Abstract
Many nonhuman proteins have useful pharmacological activities, but are infrequently effective in humans because of their high immunogenicity. A recombinant immunotoxin (HA22, CAT8015, moxetumomab pasudotox) composed of an anti-CD22 antibody variable fragment fused to PE38, a 38-kDa portion of Pseudomonas exotoxin A, has produced many complete remissions in drug-resistant hairy-cell leukemia when several cycles of the agent can be given, but has much less activity when antibodies develop. We have pursued a strategy to deimmunize recombinant immunotoxins by identifying and removing B-cell epitopes. We previously reported that we could eliminate most B-cell epitopes using a combination of point mutations and deletions. Here we show the location and amino acid composition of all of the B-cell epitopes in the remaining 25-kDa portion of Pseudomonas exotoxin. Using this information, we eliminated these epitopes to produce an immunotoxin (HA22-LR-8M) that is fully cytotoxic against malignant B-cell lines, has high cytotoxic activity against cells directly isolated from patients with chronic lymphocytic leukemia, and has excellent antitumor activity in mice. HA22-LR-8M does not induce antibody formation in mice when given repeatedly by intravenous injection and does not induce a secondary antibody response when given to mice previously exposed to HA22. HA22-LR-8M also has greatly reduced antigenicity when exposed to sera from patients who have produced antibodies to HA22. The properties of HA22-LR-8M make it an excellent candidate for further clinical development.
Keywords: protein engineering, SS1P, passive immunotherapy, target therapy/cancer therapeutics
Nonhuman proteins have properties that make them attractive therapeutic agents, but their usefulness can be limited by the development of antidrug antibodies that neutralize their biological activity, shorten their half-life in the circulation, and cause life-threatening immune responses in rare cases (1–4). Foreign proteins have been used for the treatment of cancer; mouse monoclonal antibodies (mAbs) were initially used to treat cancers, but these efforts had limited success because of a strong immune response to the mouse Fc (5). Antidrug antibodies are much less frequent with the current use of human or humanized antibodies to treat cancers, although some human antibodies and cytokines are still immunogenic (6). Many antibodies, which are ineffective at killing cancer cells alone, can be useful as immuno-conjugates to deliver cytotoxic agents to cancers. We have developed recombinant immunotoxins (RITs) for the treatment of cancer that are composed of an antibody variable fragment (Fv) fused to a bacterial toxin (7). The Fv binds to an antigen on a cancer cell, enabling the bacterial toxin, a 38-kDa fragment of Pseudomonas exotoxin A (PE38), to enter the cell by endocytosis. After cellular entry and proteolytic processing, a fragment of PE38 traffics to the cytosol, where it catalyzes the ADP ribosylation and inactivation of elongation factor 2 (EF2), arrest of protein synthesis, and cell death.
Clinical trials are ongoing with several RITs. BL22 and its improved variant moxetumomab pasudotox (HA22) [anti-CD22(Fv)-PE38] are targeted to CD22 on B-cell malignancies (8, 9), and SS1P [anti-mesothelin(Fv)-PE38] is targeted to mesothelin on mesotheliomas and ovarian, lung, and other cancers (10, 11). BL22 and moxetumomab pasudotox have produced many complete responses in patients with drug-resistant hairy-cell leukemia, where many cycles of RIT therapy can usually be given before antibodies develop and prevent further treatment (8). We suspect that the delayed antibody responses in patients with B-cell malignancies is a result of the immunosuppressive effect of prior chemotherapy and to the destruction of immune cells by tumor cells infiltrating into the bone marrow. Some hairy-cell leukemia patients, however, develop antibodies and treatment must be stopped before complete response is achieved. In patients with mesothelioma receiving SS1P, minor responses but no major responses have been observed (10). One factor contributing to the poor responses is the rapid development of neutralizing antibodies because the immune system is intact in these patients. Because it is usually necessary to give many doses of a drug to obtain a major response in cancer, we are investigating approaches that will enable us to give more doses of RITs.
Several approaches have been investigated to eliminate the immunogenicity of protein therapeutics. The most successful approach is masking B-cell epitopes by modifying the protein with high molecular-weight polyethylene glycol (PEG) (12). We have modified RITs with PEG, but the addition of PEG greatly diminished their cytotoxic activity (13). Another approach is to modify T-cell epitopes (14), and research to this end is ongoing. Because T-cell epitopes are presented in the context of the highly polymorphic major histocompatibility complex proteins, it seems difficult to identify and remove all possible T-cell epitopes.
