Abstract
Microwave reactors remain largely underutilized in the field of PET chemistry. This is particularly unfortunate since microwave synthesis elegantly addresses two of the most critical issues of PET radiochemistry with short-lived radionuclides: reaction rate and side-product formation. In this study we investigate the efficiency of synthesis of terminally [18F]fluorinated fatty acid analogs using a commercial microwave reactor in comparison with conventional heating.
Methods
The labeling precursors were methyl esters of terminally substituted alkyl bromides and iodides. Duration and temperatures of the [18F]fluorination reaction were varied. Chemical and radiochemical purities, and radiochemical yields were investigated for conventional (CH) and microwave-assisted (MW) radiosyntheses.
Results
The results demonstrate that microwave heating enhanced [18F]fluoride incorporation to >95% (up to 55% improvement), while reducing reaction times to 2 min (~10-fold reduction) or temperatures to 55–60°C (20°C reduction). Overall decay-corrected radiochemical yields of purified [18F]fluoro fatty acids were higher (MW=49.0 ± 4.5%, CH=23.6 ± 3.5%, p<0.05) with microwave heating and side-products were notably fewer.
Conclusion
For routine synthesis of [18F]fluoro fatty acid analogs, microwave heating is faster, milder, cleaner, less variable and higher yielding than conventional heating and therefore the preferred reaction method.
Keywords: Microwave-assisted [18F]fluorination, PET, [18F]fluoro fatty acid
1. Introduction
The ability to produce high yields of a target compound is of paramount importance in radiochemical synthesis. When working with short-lived PET isotopes such as 18F (~110 min) and 11C (~20 min), the radiochemist surrenders the ability to synthesize and store large quantities of a target compound for future use. Therefore, great emphasis is placed on maximizing radiochemical yields in order to provide adequate amounts of radiotracer to carry out imaging studies. Although traditional organic chemistry lacks the challenges associated with isotopic half-life, the desire for synthetic efficiency (time, fiscal expense and product purity) has driven microwave reactors to become common place in synthetic chemistry labs. Realizing that microwave reactors can address the challenges of radiochemical synthesis, radiochemists have introduced microwave reactors as an alternative to conventional heating [1]. While a considerable number of articles and reviews of microwave heating have already been compiled over the past decade [2–9], space considerations and cost have inhibited this technology from widespread use. CEM (Mathews, NC) has developed a small microwave reactor system (PETWave®) whereby microwaves are generated outside of the hot cell and transmitted into the shielded workspace via an umbilical cord to a reaction vessel housing with a footprint of 103 cm2 (as compared to 1480 cm2 for CEM’s Discover® synthetic chemistry system).
Motivated by the growing importance of radiolabelled fatty acids as metabolic probes for fatty acid oxidation [10–14], we sought to employ the PETWave® system to improve the radiochemical yields of our [18F]fatty acid analog PET probes. In this study, we compare [18F]fluoride incorporation using microwave heating (MW) and conventional heating (CH) for three different palmitate derivatives; methyl 16-bromo-hexadecanoate (BP or 3), methyl 16-iodo-hexadecanoate (IP or 4) and methyl 3-(12-bromododecylthio)propionate (BTP or 5) (Fig. 1).
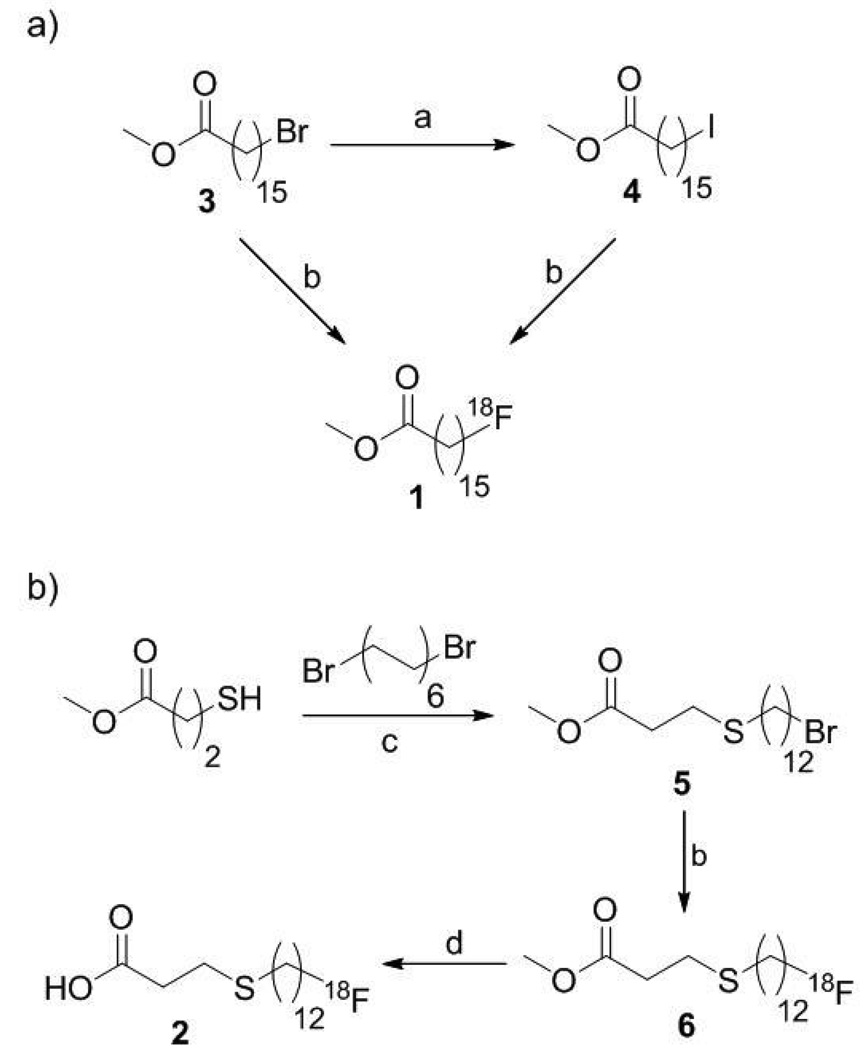
Fig. 1.
