Abstract
The social spider Anelosimus studiosus exhibits a behavioral polymorphism where colony members express either a passive, tolerant behavioral tendency (social) or an aggressive, intolerant behavioral tendency (asocial). Here we test whether asocial individuals act as colony defenders by deflecting the suite of foreign (i.e., heterospecific) spider species that commonly exploit multi-female colonies. We (1) determined whether the phenotypic composition of colonies is associated with foreign spider abundance, (2) tested whether heterospecific spider abundance and diversity affect colony survival in the field, and (3) performed staged encounters between groups of A. studiosus and their colony-level predator Agelenopsis emertoni (A. emertoni)to determine whether asocial females exhibit more defensive behavior. We found that larger colonies harbor more foreign spiders, and the number of asocial colony members was negatively associated with foreign spider abundance. Additionally, colony persistence was negatively associated with the abundance and diversity of foreign spiders within colonies. In encounters with a colony-level predator, asocial females were more likely to exhibit escalatory behavior, and this might explain the negative association between the frequency of asocial females and the presence of foreign spider associates. Together, our results indicate that foreign spiders are detrimental to colony survival, and that asocial females play a defensive role in multi-female colonies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00265-010-1112-z) contains supplementary material, which is available to authorized users.
Keywords: Behavioral syndrome, Cooperation, Personality, Task specialization, Temperament
Introduction
Cooperative defense of shared territory is a hallmark of animal societies (Wilson 1971, 1975), and is commonly cited as a contributing factor to the evolution of social behavior (Shank 1977; Farabaugh et al. 1992; Herrnkind et al. 2001; Krause and Ruxton 2002; Hölldobler and Wilson 1990, 2009). In eusocial societies, unique defensive castes boast an exquisite diversity of behavioral and morphological adaptations to counter intruders. Most social animals, however, lack such extreme adaptations, and group defense more commonly falls to large and/or dominant individuals (e.g., older individuals, male dominants). For instance, in prides of African lions (Panthera leo), resident males defend female reproductives and their young from attacks by foreign male conspecifics (Grinnell et al. 1995). Consistent individual differences in behavioral tendency might also be an important route to biased task performance, and individual specialization in defense could be one ancillary outcome (Sendova-Franks and Franks 1994; Fewell and Page 1999; Bolnick et al. 2003; Jeanson et al. 2005; Beshers and Fewell 2001; Sih et al. 2004; Bell 2007; Jandt et al. 2009; Pruitt and Riechert 2010).
Spider societies lack discrete morphological castes, but individual differences in behavior and body size may structure task performance in important ways (Ebert 1998). Characteristically, social spider colonies cooperate in prey capture, web repair, and alloparental care, and colony members are highly related (e.g., r ≈ 0.30 Anelosimus studiosus ) (Avilés 1997; Lubin and Bilde 2007; Duncan et al. 2010). Despite the finding that colonies are commonly encumbered by a rich community of kleptoparasites and colony-level predators (typically heterospecific spiders, hereafter termed “foreign spiders”), few investigations have considered the role of colony defense for individual and colony-level fitness (but see Cangialosi 1990, 1991). Instead, recent work on social spiders has focused on the foraging consequences of cooperative behavior (Avilés et al. 2007; Guevara and Avilés 2007; Powers and Avilés 2007; Purcell and Avilés 2008; Yip et al. 2008). However, some circumstantial evidence has associated dramatic mass-dispersal events (i.e., spatial “star bursts”) and colony-level extinctions with the presence of foreign spider species (Agnarsson 2006; Avilés et al. 2006; Perkins et al. 2007). Thus, colonies’ ability to identify and repel foreign species might have important implications for colony persistence and population dynamics. Additionally, colony defense could influence the viability of kleptoparasitism as a conditional strategy for other web-building araneofauna.