We have focused on the identification and removal of B-cell epitopes, using a mouse model. We developed a panel of mouse mAbs reacting with PE38, and assigned them to seven major epitope groups and 13 subgroups (15). Because we identified only seven discrete epitopes, it seemed possible that we could eliminate them by mutagenesis. Our approach to removing epitopes was to change large, surface-exposed, hydrophilic residues that are commonly involved in antibody binding, such as arginine, lysine, glutamine, and glutamate, to smaller residues like alanine, glycine, or serine. By combining mutations, we made an immunotoxin with diminished immunogenicity in mice that retained excellent cytotoxic and antitumor activity (16). Immunogenicity was further reduced by the removal of large sections of PE38, which reduced the toxin to a 25-kDa fragment (17) (Fig. 1). The resulting molecule, HA22-LR, is still immunogenic in mice, although less immunogenic than the parental molecule HA22 (18).
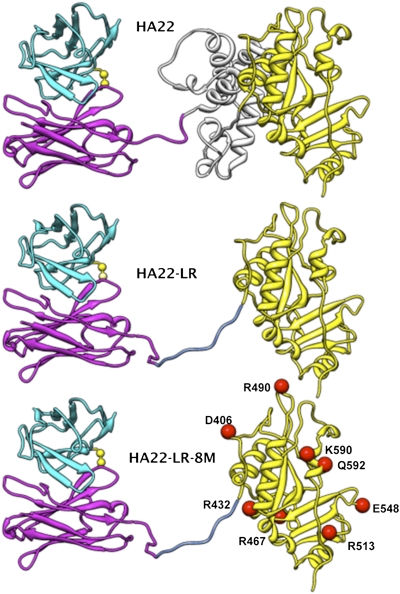
Fig. 1.
RITs. Ribbon drawing of HA22, HA22-LR, and HA22-LR-8M. The light chain is in cyan and the heavy chain is in magenta. The interchain disulfide bond is in yellow. Domain II of the toxin is in gray and domain III in yellow. The furin cleavage sequence (RHRQPRGWEQL) in the linker between Fv and the toxin is blue. The Fv part was modeled using the heavy chain of the PDB structure 1hil and the light chain of 1fbi. The toxin part is from the PDB structure 1ikq. The linker conformation was chosen arbitrarily.
Here we show the location and composition of all B-cell epitopes in HA22-LR. We have used this information to produce a RIT, HA22-LR-8M, which is fully cytotoxic to CD22+ cell lines and cells from patients, and shows excellent antitumor activity in mice. It does not induce antibody formation when given repeatedly to mice by intravenous injection, or induce a secondary antibody response. Additionally, HA22-LR-8M has significantly reduced antigenicity when tested against sera from patients who have made antibodies to HA22.
Results
Location of Amino Acids That Define Epitopes.
Fig. 1 shows the schematic structure of HA22, HA22-LR, and the recently described protein HA22-LR-8M. HA22 contains the anti-CD22 Fv linked to domains II and III of native Pseudomonas exotoxin A. HA22-LR, which has similar activity to HA22 on cell lines, contains the Fv connected to domain III by only a furin cleavage site (RHRQPRGWEQL) derived from domain II (the amino acid sequence of the LR toxin is in SI Materials and Methods). We therefore directed our attention toward de-immunizing domain III (17).
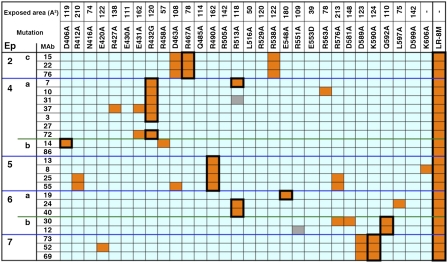
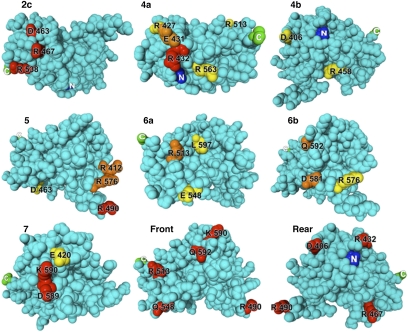
To identify the residues that make up the B-cell epitopes in domain III, we measured the reactivity of 29 mAbs with 30 different domain III mutant proteins in which large, surface-exposed residues were mutated to alanine or glycine (Fig. 2). Mutant proteins with less than 10% of the native protein's reactivity are shown in orange, and mutant proteins with reactivity similar to the native protein are in blue. The results show that when a mutation decreased antibody binding, it affected a limited number of mAbs that were located in the same epitope group or subgroup. The exact locations of the residues that affected antibody binding to each epitope are shown in a set of space-filling drawings of the structure (Fig. 3). The amino acids associated with each epitope/subepitope (2c, 4a, 4b, 5, 6a, 6b, and 7) are colored red, orange, or yellow to indicate their relative importance. If the binding of three or more mAbs is greatly reduced by the mutation, then the residue is colored red. If the binding of two mAbs is reduced the residue is colored orange, and if the binding of one mAb is reduced it is colored yellow.