Synthesis and [18F]fluorination of [18F]fatty acid precursors. a. NaI, acetone, r.t. b. K18F, K[2.2.2], K2CO3, MeCN (heating method, temperature and time were varied as described in results) c. NaH, THF, 0°C 12h. d. 1) 0.2M KOH 75°C (2 min using MW, 5 min using CH) 2), 0.2M AcOH.
2. Materials and methods
2.1 General
All chemicals and solvents were purchased from Aldrich (St. Louis, MO) and used as received, including methyl 16-bromo-hexadecanoate (3). Anhydrous solvents were also purchased from Aldrich in sure seal bottles. GC-MS data was acquired on a Shimadzu QP2010 Plus (EI) using a 1:20 split ratio and heating at 50°C for 5 min, then ramping to 280°C at a rate of 30°C/min and holding at 280°C for 3 min. TLC analysis of reaction mixtures was performed on Merck silica gel 60 F254 TLC plates. Chromatography was carried out on Merck 60 silica gel (32–63 µm). 1H and 13C NMR were recorded on a Varian 600 MHz spectrometer and referenced to CDCl3 (1H NMR: CDCl3 at 7.26 ppm and 13C NMR: CDCl3 at 77.0 ppm). Semi-preparative HPLC was performed using Phenomenex Luna C-18, 5 µm, 10 × 250 mm column; mobile phase: 90% acetonitrile, 9.995% water, 0.005% trifluoroacetic acid; flow rate 5.0 mL/min. Radioactivity-TLC (rTLC) analysis was performed on a EZ-Scan TLC scanner equipped with an Omni-Rad multimode radiation detector (Carroll & Ramsey Associates, Berkeley, CA).
2.2 Synthesis of Precursors
2.2.1 Methyl 16-iodo-hexadecanoate (4)
To a stirred solution of compound 3 (300 mg, 0.86 mmol) in acetone (5 mL) was added NaI (644 mg, 4.29 mmol). This solution was stirred for 3 days at room temperature. The crude reaction mixture was then evaporated to dryness and purified by silica gel column chromatography using dichloromethane as the eluent. The product fractions were then evaporated to dryness under vacuum giving 317 mg of compound 4 (93% yield) as a white solid. The observed 1H and 13C NMR data matched previously reported literature values [15]. 1H NMR (599.77 MHz, CDCl3) δ ppm: 1.22–1.34 (brs, 20H, CH2×10), 1.36–1.41 (m, 2H), 1.63–1.79 (m, 2H), 1.72–1.84 (m, 2H), 2.30 (t, 2H, - CH2COOCH3), 3.19 (t, 2H, -CH2CH2I), 3.68 (s, 3H, OCH3). 13C NMR (150.81 MHz, CDCl3) δ ppm: 7.3, 25.0, 28.5, 29.1–29.6, 30.5, 33.6, 34.1, 51.4(OCH3), 174.3(CO). GC-MS (EI): 13.9 min, 396 (M+) and 269 [(M+ - 127I) for C17H33O2I].