The temperate zone social spider, A. studiosus (Araneae, Theridiidae), exhibits a behavioral polymorphism—female colony members exhibit either a tolerant/social or aggressive/asocial behavioral tendency (Jones et al. 2007; Riechert and Jones 2008; Jones et al. 2010). Asocial females are more aggressive towards simulated predator threats, prey, and mates (Pruitt et al. 2008; Pruitt and Riechert 2009a, b; Pruitt et al. 2010), and consume a disproportionately high amount of shared resources in staged, group foraging bouts (Pruitt et al. 2008; Pruitt and Riechert 2009c). Despite the finding that natural colonies vary in their proportion of social and asocial females (Pruitt and Riechert 2009c), the relationship between social and asocial colony members is presently unresolved. Some data suggest asocial females parasitize social females by consuming a disproportionate amount of food (Pruitt and Riechert 2009c). An alternative hypothesis is that asocial individuals provide services for colonies by functioning as a behaviorally specialized caste (e.g., improving colony defense, prey capture) (Pruitt and Riechert 2010).
Here, we examine the second hypothesis and consider the relationship between the phenotypic composition of colonies and the presence of foreign spider species. We ask the following questions: (1) Do larger colonies contain a more genus-rich or numerically abundant araneofauna community? We define genus richness as the number of heterospecific spider genera which inhabit A. studiosus colonies. (2) Is there a relationship between the number of asocial females and the richness and abundance of foreign spider species? (3) Does the abundance or diversity of foreign araneofauna predict colony-level extinction? (4) In staged, mixed-phenotype encounters with intruding foreign spiders, do colony constituents respond non randomly (e.g., asocial females attack intruders, social individuals flee)?
Methods
Collection & transport
Anelosimus studiosus occurs in high concentrations along riparian habitats and roadways from the northeastern United States to Patagonian Argentina. We sampled colonies along two 250-m transects along Cove Lake in East Tennessee (36.30904°N, 84.21106°W).We sampled one colony every 10 m by placing a garbage bag over the colony and trimming off the supporting foliage. Colonies were then transported back to the laboratory and dissected to determine the number of social and asocial females, and to identify associated araneofauna.
Phenotypic composition and web-associate abundance and diversity
Colonies were dissected by hand picking through webbing and turning over each leaf individually. All A. studiosus were separated out and housed individually in 59-ml plastic cups before being run through a behavioral assay to determine their behavioral phenotype (described below). All foreign spiders were identified to genus and preserved in 70% ethanol.
Web-associate presence and colony extinction
To assess the effect of web associates on colony persistence, we haphazardly sampled 45 multi-female colonies along Cove Lake in May of 2009. Foreign araneofauna were censused by cutting open the colonies along one side, and visually scanning for foreign spider species. Visual surveys were performed for 10 min. Foreign spiders were identified to family. Colonies were checked again in mid-July for brooding females or middle-instar juveniles. Those no longer containing A. studiosus were considered “extinct”, presumably either (1) due to a mass dispersal event, or (2) a colony-wide die off. However, A. studiosus disperse as late instar juveniles (Brach 1977; Riechert and Jones 2008), and thus, colony disappearance late in this census period more likely reflects mortality. To confirm the accuracy of the visual scans, we collected 15 additional colonies and transported them individually in zip-lock bags to our laboratory at the University of Tennessee, Knoxville. These colonies were then fully dissected using the protocol described above, and we tested for a positive correlation between the number of foreign web associates counted by visual observations in the field and by complete colony dissections in the laboratory.
Inter-individual distance test
To determine the females’ social tendency, two female A. studiosus of unknown social tendency were individually marked with fluorescent powder and placed in the center of clear, plastic container (13 × 13.5 × 2.5 cm). After 24 h of settling time, we measured the distance between them (termed their inter-individual distance score). All females that exhibited an inter-individual distance greater than zero were run through a second confirmatory test with a known highly social female (i.e., one that previously exhibited an inter-individual distance of zero). The second trial is necessary because more aggressive females, which demand space, may chase or even attack social females (Pruitt et al. 2008). We used the between-individual distance from the second confirmatory trial when categorizing individuals for subsequent behavioral assays. By using inter-individual distance, we allow for the possibility of intermediate social phenotypes. Inter-individual distances are both repeatable and heritable (Pruitt et al. 2008; Pruitt and Riechert 2009b).
