Abstract
It is proposed that a progressive series of mutations and epigenetic events leads to human colorectal cancer (CRC) and metastasis. Furthermore, data from resequencing of the coding regions of human CRC suggests that a relatively large number of mutations occur in individual human CRC, most at low frequency. The functional role of these low-frequency mutations in CRC, and specifically how they may cooperate with high-frequency mutations, is not well understood. One of the most common rate-limiting mutations in human CRC occurs in the adenomatous polyposis coli (APC) gene. To identify mutations that cooperate with mutant APC, we performed a forward genetic screen in mice carrying a mutant allele of Apc (ApcMin) using Sleeping Beauty (SB) transposon-mediated mutagenesis. ApcMin SB-mutagenized mice developed three times as many polyps as mice with the ApcMin allele alone. Analysis of transposon common insertion sites (CIS) identified the Apc locus as a major target of SB-induced mutagenesis, suggesting that SB insertions provide an efficient route to biallelic Apc inactivation. We also identified an additional 32 CIS genes/loci that may represent modifiers of the ApcMin phenotype. Five CIS genes tested for their role in proliferation caused a significant change in cell viability when message levels were reduced in human CRC cells. These findings demonstrate the utility of using transposon mutagenesis to identify low-frequency and cooperating cancer genes; this approach will aid in the development of combinatorial therapies targeting this deadly disease.
Keywords: cancer gene discovery, transgenic mice
Human colorectal cancers (CRC) generally can be divided into two classes based on whether they display chromosomal instability (CIN) or microsatellite instability (MSI). The majority of CRC (∼80–90%) have a CIN phenotype; the remaining cases are characterized by MSI (1). CRC displaying CIN frequently harbor allelic losses or mutations in adenomatous polyposis coli (APC), v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), SMAD family member 4 (SMAD4), and tumor protein p53 (TP53), whereas MSI-type CRC usually have a mutation in one of six DNA mismatch repair genes (2). In both CIN and MSI CRC complete functional loss of a gatekeeper tumor suppressor gene typically is the rate-limiting event in intestinal cell transformation. For CIN CRC, APC plays the key gate-keeping role, and its loss underlies the great majority of CIN CRC and >80% of all CRC. Although both classes of CRC are characterized by high-frequency mutations, such as those in APC, it is evident that many more low-frequency mutations are required for CRC development, and the majority of these low-frequency mutations are unknown (3).
To identify these low-frequency mutations, we performed a forward genetic screen in mice using the Sleeping Beauty (SB) DNA transposon as a mutagen in intestinal epithelial cells. To focus on mutations that contribute to the CIN phenotype, we conducted the screen in mice carrying the ApcMin allele. ApcMin mice harbor a T→A nonsense mutation in the Apc gene (4, 5) that results in a truncated protein product that is unable to bind β-catenin and promote its degradation, thus leading to abnormal levels of β-catenin protein and up-regulation of β-catenin target genes such as cyclin D1 (Ccnd1) and myelocytomatosis oncogene (C-Myc). The Min mutation corresponds to a mutational hotspot in the human APC ortholog, and these mutations similarly result in dysregulation of the Wnt/β-catenin signaling pathway. There is strong evidence that β-catenin dysregulation is a common transformative event in tumorigenesis in the ApcMin mouse and in both the inherited form of APC-deficient CRC (familial adenomatous polyposis, FAP) and in sporadic CRC (6). Thus, the ApcMin mouse is an informative genetic model for APC-deficient intestinal cancer. ApcMin mice on the C57BL/6J background strain rarely survive beyond 120 d and can develop >100 tumors throughout the small and large intestine, with the phenotype dependent on diet, mouse strain, and other environmental factors (7, 8).
As in human CRC patients, loss of heterozygosity (LOH) leading to inactivation of both alleles of Apc is necessary for tumorigenesis to commence in ApcMin mice (9, 10). However, in contrast to LOH events in many human CRC, LOH in ApcMin tumors occurs predominantly by homologous somatic recombination (11). In this study we screened for mutations that cooperate with the ApcMin mutation by randomly mutating genes through selective activation of SB transposition in intestinal cells of ApcMin mice. The results of our screen support the importance of the loss of the second allele of Apc, because the great majority of tumors analyzed contained a transposon insertion in Apc, in particular in tumors in which there was maintenance of heterozygosity (MOH) for the Min allele. In addition to Apc, we identified 32 other genes and loci that probably facilitate the development of intestinal cancer in an ApcMin model. The function of these additional mutations could be to remove the requirement for Apc LOH, or they may function in some other manner. The majority of these genes have not been associated with CRC previously. To confirm that these genes play a causal role in tumor development, we used siRNA to knock down message levels of nine of the candidate genes in human colon cancer cell lines and demonstrated that five of these genes affected the growth rate of these cells.