2.2.2 Methyl 3-(12-bromododecylthio)propionate (5)
Synthesis of precursor 5 was carried out by adding NaH (100 mg, 4.16 mmol) to a stirred solution of methyl 3-mercaptopropanoate (500 mg, 4.16 mmol) in dry THF (80 mL) at 0°C under N2. The resulting solution was stirred for 15 min at 0°C. A solution of 1,12-dibromododecane (1.23 g, 3.75 mmol) in THF (10 mL) was slowly added and the resulting mixture was maintained at 0°C for 30 min. The mixture was stirred for 3 hrs at room temperature and quenched with water. The dichloromethane extract was dried over anhydrous Na2SO4 and solvent was evaporated under vacuum. The residue was subjected to column chromatography (5% ethyl acetate/hexane) to yield 5 (1.19 g, 77%) as a colorless oil [11]. 1H NMR (599.77 MHz, CDCl3) δ ppm: 1.26 – 1.28 (brs, 12H, CH2×6), 1.36 (m, 2H), 1.42 (m, 2H),1.58 (m, 2H, S-CH2CH2CH2-), 1.88 (m, 2H, - CH2CH2Br), 2.51 (t, 2H, -CH2CH2CO-), 2.61 (t, 2H, S-CH2CH2CH2-), 2.71 (t, 2H, S-CH2CH2CO), 3.41 (t, 2H, -CH2Br), 3.71 (s, 3H, OCH3). 13C NMR (150.81 MHz, CDCl3) δ ppm: 26.9, 28.1, 28.7, 28.8, 29.1, 29.3 – 29.5, 32.1, 32.8, 34.0, 34.7, 51.7 (OCH3), 172.4 (CO). GC-MS (EI): 14.4 min, 368 (M+) and 366 [(M+ - 2) for C16H31O2S79Br].
2.3 Synthesis and Use of Non-radioactive Fluorination Standards
Methyl 16-fluoropalmitate was synthesized by reacting compound 3 (100 mg, 0.29 mmol) with 2.9 mL 1M tetrabutyl ammonium fluoride in THF (2.9 mmol) at 80 °C overnight. The reaction mixture was then exaporated to a viscous oil, diluted with 5mL of dicholomethane, and passed through a plug of silica using dichloromethane as the eluent giving non-radiactive methyl 16-fluoropalmitate (64 mg, 77%). The observed 1H and MS data matched previously reported literature values [16]. 1H NMR (599.77 MHz, CDCl3) δ ppm: 1.2 – 1.4 (brs, 24H, CH2×12), 1.61 (m, 2H, CH2CH2F), 2.29 (t, 2H, J = 7.2Hz, CH2COOCH3), 3.66 (s, 3H, CO2CH3), 4.43 (dt, 2H, JHF = 47.4 Hz JHH =6.3 Hz, CH2F). GC-MS (EI): 12.5 min, 288 (M+) and 257 [(M+ - CH3O) for C17H33O2F]. HPLC of the product using the system described in the experimental section and 90% MeCN, 10% H2O as mobile phase gave a UV (240 nm) retention time of 14.5 min. This matched the gamma detector HPLC retention time for [18F]fluoro ester 1 using the same system and mobile phase, thus confirming the identity of the radioactive product.
The non-radioactive standards for FTP (2) and methyl FTP (6) were synthesized as previously described [11]. Using 90% MeCN, 9.995% H2O, and 0.005% trifluoroacetic acid as the mobile phase gave retention times of 6.8 min and 10.7 min for compounds 2 and 6 respectively. These time were found to match those of the corresponding [18F]fluorinated analogs.
2.4 18F-Labeling Procedure
The labeling precursors were compounds 3, 4, and 5 (Scheme 1a and 1b). Briefly, cyclotron-produced [18F]fluoride (~50 mCi) was dried down under nitrogen at 95°C in a 3 mL glass vial containing Kryptofix 2.2.2 (10 mg), acetonitrile (0.8 mL), and K2CO3 (4 mg) solution in water (0.30 mL). The residue was further dried by azeotropic distillation with anhydrous acetonitrile (2 × 1.0 mL). The [18F]fluoride residue was reconstituted in 2 mL acetonitrile. Small aliquots of this solution (200–300 µL) were then transfered to a reaction vessel, followed by a solution of the precursor (approximately 4 mg) in acetonitrile (0.5 mL). To generate [18F]fluoride incorporation data: The vial was sealed and heated at various temperatures and times in either the PETWave® microwave reactor or standard thermal heating block. Silica TLC plates were spotted with the reaction mixture at the appropriate time points and run using dichloromethane as the mobile phase. The plates were then analyzed for fluoride incorporation by rTLC. To generate overall radiochemical yield data: [18F]fluorination was carried out with conventional heating for 15 minutes at 75°C or in the PETWave® microwave reactor for 10 minutes at 75°C. Subsequent hydrolysis of the resulting [18F]fluoro ester was accomplished by adding 0.15 mL 0.2M KOH to the reaction vessel and heating at 75°C for 5 min for conventional heating, or at 75°C for 2 min for microwave heating. The mixture was cooled, acidified with 0.15 mL acetic acid (0.2M), and applied to the semi-preparative HPLC column. An in-line ultraviolet detector (240 nm) was used to monitor the elution of unlabeled materials. The [18F]fluoro fatty acid fraction was collected and diluted in 50 mL water and passed through a C-18 Sep-Pak cartridge (Accel C-18 Plus, Waters Corporation, Milford, MA). The Sep-Pak was then washed with 10 mL water, followed by elution of the [18F]fluoro fatty acid using 1 mL ethanol. The activity of the isolated product 2 was then measured.