Fig. 2.
Reactivity of anti-PE38 mAbs against HA22 point mutants. Orange, light-blue, and gray cells indicate less than 10% reactivity, greater than 10% reactivity, and not tested, respectively. The mutants are ordered by their location from the N terminus (Left) to the C terminus (Right). The ASA of each residue before mutation is also shown (Å2). Black boxes enclose residues that were mutated to generate HA22-LR-8M.
Fig. 3.
Location of residues that define epitopes in domain III. The amino acids whose mutation decreased the binding of mAbs within each epitope (2c, 4a, 4b 5, 6a, 6b, and 7) are colored red (affect the binding of three or more mAbs), orange (affect the binding of two mAbs), or yellow (affect the binding of one mAb). The model of domain III was constructed by removing residues 1 to 394 from the crystal structure of native PE (PDB File 1ikq). The location of residues mutated in HA22-LR-8M is indicated from two perspectives (front, rear) rotated 180° from each other.
Antibodies reactive with epitope 2c were affected by mutations at residues D463, R467, and R538. A mutation of any of these residues to alanine eliminates the binding of all three antibodies that recognize the epitope (Fig. 2). These residues lie spatially adjacent in the structure of domain III, even though they are separated by 71 amino acids in the sequence. Antibodies to epitope 4a are affected by mutations at five residues. R432 is the most important of them; mutating it to glycine destroys binding of five of the six mAbs that define epitope 4a. Mutating residue E431 prevents the binding of two mAbs, and mutating R513 or R427 eliminates the binding of one antibody. Structurally, R432 is at the center of a cluster with R563 on one side and E431 and R427 on the other. No mutation affecting mAb 27 was found.
Reactivity with two mAbs defines epitope 4b. A mutation at D406 or R458 affects binding of one mAb, but a mutation affecting the other (mAb 86) was not found. Epitope 5 is defined by reactivity with four mAbs, and binding to all four is eliminated by a mutation at R490. Mutations at R576 or R412 prevent the binding of two of the four mAbs, and mutations at D463 and K606 eliminate binding of one mAb. Epitope 6a is defined by reactivity with three mAbs. Binding to two of these is abolished by a mutation at R513, and binding to the other by a mutation at E548. Epitope 6b is defined by reactivity with two mAbs. Mutation of Q592 destroys the binding of both, and a mutation at D581 or R576 affects one of the two. Epitope 7 is defined by reactivity with four mAbs. Binding to all four is affected by a mutation at one of the adjacent residues D589 or K590. Mutating E420 eliminates binding of a single mAb.
In summary, mutations in domain III have identified residues important for antibody recognition, and have confirmed the prediction that epitopes are located in discrete locations on the surface of domain III. We used this information to choose which amino acids to mutate to abolish all identified epitopes.
Production of a Nonimmunogenic RIT.
We faced several challenges in choosing mutations to incorporate into domain III, with a goal of preventing immunogenicity. One challenge was to determine how many mutations were needed to destroy an epitope. Another challenge was to maintain cytotoxic activity, and a third was to produce sufficient quantities of protein to make it useful for therapeutic purposes.
In most cases we replaced residues with alanine because its small side chain reacts poorly with antibodies, and because it usually did not affect protein folding. We also used serine to avoid an especially hydrophobic surface. Mutations were added consecutively, and those that reduced the activity or yield of the purified protein were discarded in favor of others. We generated more than 50 different mutant proteins before producing HA22-LR-8M, which has eight mutations in domain III in addition to deletions removing most of domain II from PE38 (Fig. 1). The residues mutated in the final molecule are shown in large bold letters near the top of Fig. 2, and their effect on reducing mAb binding (epitope removal) is indicated by the black boxes surrounding orange squares. In most cases a single mutation reduced the binding affinity of one or more mAbs by greater than 90%. We used the mutation R467A to remove epitope 2c, R432G for epitope 4a, D406A for epitope 4b, R490A for epitope 5, Q592A for epitope 6b, and K590S for epitope 7. For epitope 6a we combined mutations at R513A and E548S. The locations of all eight mutated residues in domain III of HA22-LR-8M are shown in Figs. 1 and 3. It is important to notice that the residues are widely separated on the surface of the protein. We examined the reactivity of HA22-LR-8M with each mAb in the panel and found its reactivity with each one was reduced by more than 90% (Fig. 2). Even mAb 27 in epitope 4a and mAb 86 in epitope 4b, which were not affected by individual point mutations, were unreactive with HA22-LR-8M. Fig. S1 shows an SDS gel of the purified HA22-LR-8M protein. It is over 95% pure and runs at a molecular weight of approximately 50 kDa.
Cytotoxicity.