Results
Design of a Forward Genetic Screen for CRC Genes.
In a previous study we demonstrated that SB transposon-mediated mutagenesis in the intestinal tract of C57BL/6J Apc+/+ mice resulted in polyp formation (12). By mapping transposon insertions in DNA extracted from these tumors, we were able to identify 77 genetic loci which probably harbored genes that, when mutated, contributed to tumor development. Because APC loss is rate limiting in the development of most human CRC (13), we reasoned that SB mutagenesis in a mouse already harboring a mutation in Apc might generate more tumors with a shorter latency and reveal mutations that cooperate with Apc during tumor development. To identify these genes, we performed a forward genetic screen using SB transposon-mediated mutagenesis in ApcMin mice. The screen consisted of a cohort of ApcMin SB transgenic test mice along with three groups of ApcMin control mice. The ApcMin SB test mice harbored three transgenes required for targeting SB mutagenesis to the gastrointestinal tract (Fig. S1). The first transgene was a concatamer of oncogenic transposons (T2/Onc) that were resident on chromosome 1 (14). To enhance the mutagenic potential of the transposon, T2/Onc contains a strong viral promoter, splice acceptors in both orientations, and a bidirectional polyA signal. The second transgene was a conditionally expressed knockin SB11 transposase allele downstream of the Rosa26 promoter (Rosa26-LsL-SB11) (15, 16). Because of the presence of a floxed stop cassette, the transposase allele is not expressed unless Cre recombinase protein is present. The third transgene was Cre recombinase driven by the gastrointestinal tract-specific Villin promoter (Vil-Cre) (17). We have shown previously that these three transgenes effectively limit SB mutagenesis to the intestinal tract (12). All mice were heterozygous for the ApcMin allele, and all the transgenes were fully congenic on the C57BL/6J genetic background. The first control group contained Rosa26-LsL-SB11 and either Vil-Cre or T2/Onc; the second control group contained T2/Onc and/or Vil-Cre but not Rosa26-LsL-SB11; and the third control group harbored only the ApcMin allele. Mice were killed when moribund or at 120 d.
Intestinal Tumorigenesis Is Enhanced Significantly in ApcMin SB Test Mice.
ApcMin mice that harbored all three transgenes (Rosa26-LsL-SB11, T2/Onc, and Vil-Cre) developed an average of 360 polyps (test mice, Table 1). In contrast, mice carrying the ApcMin allele alone developed an average of 112 polyps (control group 3, Table 1), a result that is consistent with the phenotype of ApcMin mice in our colony (18). Surprisingly, we also observed an enhanced rate of polyp development in control group 1 that carried the Rosa-26-LsL-SB11 allele but not the complete combination of alleles required for transposition. This control group developed an average of 182 polyps (control group 1, Table 1), a result that was unexpected based on previous screens. It is possible that the increased polyp number in these animals is caused by one or more modifiers linked to the Rosa26-LsL-SB11 transgene, because strain-specific modifiers are known to exist (19). Control animals carrying the ApcMin allele, T2/Onc, and/or Vil-Cre, but not Rosa26-LsL-SB11 (control group 2, Table 1) developed the same number of polyps as the control mice carrying the ApcMin allele alone. Although the Rosa26-LsL-SB11 allele alone contributes to polyp formation, the effect of active SB transposition was much greater, resulting in twice as many polyps in the test mice. In addition, the tumor burden was so extensive that ApcMin SB test mice became moribund earlier than any of the three control groups (Table 1). Indeed, in a subset of ApcMin SB test mice the tumor load was very severe, with some animals developing as many as 700 tumors.
Table 1.
Polyp number and age of death for transgenic mice
| Average no. polyps per mouse† |
|||||
| Group* | Number per group | Large intestine | Small intestine | Total | Date of death‡ |
| Test | 38 | 14.7 | 345 | 360 | 85 |
| Control 1 | 57 | 4.2 | 178 | 182 | 110 |
| Control 2 | 9 | 2.4 | 113 | 115 | 120+ |
| Control 3 | 100 | 2 | 110 | 112 | 120+ |
*Groups: Test = ApcMin × Rosa26-LsL-SB11 × T2/Onc × Vil-Cre; Control 1 = littermates harboring either ApcMin × Rosa26-LsL-SB11 or ApcMin × Rosa26-LsL-SB11 × T2/Onc or ApcMin × Rosa26-LsL-SB11 × Vil-Cre; Control 2 = littermates harboring either ApcMin × T2/Onc or ApcMin × Vil-Cre or ApcMin × T2/Onc × Vil-Cre; Control 3 = contemporaneous mice harboring ApcMin only.
†Average number of polyps per mouse by large intestine, small intestine, and total.
‡Control groups 2 and 3 were killed at 120 d whether they were moribund or not.