2.5 PETWave microwave reactor settings
The PETWave microwave reactor was run in “Dynamic” mode, where microwave power was modulated in order to maintain constant temperature. Temperature was monitored by a UV sensor located adjacent to the reaction vessel. The microwave power levels fluctuated between 0 and 50 Watts in a typical synthesis. A charcoal-filtered vent needle was placed in the reaction vessel septum during the course of the reaction.
2.6 Statistical Analyses
All results were expressed as the mean ± SD. Comparison of the means was made using a two-tailed student’s t-test. The acceptable level of significance was set at p<0.05.
3. Results
3.1 Synthesis of labeling precursors
The commercially available bromo ester 3 was converted to the corresponding iodo-compound 4 via Finkelstein halide exchange. The 4-thia precursor 5 was synthesized analogously to previously published methyl 3-(12-iodododecylthio)propionate [6] by coupling 1,12-dibromododecane to methyl 3-mercaptopropanoate.
3.2 Fluoride incorporation: microwave vs conventional heating
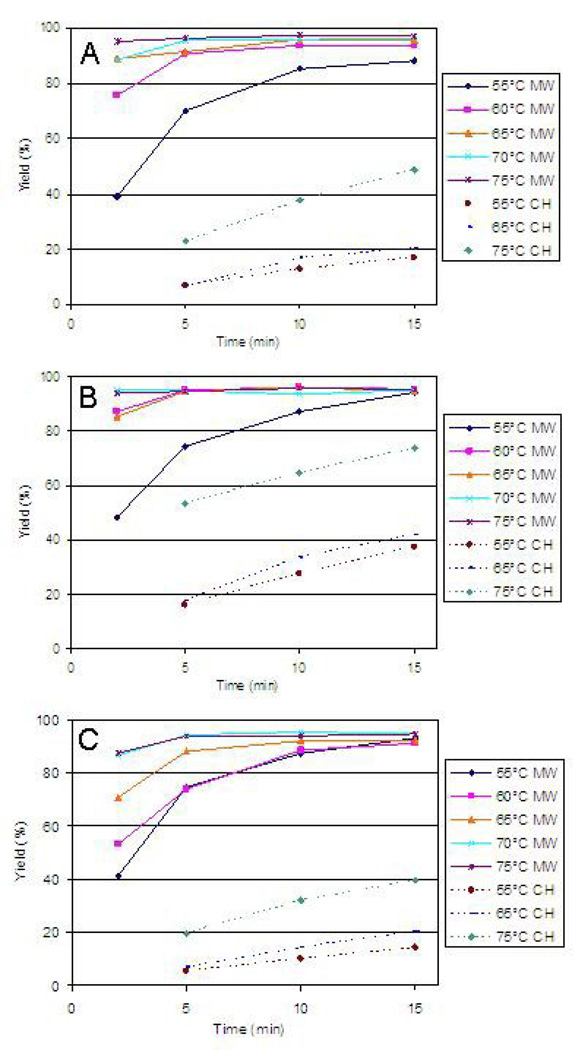
To control for variations in yield due to [18F]fluoride adsorption to the glass wall of the dry-down vessel, the [18F]fluoride/K[2.2.2] solution was dried down in a conical borosilicate glass vial, reconstituted in acetonitrile, and then transfered to a separate borosilicate glass [18F]fluorination reaction vessel. Radiochemical yields were calculated based on the radioactivity transferred to the reaction vessel. [18F]Fluoride incorporation yields depicted in Fig. 2 were calculated from radio-TLC data (averages and standard deviations can be found in Table 1). These yields were based on solubilized [18F]fluoride in the vessel at the end of the [18F]fluorination reaction. It was determined that 19.5± 6.2% (n=3) of the total 18F-radioactivity was adsorbed to the wall of the reaction vessel in the form of fluoride ion. Thus, the actual [18F]fluorination yields would be approximately 19.5% below the values in Table 1 and Figures 2 and 4.
Fig. 2.
Time- and leaving group-dependence of [18F]fluoride incorporation into [18F]fluoro fatty acid esters based on radio-TLC (A = BP (3), B = IP (4), C = BTP (5), MW = microwave heating, CH = conventional heating).
Table 1.