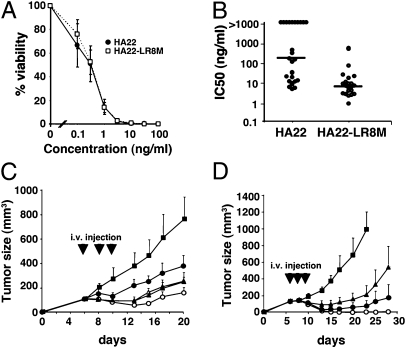
We next evaluated the activity of HA22-LR-8M both in vitro and in vivo. HA22-LR-8M was cytotoxic to several cell lines derived from B-cell malignancies. A typical cytotoxic activity assay (Fig. 4A) shows that HA22 and HA22-LR-8M have identical activity on CA46 cells. HA22-LR-8M was also cytotoxic to cell lines Daudi, Raji, Ramos, and KOPN-8, which all express CD22 (Table 1). It had no activity on the A431 cell line that does not express CD22.
Fig. 4.
Biological activities of HA22-LR-8M. (A) Cytotoxic activity of HA22 (●) and HA22-LR-8M (□) on CA46 cells. (B) Cytotoxic activity (IC50) of HA22 and HA22-LR-8M on CLL cells from 25 patients. The bar indicates the median IC50 value. (C) Antitumor activity of HA22 and HA22-LR-8M at low doses. Groups of 8 SCID mice bearing CA46 tumors were treated QODx3 (arrows), with 0.2% mouse serum albumen (MSA) in PBS (■), HA22 at 0.3 mg/kg (▲), or HA22-LR-8M at 0.3 mg/kg (●), 0.6 mg/kg (△) or 1.0 mg/kg (○). (D) Antitumor activity of HA22 and HA22-LR-8M at high doses. Groups of eight mice with CA46 tumors were treated QODx3 (arrows), with 0.2% MSA in PBS (■), HA22 at 0.4 mg/kg (▲), or HA22-LR-8M at 2.5 mg/kg (●) or 5.0 mg/kg (○). Data are expressed as the mean ± SD.
Table 1.
Cytotoxic activities of HA22-LR-8M
| Cell line | IC50 (ng/mL) |
| Daudi | 0.2 |
| KOPN-8 | 0.45 |
| Raji | 0.18 |
| Ramos | 0.9 |
| A431 | >100 |
We then assessed the activity of HA22-LR-8M on cells directly isolated from patients with chronic lymphotic leukemia (CLL). Fig. 4B shows that HA22-LR-8M is highly cytotoxic to CLL cells, with a median IC50 of 6.6 ng/mL that greatly exceeds that of HA22 (105 ng/mL). Furthermore, all patient cells were sensitive to HA22-LR-8M, whereas several samples from CLL patients were not sensitive to HA22 (IC50 > 1,000 ng/mL).
In addition to in vitro cytotoxicity, the antitumor activity of HA22-LR-8M was investigated in a mouse CA46 xenograft tumor model (Fig. 4 C and D). CA46 cells were equally sensitive to HA22 and HA22-LR-8M in vitro (Fig. 4A). We first determined the dose of HA22-LR-8M that elicited the same antitumor effect as HA22. When mice were treated with 0.4 mg/kg HA22 every other day times three, modest tumor shrinkage was observed. This is the maximum amount of HA22 that can be given safely to mice under this dosing schedule; larger doses cause liver toxicity and death (17, 19). We observed an equivalent antitumor response with HA22-LR-8M at 0.8 mg/kg (Fig. 4C). The decreased antitumor activity of HA22-LR-8M is likely because of its more rapid clearance from the blood, because it is smaller than HA22. We previously found that HA22-LR has a half-life of ∼8 min, compared with ∼15 min for HA22 (17). Because high doses of HA22-LR-8M, like HA22-LR, can be given safely to mice, HA22-LR-8M was given at 2.5 mg/kg or 5.0 mg/kg every other day times three (Fig. 4D). These doses were well tolerated with no signs of illness in the mice. HA22-LR-8M at 5.0 mg/kg produced complete remissions in all eight mice, and the 2.5 mg/kg dose also had excellent activity.
Immunogenicity.
The immune response of mice treated with HA22-LR-8M was also evaluated. Mice were dosed intravenously with either 5 μg of HA22 or 10 μg of HA22-LR-8M every 14 d, and serum was collected from each mouse 10 d after each injection. We based the dose on the antitumor activity of each molecule. Thus, we gave twice as much HA22-LR-8M as HA22 because the antitumor experiments required twice as much HA22-LR-8M as HA22 to achieve the same effect. Antibody responses were measured by immune complex captured (ICC)-ELISA, which measures conformational epitopes (15). Antibody responses are expressed in microgram per milliliter using a mAb to PE38 (IP30) as a standard (15, 16). We observed that the antibody levels of HA22-LR-8M were not significantly above background (less than 0.1 μg/mL) after the third, fourth, and fifth injections (Fig. 5A). In contrast, the levels of antibodies to HA22 were significantly elevated after the fourth and fifth injections, reaching a maximum level of 40 μg/mL (Fig. 5A). In addition, we explicitly measured IgM levels and found that HA22 induced a specific increase in IgM reactive with HA22, whereas the mutant protein showed no such increase (Fig. 5B).