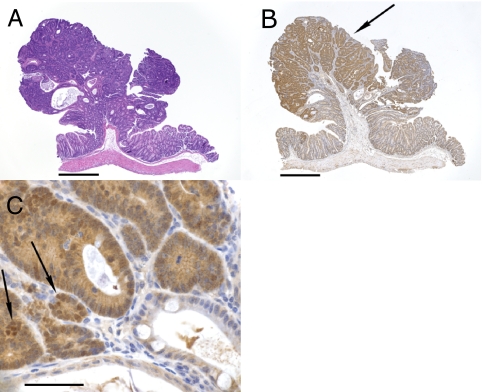
Although polyp number was greatly increased by SB mutagenesis, there was no evidence of local or systemic metastasis in experimental or control mice. We performed histopathologic analysis of tumors collected from 10 animals. These analyses identified numerous microadenomas and adenomas in the small intestine and a much smaller number of these lesions in the large intestines. No adenocarcinomas were identified, perhaps because of the short lifespan of ApcMin SB test mice. Immunohistochemistry for β-catenin was performed on 24 adenomas from seven animals. There was increased expression of β-catenin in all tumors compared with the adjacent normal mucosa epithelium (Fig. 1).
Fig. 1.
A pedunculated adenoma stained with H&E (A) or immunostained for β-catenin (B and C). (B) There is increased staining for β-catenin (arrow) in the adenoma. (C) Higher-power magnification of a different section showing increased cytoplasmic and nuclear (arrows) staining for β-catenin in tumor cells compared with adjacent normal tissue seen in lower right and bottom of picture. (Scale bars: A and B, 500 μm; C, 50 μm.)
Analysis of Common Insertion Sites Identifies 30 Candidate Cancer Genes.
To identify genes that contribute to tumor initiation and development in ApcMin mice, we analyzed transposon insertions in 96 polyps, representing all regions of the intestines, from 12 mice to find common insertion sites (CIS). A CIS is defined by analyzing transposon insertions in many tumors and identifying genomic loci that contain transposon insertions at a higher rate than would be expected by chance (SI Materials and Methods). The presence of a CIS indicates that a transposon-mediated mutation in that locus probably has contributed to tumor development. By analyzing the genes within the CIS, one can identify candidate cancer genes.
To map transposon insertions, we isolated DNA from the 96 tumors, digested the DNA with restriction enzymes, and performed ligation-mediated PCR (LM-PCR) to amplify transposon-genomic fragments specifically (20). Barcodes and fusion sequences were attached to the LM-PCR primers to enable pooling of the amplicons, which then were sequenced using the Roche GS FLX pyrosequencing machine. Six separate sequencing runs produced 347,993 sequence reads, 93% of which (324,898) contained a barcode, the transposon sequence, and sufficient genomic sequence (>16 bp) for BLAST analysis. We were able to map more than half of these sequences (53%) unambiguously to the mouse genome. Of the 173,101 mapped sequences, 100,171 (67%) were redundant, leaving 72,930 nonredundant mapped insertions. Roughly half of the nonredundant insertions mapped to the same chromosome as the donor transposon concatamer (Chr 1), as expected because of the phenomenon of local hopping seen in other SB screens (12, 14, 21). To eliminate statistical bias in the dataset, these sequences were eliminated along with a smaller number of insertions that probably represent PCR artifacts (SI Materials and Methods). The remaining 30,088 insertions (Dataset S1) were analyzed to determine CIS. We used Monte Carlo simulations to find insertion rates in a given genomic window size that would not be expected to occur by chance (12). For example, based on a random assignment of 30,088 insertions to the mouse genome, one would not expect to find five or more insertions within a 12-kb window. Using these Monte Carlo-defined parameters, we identified 37 CIS. Two of these CIS were removed from further analysis because all the tumors contributing to these two CIS originated from a single mouse, indicating the tumors may be clonally related. Two more CIS were removed because they also were identified in a control dataset of tail-snip DNA from mice harboring unselected SB insertions and may represent hotspots for SB insertions (12) Because this control dataset was generated from tail snips, it is possible that other hotspots exist in other types of cells. After removal of these possible artifacts, 33 CIS remained (Table 2).
Table 2.