[18F]Fluoride incorporation into fatty acid esters based on radio-TLC (%)
| Microwave Heating | Conventional Heating | ||||||
|---|---|---|---|---|---|---|---|
| Temp (°C) | Time (min) | BP (3) | BTP (5) | IP (4) | BP (3) | BTP (5) | IP (4) |
| 55 | 2 | 39.2 ± 3.0 | 41.1 ± 16.1 | 48.2 ± 11.5 | |||
| 55 | 5 | 70.2 ± 5.3 | 74.4 ± 3.7 | 74.6 ± 13.8 | 7.1 ± 3.2 | 5.2 ± 1.9 | 15.9 ± 3.7 |
| 55 | 10 | 85.4 ± 6.0 | 87.4 ± 2.7 | 87.3 ± 3.6 | 12.7 ± 5.0 | 9.8 ± 3.1 | 27.8 ± 8.4 |
| 55 | 15 | 88.4 ± 6.1 | 93.1 ± 2.5 | 94.2 ± 1.2 | 17.1 ± 4.1 | 14.4 ± 6.2 | 37.5 ± 8.3 |
| 60 | 2 | 75.5 ± 3.0 | 53.2 ± 22.5 | 87.2 ± 8.3 | |||
| 60 | 5 | 90.6 ± 1.2 | 73.9 ± 10.6 | 95.1 ± 1.5 | |||
| 60 | 10 | 93.6 ± 2.4 | 88.3 ± 4.1 | 96.1 ± 0.7 | |||
| 60 | 15 | 93.7 ± 3.4 | 91.2 ± 1.2 | 95.5 ± 1.6 | |||
| 65 | 2 | 89.0 ± 2.4 | 70.8 ± 9.2 | 85.5 ± 11.2 | |||
| 65 | 5 | 91.6 ± 4.2 | 88.1 ± 1.8 | 94.9 ± 0.5 | 6.5 ± 5.5 | 6.9 ± 3.6 | 17.4 ± 16.6 |
| 65 | 10 | 95.9 ± 2.0 | 92.1 ± 2.8 | 96.3 ± 0.9 | 17.1 ± 16.4 | 14.3 ± 7.4 | 33.6 ± 24.5 |
| 65 | 15 | 95.6 ± 0.1 | 92.4 ± 1.2 | 94.7 ± 3.0 | 20.3 ± 13.5 | 20.3 ± 7.0 | 41.9 ± 27.9 |
| 70 | 2 | 88.7 ± 1.0 | 86.4 ± 2.4 | 95.2 ± 1.7 | |||
| 70 | 5 | 95.6 ± 1.2 | 94.2 ± 0.9 | 95.1 ± 0.3 | |||
| 70 | 10 | 95.9 ± 0.9 | 95.3 ± 1.4 | 93.8 ± 0.9 | |||
| 70 | 15 | 96.2 ± 1.2 | 94.5 ± 3.4 | 95.1 ± 2.1 | |||
| 75 | 2 | 95.1 ± 0.9 | 87.4 ± 3.1 | 94.0 ± 2.4 | |||
| 75 | 5 | 96.4 ± 0.8 | 94.0 ± 1.3 | 94.8 ± 2.1 | 22.9 ± 15.7 | 19.6 ± 15.4 | 53.5 ± 25.9 |
| 75 | 10 | 97.6 ± 0.2 | 93.7 ± 3.1 | 95.8 ± 0.6 | 37.6 ± 21.8 | 32.1 ± 24.1 | 64.7 ± 25.1 |
| 75 | 15 | 97.0 ± 1.0 | 94.6 ± 3.5 | 95.6 ± 1.0 | 48.6 ± 24.8 | 39.5 ± 19.6 | 73.6 ± 22.1 |
n=3 at each condition
Labeling precursor abbreviations as described in Figure 2 caption.
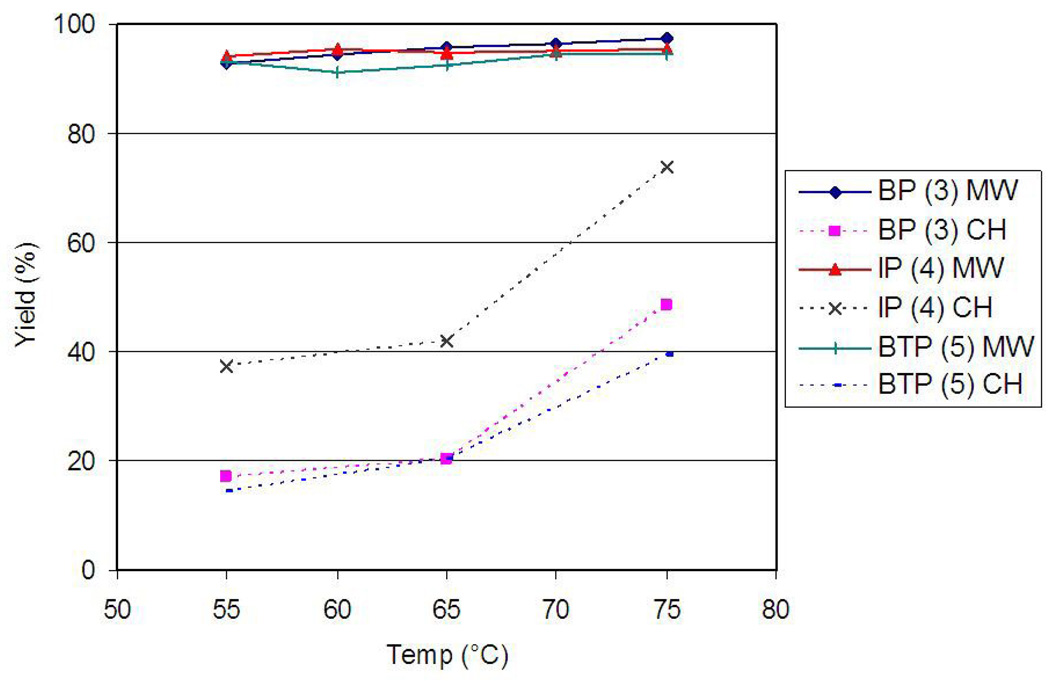
Fig. 4.