Fig. 5.
Immunogenicity of HA22-LR-8M in mice. (A) Groups of BALB/c mice (n = 9) received HA22 (250 μg/kg, ●), or HA22-LR-8M (500 μg/kg, ▲) intravenously every 14 d (arrows) and were bled 10 d after each injection. Antibody levels were measured as described in Materials and Methods. IgG antibody levels are expressed relative to IP30, a mAb against PE38. (B) IgM response. BALB/c mice (10 per group) were injected with HA22 (250 μg/kg, ●) or HA22-LR-8M (500 μg/kg, ▲). Mice were bled 10 d after injection and the 50-fold diluted sera were assayed for IgM by ELISA. (C) Secondary immune response to HA22 and HA22-LR-8M. Four weeks after primary immunization with HA22 (two injections of 5 μg with a 2-wk interval between injections; black arrows), mice were reimmunized with HA22 (C-a; black arrows) or HA22-LR-8M (C-b; gray arrows), and anti-PE IgG levels were measured. The SD of the HA22 group and HA22-LR-8M group on day 66 is 42.5 and 0.47, respectively. (D) Immune responses of mice with preexisting antibodies to HA22. Fifteen weeks after initial immunizations with HA22 (5 μg per mouse), eight mice with low titers (∼103) of anti-HA22-specific IgG were reimmunized with either HA22 (5 μg per mouse) or HA22-LR-8M (10 μg per mouse). Blood was drawn at week 17 and antibody levels were measured. HA22-specific IgG titer is shown. Data are expressed as the mean ± SD; ns, not significant; *P < 0.05.
Secondary Antibody Response.
To determine if prior immunization with HA22 primed the mice to respond to HA22-LR-8M, we immunized mice with HA22 and boosted with HA22 or HA22-LR-8M. Fig. 5C shows that antibodies reactive with HA22 developed in mice boosted with HA22 (35 μg/mL of HA22-specific IgG), but no antibodies developed in mice boosted with HA22-LR-8M. This finding demonstrates that B cells activated by a primary immunization with HA22 were not stimulated to produce antibodies by HA22-LR-8M. To further study the secondary B-cell responses to HA22-LR-8M, we produced mice with low but detectable titers of anti-HA22 specific IgG and boosted them with HA22 or HA22-LR-8M. Fig. 5D shows that these mice responded to 5 μg of HA22 but not to 10 μg of HA22-LR-8M. In summary, HA22-LR-8M did not induce antibody responses alone, and did not stimulate a secondary antibody response in mice previously immunized with HA22.
Antigenicity.
Antigenicity is defined as the binding of immunogens to preexisting antibodies. To assess the antigenicity of HA22-LR-8M to mouse antisera against HA22, we carried out competition experiments to measure the concentration of free HA22 or HA22-LR-8M that reduced the level of antibodies reacting with HA22 by 50%. Typical competition results with two mouse sera are shown in Fig. S2 and summarized in Table 2.
Table 2.
Antigenicity of HA22 and HA22-LR-8M to mouse antisera
| Mouse* | IC50 of HA22 (nM) | IC50 of HA22-LR-8M (nM) | Binding† (%) |
| 1 | 1.3 | >200 | <0.65 |
| 2 | 2.7 | >200 | <1.4 |
| 3 | 3.5 | >200 | <1.8 |
| 4 | 8.8 | >200 | <4.4 |
| 5 | 11 | >200 | <5.5 |
| 6 | 2.0 | >200 | <1.0 |
| 7 | 1.5 | 110 | 1.4 |
*Mice were treated with HA22 (250 μg/kg i.v. at 2-wk intervals for five cycles).
†IC50 ratio(HA22/HA22-LR-8M) x 100.
We examined the sera of seven mice with high anti-HA22 titers and found that in five mice, HA22-LR-8M failed to significantly reduce the binding of anti-HA22 antibodies. In one mouse, only 1.4% Ab could bind HA22-LR-8M, and in the other less than 6% could bind. These data show HA22-LR-8M has very low antigenicity with mouse antibodies to HA22.