Colony defense
Forty experimentally reconstituted colonies were constructed by randomly selecting two social individuals and two asocial, and placing them in a 490-ml plastic container. This test colony size resembles the average sized colony (5.41 females/colony) from this latitude (Pruitt and Riechert 2009c). Two days after a group was placed together, they began a maintenance diet of six termite workers twice weekly. One week after a colony was established, one juvenile A. emertoni (Agelenidiae; Araneae) was placed onto the edge of a colony by removing the container’s lid and guiding the foreign spider onto the web using an open-tipped syringe (after Pruitt and Husak 2010). A. emertoni is a common predator and web-associate in A. studiosus colonies (Perkins et al. 2007). The behavior of the colony constituents was observed for the next 20 min and colonies were checked again 24 h later. Specifically we note (1) which individuals fled or attacked the intruding spider, (2) whether the intruder remained in the web after 24 h, and (3) instances of mortality.
Statistical methods
To test for relationships between colony size, phenotypic composition of colonies, and the richness/abundance of foreign spiders, we used a general linear models (ANCOVAs) with the number of asocial colony members as our independent variable, colony size (i.e., the number of resident females) as a covariate, and web-associate abundance and genus richness as response variables.
To assess the effect of web-associate abundance and diversity on colony persistence, we used multiple logistic regression with colony persistence (1,0) as our response variable. We used the following predictor variables in our model: combined web-associate abundance, the abundance of each family, and the total number of foreign spider families. We identified the best single model predicting persistence using AIC model selection criterion.
We used chi-square statistics to test for phenotypic bias in attack/flight behavior and colony member mortality in staged, mixed-phenotype encounters with foreign web associates. All statistical analyses were conducted using Sigmaplot 11.0.
Results
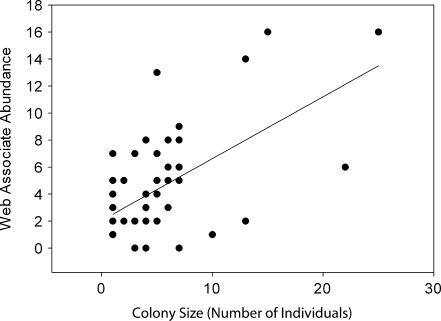
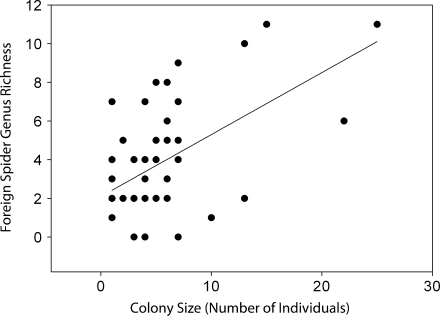
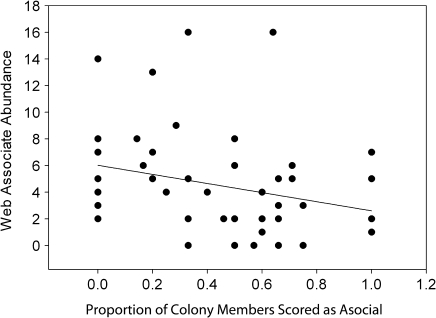
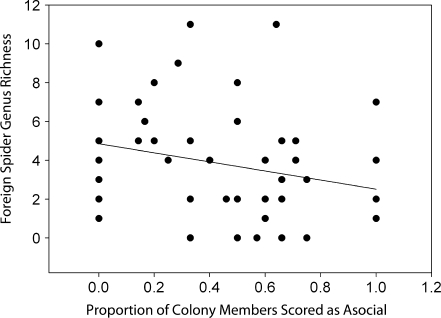
We detected significant effects of colony size and phenotypic composition on the number of genera and the total abundance of foreign spider species. Larger colonies harbored a more abundant (F 3,49 = 6.00, P < 0.0001) and genus-rich (F 3,49 = 5.50, P < 0.0001) community of foreign araneofauna (Figs. 1 & 2), and the number of asocial females was negatively associated with the total abundance (F 3,49 = −4.32, P < 0.001) and genus richness (F 3,49 = −3.81, P < 0.001) of associated araneofauna (Figs. 3 & 4). The interaction term between colony size and the number of asocial females was nonsignificant in both models: web-associate abundance (F 3,49 = 1.85, P = 0.071), genus richness (F 3,49 = 1.44, P = 0.157).
