptors. In support of this, in one study, time domain OCT showed a loss or irregularity of the IS/OS line in a patient with AZOOR, and the authors suggested that the primary lesion occurred in the photoreceptor outer segment [5].
Recent improvements in the resolution of OCT instruments have allowed investigators to obtain higher-resolution images of the intraretinal microstructure in several retinal diseases [12]. The OCT images have shown that the existence of a continuous IS/OS line is due to well-recovered photoreceptor cells [10], and that the integrity of the ELM is correlated with the morphologic changes in the photoreceptor cell bodies and Müller cells [13, 14]. Wakabayashi et al. [14] focused on the microstructure of the outer retinal layer in eyes with surgically closed macular holes, and they reported that the ELM and IS/OS line can recover in some cases, but that the latter rarely recovered without a recovery of the ELM. Spaide et al. [4] found that defects in the IS/OS line were associated with an enlargement of the blind spot in eyes with AZOOR complex diseases.
More recently, ultrahigh-resolution OCT and SD-OCT have been used to examine patients with AZOOR complex disorders, and not only a thinning of the ONL but also a loss of the IS/OS was observed [4, 9]. Our patient presented similar findings. The decrease in the thickness of the ONL plus the thinning of the inner nuclear layer in severe AZOOR cases have been thought to reflect a secondary degeneration after damage of the outer segments [5, 9]. The possibility that in some cases, the loss of outer segments in the COST line is secondary to a loss of photoreceptor cell bodies in the ONL cannot be denied. Further studies are needed to confirm this hypothesis.
Srinivasan et al. [10] reported that the highly reflective line between the IS/OS junction and the retinal pigment epithelium (RPE) was the COST line, and that it was visible because of the different lengths of the cone and rod outer segments. Recently, a third line between the IS/OS line and the RPE was noted and named the intermediate line or Verhoeff's line in several retinal diseases [11, 12, 15, 16, 17]. A correlation was found between the site of the visual impairments and the loss of the foveal COST line [15, 16].
Our findings are based on a single case of AIBSE, and thus they should not be extended to all AZOOR complex disorders until further studies are conducted. However, to the best of our knowledge, cases with similar findings have not been reported for any retinal disease. Our results support the earlier suggestion that the photoreceptors are the site of the primary lesion in AZOOR complex diseases [2, 3, 4]. In addition, the selective absence of the COST line and the regional cone dysfunction detected by mfERGs suggest that the photoreceptor cell bodies and rod outer segments were most likely morphologically intact. Whether the intact ELM and IS/OS line only appeared after the recovery or had been intact throughout the course of the disease process could not be determined. Further longitudinal studies with large numbers of patients are required to identify the underlying pathology.
We conclude that examinations of the integrity of outer retinal microstructures including the ELM, IS/OS, and COST lines should provide information on the disease process and be clinically relevant to the understanding of the pathologic mechanisms and the severity or stage of different retinal diseases.
Disclosure Statement
None of the authors has a financial or proprietary interest in any material or method mentioned.
Acknowledgements
Support of this study was provided by Research Grants on Sensory and Communicative Disorders from the Ministry of Health, Labor, and Welfare, Japan, and from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Fletcher WA, Imes RK, Goodman D, Hoyt WF. Acute idiopathic blind spot enlargement. A big blind spot syndrome without optic disc edema. Arch Ophthalmol. 1988;106:44–49. doi: 10.1001/archopht.1988.01060130050026. [DOI] [PubMed] [Google Scholar]
- 2.Gass JD, Agarwal A, Scott IU. Acute zonal occult outer retinopathy: a long-term follow-up study. Am J Ophthalmol. 2002;134:329–339. doi: 10.1016/s0002-9394(02)01640-9. [DOI] [PubMed] [Google Scholar]
- 3.Gass JD. Are acute zonal occult outer retinopathy and the white spot syndromes (AZOOR complex) specific autoimmune diseases? Am J Ophthalmol. 2003;135:380–381. doi: 10.1016/s0002-9394(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 4.Spaide RF, Koizumi H, Freund KB. Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol. 2008;146:111–120. doi: 10.1016/j.ajo.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Li D, Kishi S. Loss of photoreceptor outer segment in acute zonal occult outer retinopathy. Arch Ophthalmol. 2007;125:1194–1200. doi: 10.1001/archopht.125.9.1194. [DOI] [PubMed] [Google Scholar]
- 6.Takai Y, Ishiko S, Kagokawa H, Fukui K, Takahashi A, Yoshida A. Morphological study of acute zonal occult outer retinopathy (AZOOR) by multiplanar optical coherence tomography. Acta Ophthalmol. 2009;87:408–418. doi: 10.1111/j.1755-3768.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 7.Zibrandtsen N, Munch IC, Klemp K, J⊘rgensen TM, Sander B, Larsen M. Photoreceptor atrophy in acute zonal occult outer retinopathy. Acta Ophthalmol. 2008;86:913–916. doi: 10.1111/j.1600-0420.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara T, Imamura Y, Giovinazzo VJ, Spaide RF. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina. 2010;30:1206–1216. doi: 10.1097/IAE.0b013e3181e097f0. [DOI] [PubMed] [Google Scholar]
- 9.Ohta K, Sato A, Fukui E. Spectral domain optical coherence tomographic findings at convalescent stage of acute zonal occult outer retinopathy. Clin Ophthalmol. 2009;3:423–428. doi: 10.2147/opth.s6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan VJ, Monson BK, Wojtkowski M, Bilonick RA, Gorczynska I, Chen R, Duker JS, Schuman JS, Fujimoto JG. Characterization of outer retinal morphology with high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49:1571–1579. doi: 10.1167/iovs.07-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marmor MF, Choi SS, Zawadzki RJ, Werner JS. Visual insignificance of the foveal pit: reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol. 2008;126:907–913. doi: 10.1001/archopht.126.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byeon SH, Kang SY. Interpretation of outer retina appearance in high-resolution optical coherence tomography. Am J Ophthalmol. 2009;147:185–186. doi: 10.1016/j.ajo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Lim JI, Tan O, Fawzi AA, Hopkins JJ, Gil-Flamer JH, Huang D. A pilot study of Fourier-domain optical coherence tomography of retinal dystrophy patients. Am J Ophthalmol. 2008;146:417–426. doi: 10.1016/j.ajo.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakabayashi T, Oshima Y, Fujimoto H, Murakami Y, Sakaguchi H, Kusaka S, Tano Y. Foveal microstructure and visual acuity after retinal detachment repair: imaging analysis by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:519–528. doi: 10.1016/j.ophtha.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S, Oshima Y. Foveal microstructure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117:1815–1824. doi: 10.1016/j.ophtha.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Park SJ, Woo SJ, Park KH, Hwang JM, Chung H. Morphologic photoreceptor abnormality in occult macular dystrophy on spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:3673–3679. doi: 10.1167/iovs.09-4169. [DOI] [PubMed] [Google Scholar]
- 17.Ooto S, Hangai M, Sakamoto S, Sakamoto A, Tsujikawa A, Yamashiro K, Ojima Y, Yamada Y, Mukai H, Oshima S, Inoue T, Yoshimura N. High-resolution imaging of resolved central serous chorioretinopathy using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 2010;117:1800–1809. doi: 10.1016/j.ophtha.2010.01.042. [DOI] [PubMed] [Google Scholar]