We then examined the reactivity of sera from nine patients with neutralizing antibodies to HA22 that made them ineligible to receive additional treatment. We found the antigenicity of HA22-LR-8M with human sera was also substantially reduced (Table 3 and Fig. S3). In three patients, reactivity could not be detected, and in an additional three patients reactivity was below 5% of HA22. In the other three the reactivity was 28 to 42% of the level with HA22. These data show that the antigenicity of HA22-LR-8M is greatly reduced with human antisera, but residual antigenicity with human antisera is still present.
Table 3.
Antigenicity of HA22 and HA22-LR-8M to human antisera
| Patient* | IC50 of HA22 (nM) | IC50 of HA22-LR-8M (nM) | Binding† (%) |
| 1 | 0.021 | 97 | 0.022 |
| 2 | 0.13 | >400 | <0.033 |
| 3 | 0.56 | >400 | <0.14 |
| 4 | 2.8 | 140 | 1.9 |
| 5 | 9.1 | >400 | <2.3 |
| 6 | 0.35 | 8.3 | 4.2 |
| 7 | 0.16 | 0.57 | 28 |
| 8 | 0.096 | 0.26 | 37 |
| 9 | 0.08 | 0.19 | 42 |
*Patients were treated with HA22 (three to five cycles) and developed neutralizing antibodies.
†IC50 ratio (HA22/HA22-LR-8M) x 100.
Discussion
In this study we describe the design, production, and characterization of a RIT targeting CD22 that does not induce a primary or secondary response when repeatedly injected intravenously into mice, yet retains excellent cell killing and antitumor activity. Our strategy was to identify and remove conformational epitopes in PE38, as the reaction that neutralizes the activity of the immunotoxin occurs in solution in the bloodstream. We reasoned that this approach could only be successful if there were a relatively small number of epitopes located at discrete regions of the protein. Fig. 3 shows the location of the residues making up each epitope in the toxin portion of HA22-LR. Several features are of special interest. Each epitope is made up of several amino acids. In two cases, epitope 2c and epitope 7, a mutation in more than one residue completely eliminated antibody recognition. For epitopes 4a, 5, and 6b, only one key residue could be identified, and that residue was mutated. For epitope 6a we needed to mutate two residues to eliminate mAb binding. We did not identify point mutations that prevented the binding of mAbs 27 and 86, but they failed to react with HA22-LR-8M, suggesting the need for multiple mutations to eliminate binding (Fig. 2). Our primary selection criterion for residues to mutate was based on the accessible surface area (ASA) from a crystal structure of domain III. Residues with a large ASA spaced widely on the surface of the protein were changed into smaller residues (20). This strategy was successful. All eight residues mutated in HA22-LR-8M have large ASAs, and seven have ASAs over 100 Å2 (Fig. 2). Also, as shown in Fig. 3, the residues mutated are widely separated from each other in the protein structure.
For the mutated RIT to kill target cells several important functions of the toxin had to be preserved. These include cleavage by furin, movement to the endoplasmic reticulum initiated by a C-terminal endoplasmic reticulum-retention signal, translocation from endoplasmic reticulum to the cytosol, binding to NAD, binding to EF2, and transfer of ADP ribose to EF2. None of these steps were obviously affected by the mutations introduced into HA22-LR-8M.
Because humans are frequently exposed to Pseudomonas aeruginosa, some subjects have preexisting antibodies to the toxin. In the vast majority of cases, the levels are low and do not prevent patients from enrolling in clinical trials. Prior antibody-producing exposure to the toxin can affect the timing and the likelihood of a secondary response. To determine the ability of HA22-LR-8M to generate a secondary response, we immunized mice with HA22 and boosted with HA22-LR-8M, either before detectable antibodies developed or when the antibody levels were low. In both cases we did not see a significant increase in antibodies, indicating that HA22-LR-8M was not stimulating antigen receptor sufficiently to produce an immune response.
We currently do not have an assay system that can predict the response of the human immune system to nonhuman antigens like HA22 or HA22-LR-8M. We did, however, measure the antigenicity of HA22 using sera from patients treated with HA22 and compare it with sera from mice treated with HA22. With both mouse and human antisera the antigenicity was decreased, showing that mice and humans share many B-cell epitopes. Our findings show that antigenicity was decreased with all nine human antisera, and in six patients it fell to undetectable or very low levels. This finding suggests that we have eliminated important human epitopes. Additional studies will be required to determine if it is sufficient to delay or diminish the antibody response of patients to HA22-LR-8M. Another approach we are pursuing is to eliminate T-cell epitopes (21) and eventually to produce a RIT with both T- and B-cell epitopes removed.
Although HA22-LR-8M demonstrated consistently lower antigenicity than HA22 with patient serum samples in the competition assay, three of the nine samples showed significant levels of antigenicity. This finding indicates the presence of human epitopes that were not identified from the mouse model, or human antibodies with a greater affinity than their mouse counterparts. Single-point mutations in an epitope may not be sufficient to detect a decrease in antigenicity with the human sera. We are making additional mutations to investigate both possibilities.