Fig. 1.
A scatter plot and best fit regression line between colony size (the number of female colony members) and the total abundance of foreign spider web associates
Fig. 2.
A scatter plot and best fit regression line between colony size (the number of female colony members) and the genus richness of foreign spider web associates
Fig. 3.
A scatter plot and best fit regression line between the phenotypic composition of colonies (i.e., the proportion of colony members scored as asocial) and the total abundance of foreign spider web associates
Fig. 4.
A scatter plot and best fit regression line between the phenotypic composition of colonies (i.e., the proportion of colony members scored as asocial) and the genus richness of foreign spider web associates
As predicted, both web-associate abundance (χ 21 = 17.35, P < 0.001) and genus richness (χ 21 = 22.72, P < 0.0001) were negatively associated with colony persistence. Using AIC model selection criterion, the single best model predicting colony persistence included family richness, the abundance agelenids, and the abundance of mimetids (F 3,44 = 21.64, P < 0.0001, Table 1). A list of AIC values for other models is available in our online supplementary materials (Online Resource 1). Additionally, we found that the web-associate abundance (r = 0.79, df = 14, P < 0.001) and genus richness (r = 0.89, df = 14, P < 0.001) estimates derived from visual scans, and laboratory dissection were positively correlated.
Table 1.
Results of multiple logistic regression predicting colony persistence (N = 45, Adj R 2 = 0.54)
| Term | Estimate | Standard Error | T-ratio | P-value |
|---|---|---|---|---|
| Family Richness | −0.1533 | 0.0301 | −5.08 | <0.001 |
| Agelenidae | −0.1693 | 0.0779 | −2.17 | 0.036 |
| Mimetidae | 0.2926 | 0.1363 | 2.04 | 0.05 |
| Intercept | 1.3178 | 0.1055 | 12.49 | <0.001 |
In staged, mixed-phenotype encounters with intruding spiders, asocial females more frequently responded to intrusion by approaching and making contact with the intruder (Asocial 43.8%, Social 15%, χ 21 = 11.25, P = 0.001). Social and asocial individuals were indistinguishable in their frequencies of unresponsiveness (Asocial 48.7%, Social 62.5%, χ 21 = 1.34, P = 0.25), but social individuals tended to flee more frequently than asocial individuals (Asocial 7.5%, Social 22.5%, χ 21 = 6.00, P = 0.01). Asocial individuals were also more likely to die than social individuals in our staged encounters (Asocial 17.4%, Social 6.25%, χ 21 = 4.26, P = 0.04), presumably from differential predation by the invading spider. The invading spider was repelled in only 7.5% of the trials, and the intruding spider was never killed.
Discussion
Anelosimus colonies commonly harbor a suite of exploitative araneofauna (Perkins et al. 2007), and thus, factors diminishing foreign spider abundance likely influence colony-wide fitness. Our results indicate that the presence of aggressive, asocial females is negatively associated with foreign spider abundance and diversity (i.e., genus richness). Asocial females were also more likely to approach and make contact with (e.g., touch, bite) foreign spiders in mixed-phenotype encounters. Our data on colony persistence demonstrated that colonies harboring more abundant and diverse foreign spider communities are more likely to go extinct, and this finding corroborates hypotheses from previous studies that foreign spiders negatively affect colony-wide survival (Avilés et al. 2006; Perkins et al. 2007). Taken together, our results suggest that asocial females play a defensive role in multi-female colonies, and could be described as a behaviorally predisposed soldier caste.