[18F]fluoride incorporation temperature dependence for 15 min reactions based on radio-TLC (MW = microwave heating, CH = conventional heating).
Initial experiments showed insignificant improvement of [18F]fluoride incorporation after 15 min for both conventional and microwave heating. Therefore, reactions were only monitored up to 15 min. Since the PETWave® system is not designed to accomodate a pressurized reaction vessel, the vessel was vented and reaction temperatures were kept below the boiling point of the solvent. For all temperatures and precursors examined, the fluoride incorporation was enhanced using microwaves as compared to conventional heating. Of note, greater than 10-fold improvements in yield with microwave heating were found for the bromo precursors (3 and 5) at lower temperatures (≤65°C) at 5 min as compared to conventional heating reactions. Somewhat lesser enhancements were seen with the iodo precursor (4) due to higher yields with conventional heating. However, at temperatures ≥65°C with microwave heating, there was not a significant dependence of yield on the leaving group.
3.3 Comparison of overall radiochemical yields
To confirm that higher [18F]fluoride incorporation with microwave heating directly translates to increases in radiochemical yield of 4-thia substituted fatty acid analogs, several [18F]FTP (2) syntheses (starting from substrate 5) using both conventional and microwave heating were then hydrolyzed and purified via semi-preparative HPLC. Yields were decay corrected relative to the radioactivity present in the vessel at [18F]fluorination. The results are displayed in Table 2. The overall corrected yields of [18F]FTP (2) were improved by >100% using the microwave reactor, while the coefficient of variation (variability) was reduced from 14.8% to 9.2%. The lower overall yield for the complete synthesis of [18F]FTP (49%) relative to the [18F]fluorination yield (>90%) is explained by release of adsorbed [18F]fluoride from the reaction vessel (~19.5%) by KOH solution that is not accounted for during the rad-TLC measurement of [18F]fluorination yield, and normal losses of product experienced during transfers, HPLC purification, and the post-HPLC trap and release process.
Table 2.
[18F]FTP (2) isolated radiochemical yields (RCYs) using microwave and conventional heating
| Heating Method | Decay Corrected RCY(%) |
Coefficient of Variation (%) |
|---|---|---|
| Microwave assisteda | 49.0 ± 4.5* | 9.2 |
| Conventionalb | 23.6 ± 3.5 | 14.8 |
n=3 at each condition
Fluorination conditions = 10 min at 75°C, Hydrolysis conditions = 2 min at 75°C
Fluorination conditions = 15 min at 75°C, Hydrolysis conditions = 5 min at 75°C
p<0.05 versus conventional
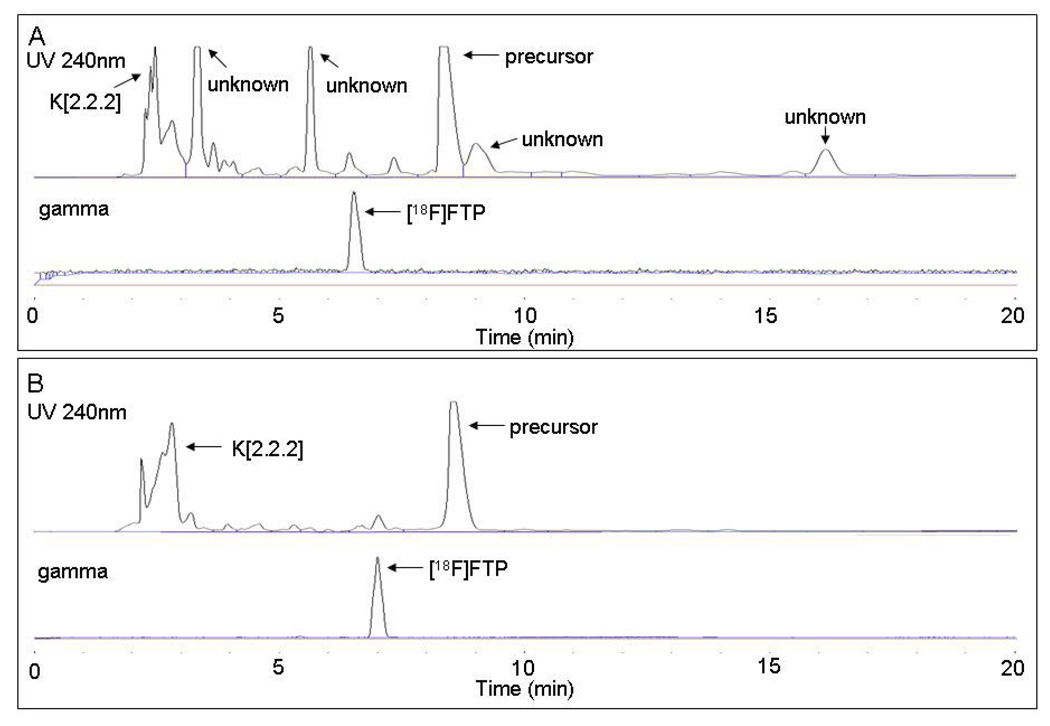
In addition to examining radiochemical yield, the chemical and radiochemical purities with both heating methods were compared. Undesired side product formation can often complicate or prohibit efficient purification of the target radiotracer. Representative HPLC chromatograms are shown in Fig. 3A and 3B. Several large UV peaks representing unidentified chemical impurities are marked as “unknown” in the conventional heating traces. These impurites were not present when microwave heating was employed. They were primarily formed during the hydrolysis reaction, as HPLC-UV traces of the reaction mixture prior to hydrolysis showed far fewer impurities than after hydrolysis (data not shown). In both conventional and microwave heating methods, high radiochemical purity is observed on the radio-chromatograms.