There are previous reports successfully reducing the immunogenicity of nonhuman proteins that contain a single catalytic site by B-cell epitope modification. Staphylokinase is a 136-residue profibrinolytic agent used for thrombolytic therapy of acute myocardial infarction. Three nonoverlapping immunodominant epitopes have been identified in staphylokinase (22). Immunogenicity was decreased by mutating 5 to 12 amino acids in these epitopes to alanine, and the resulting molecule induced neutralizing antibodies in 47% of patients, compared with 81% given native protein (23, 24). Additionally, the immunogenicity of bacterial carboxypeptidase G2, which is used for antibody-directed enzyme prodrug therapy, was reduced in mice and humans by identification and modification of a major B-cell epitope (25). These results support the hypothesis that immunogenicity can be reduced by site-directed mutagenesis without reducing activity. Our success in deimmunizing HA22, and previous results with other nonhuman proteins, indicate that the identification and removal of B-cell epitopes, in combination with structural and functional studies, is a rational way to deimmunize foreign proteins for therapeutic use.
Materials and Methods
RITs.
The expression plasmids for the RITs were derived from plasmids pEM15 and pEM16 (26, 27). RITs were prepared as previously described (28). Cell lines were grown and cytotoxicity measured by WST-8 (29–31). Competition assays to measure the reactivity of anti-PE38 mAbs with RITs were previously described (15).
Immunizations.
Female BALB/c mice were immunized intravenously with HA22 (5 μg) or HA22-LR-8M (10 μg) in 0.1 mL of PBS/0.2% mouse serum albumin. Mice were immunized every 2 wk for five injections (16). Blood was collected 10 d after each immunization. Animal protocols were approved by the Animal Care and Use Committee of the National Cancer Institute.
To check secondary Ab responses, female BALB/c mice were immunized twice intravenously with 5 μg HA22 and boosted every 2 wk with either 5 μg HA22 or 10 μg HA22-LR-8M. Blood was collected 10 d after each immunization. To assess responses in mice with measurable preexisting antibodies, mice were immunized intravenously with 5 μg HA22 every 2 wk two or three times until PE-specific antibodies were detected. Mice were boosted with either 5 μg HA22 or 10 μg HA22-LR-8M 15 wk after the initial immunization. Blood was collected at week 17 and assayed.
ICC-ELISA.
ICC-ELISA was performed as previously described (16).
Antigenicity.
Binding of HA22 or HA22-LR-8M to antibodies in mouse or human sera was analyzed in a displacement assay. Human sera were obtained under protocol 10C0066. Mesothelin-rFc was added to the ELISA plate (100 ng in 50 μL PBS/well) and incubated overnight at 4 °C. After washing, an antimesothelin/PE38 RIT SS1P (100 ng in 50 μL PBS/well) was added for 1 h to capture unbound human anti-PE38 antibodies (15). In separate tubes, sera (200- to 5,400-fold dilutions) was mixed with 2 μg/mL of HA22 or HA22-LR-8M and incubated overnight at 4 °C. After washing the plate, 50 μL of immunotoxin-antibody mixtures were transferred to each well. The human antibodies not bound to HA22 or HA22-LR-8M were captured by SS1P and detected by HRP-conjugated rabbit anti-human IgG Fc (Jackson ImmunoResearch Laboratories), followed by TMB substrate kit (Pierce). Binding curves were fitted using a four-parametric logistic curve model by SoftMaxPro 4.0 (Molecular Devices). The IC50 values indicate the concentration of RIT (HA22 or HA22-LR-8M) that inhibits 50% of the antibody reactivity with SS1P.
Statistics.
Mann-Whitney nonparametric method was used; P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. B. K. Sathyanaraya for making the structure drawings shown in Figs. 1 and 3. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and by a Cooperative Research and Development Agreement with MedImmune, LLC.
Footnotes
Conflict of interest statement: B.L., R.J.K., and I.P. are inventors on patents describing mutations that make immunotoxins more active and less immunogenic that are assigned to the National Institutes of Health.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102746108/-/DCSupplemental.