Our results suggest asocial females defend colonies from intrusion by foreign spiders. First, we found that colonies with more asocial females tended to have less abundant and diverse foreign spider communities. This relationship could be observed (1) if foreign spiders are more readily identified and repelled by colonies with more asocial females or (2) if colonies with more asocial females are somehow less attractive to other spiders. The former receives some support from our behavioral observations. In staged, mixed-phenotype encounters with the foreign spider A. emertoni, asocial females more frequently approached and made contact with the intruder. In contrast, social females tended to flee from the intruder or remain unresponsive. Our results also indicate that the escalatory, defensive behavioral tendency of asocial females places them at a higher risk of predation by foreign spiders. Thus, their defensive role could have significant individual fitness costs. Unfortunately, intruders were very rarely repelled in our staged encounters, and thus, we lack the statistical power to determine what factors lead to successful colony defense.
Interestingly, it is not merely foreign spider abundance which predicts colony persistence. Instead, our data demonstrate that it is foreign spider diversity that is most tightly negatively associated with colony extinction. A previous study by Perkins et al. (2007) assayed the diversity of foreign araneofauna that inhabit A. studiosus webs, and estimated the ecological interactions between A. studiosus and its associates using staged pairwise interactions. The authors found that A. studiosus commonly fall victim to predation by foreign spiders, and that funnel-web spiders (Agelenidae) and sac spiders (Clubionidae) were the most lethal families in these interactions. Our data generally corroborate the notion that foreign spider species are detrimental to colony performance, and that agelenids are particularly detrimental to whole-colony survival; however, the abundance of clubionids was not significantly associated with colony persistence. Instead, we found that the abundance of mimetids was positively associated with colony survival. Interestingly, spiders of family Mimetidae, or pirate spiders, are known for their high incidence of araneophagy. Though speculative, our data are consistent with the hypothesis that mimetids might not target A. studiosus at all, but may instead feed on other predatory web associates. However, further field experiments are required to confirm this indirect-effect hypothesis.
In general, our data suggest that it is the diversity of foreign spider associates which best predicts colony persistence. While we lack data on the mechanism behind this pattern, one viable hypothesis is that colonies are capable of accommodating most associates individually, but collapse when encumbered by many exploiters: some web associates lay their eggs within colonies, and the resulting offspring compete with juvenile A. studiosus for food (Hyctia, Phidippus: JN Pruitt personal observation), other spiders steal prey from adults (Argyrodes: Cangiolosi 1990), and others have been observed preying on adult colony members (Agelenopsis: Perkins et al. 2007). We propose that colonies are able to survive single exploitative interactions, but combinations might become insurmountable. Future work might consider the colonization order of foreign spiders, and how specific web associates effect the volumetric growth of colonies and per capita fitness of colony members.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
AIC scores of alternative models predicting colony extinction. Predictor families: abundance of each family, family richness, total spider abundance. (DOC 118 kb)
Acknowledgments