Fig. 3.
HPLC traces of conventional (A) and microwave-assisted (B) [18F]FTP reaction mixtures.
4. Discussion
Achieving high [18F]fluoride incorporation is a central goal in radiosynthesis of 18F-labeled radiopharmaceuticals. In designing nucleophilic [18F]fluorination precursors, chemists have modified halogen leaving groups to maximize the fluoride incorporation and thus enhance radiochemical yields. By comparing reactions of terminal bromo- and iodo-palmitate ester precursors, this study provides valuable insight into leaving group behavior in microwave-assisted fluorinations of [18F]fatty acid analogs. This study also addresses the utility of microwave heating to minimize the formation of undesirable byproducts during the synthetic procedure.
At the reaction temperature of 75°C, the iodo-ester precursor (4) gave [18F]fluoride incorporations under conventional heating that approached the values achieved using the microwave reactor, albeit with longer reaction time (15 min versus 2 min). However, the discrepancy increases dramatically as reaction temperature is decreased. Microwave-assisted [18F]fluorination provided 85% incorporation at only 65°C in just 2 minutes, while conventional heating at 65°C required 15 minutes to achieve just 42% incorporation. Efficiency of [18F]fluoride incorporation for BP (3) and BTP (5) trailed IP (4) using either mode of heating. As expected based on similarities in structure, BTP (5) and BP (3) share similar [18F]fluoride incorporation trends using microwave or conventional heating.
Microwave reactors provide a means to run reactions at much lower temperatures than required when using conventional heating. For all of the precursors examined, conventional fluorination reactions at 55°C produced limited quantities of product (~10–40% after 15 min), however, at the same temperature, all microwave-assisted fluorination reactions gave >85% [18F]fluoride incorporation after 10 min. If a substrate or product is succeptable to thermal decomposition, it may be beneficial to run the reaction in a microwave reactor at a lower temperature so as to avoid excessive thermal heating without a sacrifice in yield.
When developing a new radiosynthesis, optimizing reaction conditions can be a very time consuming process. Many [18F]fluorinations are highly temperature sensitive and can only be efficiently performed within a small temperature range. Using microwave heating greatly widens the range of temperature within which efficient fluorination can be carried out. To illustrate this, we have plotted all the 15-min [18F]fluorination yields in Fig. 4, demonstrating that decreasing the temperatures of conventionally heated reactions resulted in marked reductions in yield, while microwave-assisted yields were consistently above 90% over 55–75°C.
As expected, enhanced [18F]fluoride incorporation using microwave heating translated to increased overall radiochemical yield of [18F]FTP. Decay corrected yields were significantly increased (p = 0.0039) from 23.6% to 49.0% using microwave heating (>100% improvement). Reproducibility of radiochemical yields is another important aspect of radiopharmaceutical product. Coefficient of variation values showed that radiochemical yields of conventional heating were more variable than microwave heating (CVCH=14.8%, CVMW=9.2%). This was further supported by the observation of large fluctuations in [18F]fluoride incorporation using conventional heating as compared to microwave. Additionally, the chemical purity (HPLC UV trace) of the crude reaction mixture was much higher for the microwave assisted reaction than for the conventional reaction (Fig. 3A and 3B). The decreased formation of unlabeled impurities with microwave heating may be attributed to shorter reaction times and differences in mechanism of heating. The microwave mode of heating appears capable of facilitating a cleaner, more specific reaction path than is achievable using conventional heating. This observation corroborates previous findings in other laboratories [2]. The HPLC radioactivity chromatograms for both conventional and microwave reactions contained only the expected product. In the case of [18F]FTP (2), the impurities from the conventional reaction eluted from the HPLC at different times than the product, however, for other radiosyntheses, it is feasible that the elution of impurities may encroach upon the expected product making purification more complicated or impossible to accomplish given the half-life of the isotope at hand.