References
- 1.Chirino AJ, Ary ML, Marshall SA. Minimizing the immunogenicity of protein therapeutics. Drug Discov Today. 2004;9(2):82–90. doi: 10.1016/S1359-6446(03)02953-2. [DOI] [PubMed] [Google Scholar]
- 2.Kreitman RJ, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 3.Roscoe DM, Pai LH, Pastan I. Identification of epitopes on a mutant form of Pseudomonas exotoxin using serum from humans treated with Pseudomonas exotoxin containing immunotoxins. Eur J Immunol. 1997;27:1459–1468. doi: 10.1002/eji.1830270624. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall N, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346:469–475. doi: 10.1056/NEJMoa011931. [DOI] [PubMed] [Google Scholar]
- 5.Koren E, Zuckerman LA, Mire-Sluis AR. Immune responses to therapeutic proteins in humans—Clinical significance, assessment and prediction. Curr Pharm Biotechnol. 2002;3:349–360. doi: 10.2174/1389201023378175. [DOI] [PubMed] [Google Scholar]
- 6.Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36(1):3–10. doi: 10.1016/j.ymeth.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 8.Kreitman RJ, et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:2983–2990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreitman RJ, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 10.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan R, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 12.Hershfield MS, et al. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N Engl J Med. 1987;316:589–596. doi: 10.1056/NEJM198703053161005. [DOI] [PubMed] [Google Scholar]
- 13.Filpula D, et al. Releasable PEGylation of mesothelin targeted immunotoxin SS1P achieves single dosage complete regression of a human carcinoma in mice. Bioconjug Chem. 2007;18:773–784. doi: 10.1021/bc060314x. [DOI] [PubMed] [Google Scholar]
- 14.Yeung VP, et al. Elimination of an immunodominant CD4+ T cell epitope in human IFN-beta does not result in an in vivo response directed at the subdominant epitope. J Immunol. 2004;172:6658–6665. doi: 10.4049/jimmunol.172.11.6658. [DOI] [PubMed] [Google Scholar]
- 15.Onda M, et al. Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J Immunol. 2006;177:8822–8834. doi: 10.4049/jimmunol.177.12.8822. [DOI] [PubMed] [Google Scholar]
- 16.Onda M, et al. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci USA. 2008;105:11311–11316. doi: 10.1073/pnas.0804851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weldon JE, et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood. 2009;113:3792–3800. doi: 10.1182/blood-2008-08-173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen JK, et al. A recombinant immunotoxin targeting CD22 with low immunogenicity, low nonspecific toxicity, and high antitumor activity in mice. J Immunother. 2010;33:297–304. doi: 10.1097/CJI.0b013e3181cd1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onda M, et al. Inhibition of TNF-alpha produced by Kupffer cells protects against the nonspecific liver toxicity of immunotoxin anti-Tac(Fv)-PE38, LMB-2. J Immunol. 2000;165:7150–7156. doi: 10.4049/jimmunol.165.12.7150. [DOI] [PubMed] [Google Scholar]
- 20.Bang S, Nagata S, Onda M, Kreitman RJ, Pastan I. HA22 (R490A) is a recombinant immunotoxin with increased antitumor activity without an increase in animal toxicity. Clin Cancer Res. 2005;11:1545–1550. doi: 10.1158/1078-0432.CCR-04-1939. [DOI] [PubMed] [Google Scholar]
- 21.Stickler M, et al. An in vitro human cell-based assay to rank the relative immunogenicity of proteins. Toxicol Sci. 2004;77:280–289. doi: 10.1093/toxsci/kfh021. [DOI] [PubMed] [Google Scholar]
- 22.Collen D, et al. Recombinant staphylokinase variants with altered immunoreactivity. I: Construction and characterization. Circulation. 1996;94:197–206. doi: 10.1161/01.cir.94.2.197. [DOI] [PubMed] [Google Scholar]
- 23.Collen D, Moreau H, Stockx L, Vanderschueren S. Recombinant staphylokinase variants with altered immunoreactivity. II: Thrombolytic properties and antibody induction. Circulation. 1996;94:207–216. doi: 10.1161/01.cir.94.2.207. [DOI] [PubMed] [Google Scholar]
- 24.Laroche Y, et al. Recombinant staphylokinase variants with reduced antigenicity due to elimination of B-lymphocyte epitopes. Blood. 2000;96:1425–1432. [PubMed] [Google Scholar]
- 25.Mayer A, et al. Modifying an immunogenic epitope on a therapeutic protein: A step towards an improved system for antibody-directed enzyme prodrug therapy (ADEPT) Br J Cancer. 2004;90:2402–2410. doi: 10.1038/sj.bjc.6601888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvatore G, Beers R, Margulies I, Kreitman RJ, Pastan I. Improved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 27.Horton RM, Cai ZL, Ho SN, Pease LR. Gene splicing by overlap extension: Tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- 28.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–518. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
- 29.Kreitman RJ, et al. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) toward fresh malignant cells from patients with B-cell leukemias. Clin Cancer Res. 2000;6:1476–1487. [PubMed] [Google Scholar]
- 30.Du X, Beers R, Fitzgerald DJ, Pastan I. Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onda M, et al. Lowering the isoelectric point of the Fv portion of recombinant immunotoxins leads to decreased nonspecific animal toxicity without affecting antitumor activity. Cancer Res. 2001;61:5070–5077. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.