We would like to acknowledge Jennifer Krauel, Dylan Dittrich-Reed, Kyle Demes, the editor, and one anonymous reviewer for their constructive comments on previous versions of this manuscript. The experiment presented herein complies with all laws of the USA.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Agnarsson I. A revision of the New World eximius lineage of Anelosimus (Araneae, Theridiidae) and a phylogenetic analysis using worldwide exemplars. Zool J Linn Soc. 2006;146:453–593. doi: 10.1111/j.1096-3642.2006.00213.x. [DOI] [Google Scholar]
- Avilés L. Causes and consequences of cooperation and permanent sociality in spiders. In: Choe J, Crespi B, editors. The evolution of social behavior in insects and arachnids. Cambridge, UK: Cambridge University Press; 1997. pp. 476–498. [Google Scholar]
- Avilés L, Maddison WP, Agnarsson I. Theridion nigroannulatum—an independently-derived highly social spider with explosive colony proliferation and a female size dimorphism. Biotropica. 2006;38:743–753. doi: 10.1111/j.1744-7429.2006.00202.x. [DOI] [Google Scholar]
- Avilés L, Agnarsson I, Salazar P, Purcell J, Iturralde G, Yip E, Powers KS, Bukowski T. Altitudinal pattern of sociality in the spider genus Anelosimus and the biology of a new mid-elevation social species in Ecuador. Am Nat. 2007;170:783–792. doi: 10.1086/521965. [DOI] [PubMed] [Google Scholar]
- Bell AM (2007) Future directions in behavioural syndromes research. Proc R Soc Lond B 274:755–761 [DOI] [PMC free article] [PubMed]
- Beshers SN, Fewell JH (2001) Models of division of labor in social insects. Ann Rev Entomol 46:413–440 [DOI] [PubMed]
- Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forrister ML. The ecology of individuals: incidence and implications of individual specialization. Am Nat. 2003;161:1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- Brach V. Anelosimus studiosus (Araneae: Theridiidae) and the evolution of quasisociality in theridiid spiders. Evolution. 1977;31:154–161. doi: 10.2307/2407553. [DOI] [PubMed] [Google Scholar]
- Cangialosi KR (1990) Social spider defense against kleptoparasitism. Behav Ecol Sociobiol 27:49–54
- Cangialosi KR (1991) Attack strategies of a spider kleptoparasite: effects of prey availability and host colony size. Anim Behav 41:639–647
- Duncan SI, Riechert SE, Fitzpatrick BM, Fordyce JA. Relatedness and genetic structure in a socially polymorphic population of the spider Anelosimus studiosus. Mol Ecol. 2010;19:810–818. doi: 10.1111/j.1365-294X.2010.04523.x. [DOI] [PubMed] [Google Scholar]
- Ebert D. Asymmetry in Relation to body weight and hunger in the tropical social spider Anelosimus eximius (Araneae, Theridiidae) J Arachnol. 1998;26:70–80. [Google Scholar]
- Farabaugh SM, Brown ED, Hughes JM. Cooperative terri-torial defense in the Australian magpie, Gymnorhina tibicen (Passeriforms, Cracticidae), a group living songbird. Ethology. 1992;92:283–292. doi: 10.1111/j.1439-0310.1992.tb00966.x. [DOI] [Google Scholar]
- Fewell JH, Page RE. The emergence of division of labour in forced associations of normally solitary ant queens. Evol Ecol Res. 1999;1:537–548. [Google Scholar]
- Grinnell J, Packer CA, Pusey AE. Cooperation in male lions: kinship, reciprocity or mutualism? Anim Behav. 1995;49:95–105. doi: 10.1016/0003-3472(95)80157-X. [DOI] [Google Scholar]
- Guevara J, Avilés L. Multiple sampling techniques confirm differences in insect size between low and high elevations that may influence levels of spider sociality. Ecology. 2007;88:2015–2023. doi: 10.1890/06-0995.1. [DOI] [PubMed] [Google Scholar]
- Herrnkind WF, Childress MJ, Lavalli K. Cooperative defence and other benefits among exposed spiny lobsters: inferences from group size and behaviour. Mar Freshw Res. 2001;52:1113–1124. doi: 10.1071/MF01044. [DOI] [Google Scholar]
- Hölldobler B, Wilson EO (1990) The Ants. Belknap Press, Harvard, MA
- Hölldobler B, Wilson EO (2009) The Superorganism: The Beauty, Elegance, and Strageness of Insect Societies. W.W. Norton, London
- Jandt JM, Huang E, Dornhaus A. Weak specialization of workers inside a bumble bee (Bombus impatiens) nest. Behav Ecol Sociobiol. 2009;63:1829–1836. doi: 10.1007/s00265-009-0810-x. [DOI] [Google Scholar]