5. Conclusions
A comprehensive comparison of microwave assisted heating using CEM’s PETWave® system and conventional heating has been performed for the [18F]fluorination of various fatty acid analogs. Microwave heating resulted in increased [18F]fluoride incorporation (up to 55% increase) and overall radiochemical yield of isolated [18F]FTP (2) (49.0% improved from 23.6%). Microwave heating further displayed clear advantages over traditional heating by allowing significant reductions in reaction times and/or temperatures, while maintaining yield reproducibility and chemical purity. Therefore, microwave-assisted radiosynthesis is the preferred method for producing [18F]fatty acid analogs.
Acknowledgements
The authors wish to acknowledge CEM for providing a PETWave® demo unit to carry out our initial studies and the support of NIH (CA-108620).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elander N, Jones JR, Lu SY, Stone-Elander S. Microwave-enhanced radiochemistry. Chem. Soc. Rev. 2000;29:239–249. [Google Scholar]
- 2.Stone-Elander S, Elander N. Microwave applications in radiolabelling with short-lived positron-emitting radionuclides. J Label Compd Radiopharm. 2002;45:715–746. [Google Scholar]
- 3.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 4.Cai L, Lu S, Pike VW. Chemistry with [18F]fluoride ion. Eur J Org Chem. 2008:2853–2873. [Google Scholar]
- 5.McCarthy TJ, Dence CS, Welch MJ. Application of microwave heating to the synthesis of [18F]fluoromisonidazole. Appl Rad Isot. 1993;44:1129–1132. doi: 10.1016/0969-8043(93)90118-t. [DOI] [PubMed] [Google Scholar]
- 6.La Beaume P, Placzek M, Daniels M, Kendrick I, Ng P, McNeel M, et al. Microwave-accelerated fluorodenitrations and nitrodehalogenations: expeditious routes to labeled PET ligands and fluoropharmaceuticals. Tetrahedron Lett. 2010;51:1906–1909. [Google Scholar]
- 7.Riss PJ, Roesch F. Efficient microwave-assisted direct radiosynthesis of [18F]PR04.MZ and [18F]LBT999: Selective dopamine transporter ligands for quantitative molecular imaging by means of PET. Bioorg Med Chem. 2009;17:7630–7634. doi: 10.1016/j.bmc.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 8.Mandap KS, Ido T, Kiyono Y, Kobayashi M, Lohith TG, Mori T, et al. Development of microwave-based automated nucleophilic [18F]fluorination system and its application to the production of [18F]flumazenil. Nucl Med Biol. 2009;36:403–409. doi: 10.1016/j.nucmedbio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Kallmerten AE, Jones GB. Microwave accelerated synthesis of PET image contrast agents for AD research. Curr Alz Res. 2010;7:251–254. doi: 10.2174/156720510791050830. [DOI] [PubMed] [Google Scholar]
- 10.DeGrado TR, Kitapci MT, Wang S, Ying J, Lopaschuk GD. Validation of 18F-fluoro-4-thia-palmitate as a PET probe for myocardial fatty acid oxidation: Effects of hypoxia and composition of exogenous fatty acids. J Nucl Med. 2006;47:173–181. [PubMed] [Google Scholar]
- 11.DeGrado TR, Wang S, Holden JE, Nickles RJ, Taylor M, Stone CK. Synthesis and preliminary evaluation of 18F-labeled 4-thia palmitate as a PET tracer of myocardial fatty acid oxidation. Nucl Med Biol. 2000;27:221–231. doi: 10.1016/s0969-8051(99)00101-8. [DOI] [PubMed] [Google Scholar]
- 12.DeGrado TR. Synthesis of 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid (FTHA) J Label Compd Radiopharm. 1991;29:989–995. [Google Scholar]
- 13.DeGrado TR, Stöcklin G, Coenen HH. 14(R,S)-[18F]Fluoro-6-thia-heptadecanoic acid (FTHA): Evaluation in mouse of a new in vivo probe of myocardial utilization of long-chain fatty acids. J Nucl Med. 1991;32:1888–1896. [PubMed] [Google Scholar]
- 14.Ebert A, Herzog H, Stöcklin G, Henrich M, DeGrado TR, Coenen HH, Feinendegen LE. Kinetics of 14(R,S)-[F-18]fluoro-6-thia-heptadecanoic acid (FTHA) in normal human heart at rest, during exercise and after dipyridamole. J Nucl Med. 1994;35:51–56. [PubMed] [Google Scholar]
- 15.Renzanka T, Sigler K. Identification of very long chain unsaturated fatty acids from Ximenia oil by atmospheric pressure chemical ionization liquid chromatography–mass spectroscopy. Phytochemistry. 2007;68:925–934. doi: 10.1016/j.phytochem.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 16.Buist PH, Alexopoulos KA, Behrouzian B, Dawson B, Black B. Synthesis and desaturation of monofluorinated fatty acids. J Chem Soc, Perkin Trans. 1997:2617–2624. [Google Scholar]