- Jeanson R, Kukuk PF, Fewell JH. Emergence of division of labour in halictine bees: contributions of social interactions and behavioural variance. Anim Behav. 2005;70:1183–1193. doi: 10.1016/j.anbehav.2005.03.004. [DOI] [Google Scholar]
- Jones TC, Pruitt JN, Riechert SE (2010) Reproductive success in a socially polymorphic spider: social individuals experience depressed reproductive success in isolation. Ecol Entomol 35:684–690
- Krause J, Ruxton GD. Living in groups. Oxford: Oxford University Press; 2002. [Google Scholar]
- Lubin Y, Bilde T. The evolution of sociality in spiders. Adv Study Behav. 2007;27:83–145. doi: 10.1016/S0065-3454(07)37003-4. [DOI] [Google Scholar]
- Perkins TA, Riechert SE, Jones TC. Interactions between the social spider Anelosimus studiosus (Araneae, Theridiidae) and foreign spiders that frequent its nests. J Arachnol. 2007;35:143–152. doi: 10.1636/T06-43.1. [DOI] [Google Scholar]
- Powers KS, Avilés L. The role of prey size and abundance in the geographical distribution of spider sociality. J Anim Ecol. 2007;76:995–1003. doi: 10.1111/j.1365-2656.2007.01267.x. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Riechert SE. Male mating preference is associated with risk of pre copulatory cannibalism in a socially polymorphic spider. Behav Ecol Sociobiol. 2009;63:1573–1580. doi: 10.1007/s00265-009-0751-4. [DOI] [Google Scholar]
- Pruitt JN, Riechert SE. Sex matters: sexually dimorphic fitness consequences of a behavioural syndrome. Anim Behav. 2009;78:175–181. doi: 10.1016/j.anbehav.2009.04.016. [DOI] [Google Scholar]
- Pruitt JN, Riechert SE. Frequency-dependent success of cheaters during foraging bouts might limit their spread within colonies of a socially polymorphic spider. Evolution. 2009;63:2966–2973. doi: 10.1111/j.1558-5646.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Husak JF. Context-dependent running speed in funnel-web spiders from divergent populations. Funct Ecol. 2010;24:165–171. doi: 10.1111/j.1365-2435.2009.01623.x. [DOI] [Google Scholar]
- Pruitt JN, Riechert SE (2010) How within-group behavioural variation and task efficiency enhance fitness in a social group. Proc R Soc B. doi:10.1098/rspb.2010.1700 [DOI] [PMC free article] [PubMed]
- Pruitt JN, Riechert SE, Jones TC. Behavioral syndromes and their fitness consequences in a socially polymorphic spider, Anelosimus studiosus. Anim Behav. 2008;76:871–879. doi: 10.1016/j.anbehav.2008.05.009. [DOI] [Google Scholar]
- Pruitt JN, Riechert SE, Iturralde G, Vega M, Fitzpatrick BM, Avilés L. Population differences in behaviour are explained by shared within-population trait correlations. J Evol Biol. 2010;23:748–756. doi: 10.1111/j.1420-9101.2010.01940.x. [DOI] [PubMed] [Google Scholar]
- Purcell J, Avilés L. Gradients of precipitation and ant abundance may contribute to the altitudinal range limit of subsocial spiders: insights from a transplant experiment. Proc Biol Sci. 2008;275:2617–2625. doi: 10.1098/rspb.2008.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechert SE, Jones TC. Phenotypic variation in the behaviour of the spider, Anelosimus studiosus, facilitates shift from single female to multiple female nests in colder environments. Anim Behav. 2008;75:1893–1902. doi: 10.1016/j.anbehav.2007.10.033. [DOI] [Google Scholar]
- Sendova-Franks AB, Franks NR. Social resilience in individual worker ants and its role in division of labor. Proc Biol Sci. 1994;256:305–309. doi: 10.1098/rspb.1994.0085. [DOI] [Google Scholar]
- Shank CC. Cooperative defense by bighorn sheep. J Mammal. 1977;58:243–244. doi: 10.2307/1379589. [DOI] [Google Scholar]
- Sih A, Bell AM, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378 [DOI] [PubMed]
- Wilson EO. The insect societies. Cambridge MA: Belknap Press of Harvard University Press; 1971. [Google Scholar]
- Wilson EO. Sociobiology: the new synthesis. Cambridge MA: Belknap Press of Harvard University Press; 1975. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AIC scores of alternative models predicting colony extinction. Predictor families: abundance of each family, family richness, total spider abundance. (DOC 118 kb)