List of 33 CIS
| Candidate gene | Chromosome | Start address* | End address* | Number of insertions† | Tumors with insertions‡ | Predicted effect on gene§ |
| 4930422G04Rik | 3 | 127241424 | 127431287 | 23 | 12 | NP |
| AC115907.7 | 3 | 127697338 | 127730035 | 8 | 8 | NP |
| AC131780.5 | 9 | 3001410 | 3030207 | 7 | 7 | NP |
| Adamts6 | 13 | 105093822 | 105104050 | 5 | 3 | NP |
| Ap1ar | 3 | 127454370 | 127633377 | 17 | 10 | NP |
| Apc | 18 | 34324389 | 34514767 | 185 | 72 | Loss |
| Atf2 | 2 | 73708689 | 73773260 | 9 | 9 | NP |
| Atl2 | 17 | 80239637 | 80306988 | 10 | 7 | Loss |
| Cnot1 | 8 | 98254541 | 98300340 | 8 | 8 | Loss |
| Csnk1a1 | 18 | 61726208 | 61750047 | 7 | 6 | Loss |
| Elac1 | 18 | 73903365 | 73913215 | 5 | 3 | Gain |
| Emcn | 3 | 136918543 | 137092521 | 17 | 9 | NP |
| Esco1 | 18 | 10578932 | 10773953 | 15 | 11 | Loss |
| Fnbp1l | 3 | 122217801 | 122319064 | 11 | 11 | Loss |
| Itgam | 7 | 135180978 | 135240752 | 11 | 10 | NP |
| Myo5b | 18 | 74613029 | 74796289 | 18 | 17 | Gain |
| No Gene 16 | 16 | 29019897 | 29031672 | 5 | 3 | NP |
| No Gene 18 | 18 | 26169184 | 26282443 | 12 | 7 | NP |
| No Gene 4 | 4 | 131170124 | 131238909 | 12 | 8 | NP |
| No Gene Y | Y | 2781406 | 2897989 | 16 | 15 | NP |
| Nsd1 | 13 | 55352993 | 55372133 | 6 | 6 | Gain |
| Pdcd6ip | 9 | 113560446 | 113723693 | 14 | 14 | Loss |
| Pde4dip | 3 | 97593369 | 97718572 | 14 | 11 | NP |
| Pigl | 11 | 62203495 | 62369763 | 16 | 16 | NP |
| Setd5 or Lhfpl4 | 6 | 113039133 | 113100176 | 9 | 9 | NP |
| Sfi1 | 11 | 3004743 | 3179859 | 21 | 19 | Loss |
| Snx24 | 18 | 53440600 | 53638364 | 19 | 12 | Loss |
| Srfbp1 | 18 | 52654288 | 52675270 | 6 | 5 | NP |
| Stag1 | 9 | 100583003 | 100698850 | 12 | 12 | Loss |
| Tmem132b | 5 | 126077980 | 126153976 | 11 | 4 | Loss |
| Wac | 18 | 7855248 | 8046126 | 17 | 15 | Loss |
| Zfp397 | 18 | 24110107 | 24157877 | 8 | 7 | NP |
| Zfp609 | 9 | 65561432 | 65607064 | 8 | 8 | Loss |
*Genomic address based on National Center for Biotechnology Information Mouse genome Build 37.
†Number of nonredundant SB transposon insertions within the locus.
‡Number of independent tumors with an insertion within the locus.
§Predicted effect is based on an analysis of the location and orientation of SB transposon insertions in all tumors in a single CIS (see text for discussion). Gain, gain of function; Loss, loss of function; NP, no prediction.
We assigned a candidate gene to each CIS if the majority of the insertions were in or near a single gene (Table 2). Four of the 33 CIS did not have an annotated gene within 40 kb and were not assigned a candidate gene. Another CIS contained two overlapping genes, SET domain-containing 5 (Setd5) and lipoma HMGIC fusion partner-like 4 (Lhfpl4), and all insertions in this CIS were in both genes. Notably, this CIS is located adjacent to the Rosa26 locus where the conditional SB11 knockin is located, and eight of nine insertions in this CIS are oriented with the internal promoter in the direction that would cause overexpression of the transgene. Rather than tagging an endogenous cancer gene, this CIS could represent selective pressure for increased mutagenesis via overexpression of SB11 transposase. Whether the transposon insertion caused a gain- or loss-of-function mutation sometimes can be predicted by analyzing the location and orientation of the insertions in all the tumors that comprise a single CIS. If all the tumors in a single CIS have transposon insertions in the same intron, and all the transposons are oriented in the direction of transcription, we predict the insertion causes a gain-of-function mutation. If the distribution of transposon insertions in all the tumors of a CIS is apparently random, and there is no bias in orientation, we predict a loss-of-function effect. Table 2 lists the predictions for the CIS. In total we identified 30 genes and four genomic loci with no annotated genes that probably contribute to intestinal tract cancer when mutated.
Transposon Insertions Implicated in LOH of the Wild-Type Allele of Apc.
The most commonly mutated gene in this study was Apc (in 72 of 96 tumors), reflecting the strong selective pressure for loss of the wild-type allele in ApcMin mice. Previous studies have demonstrated that loss of the Apc+ allele is an early event that occurs in almost every adenoma in ApcMin mice (9, 10). In addition, inactivation of the wild-type Apc allele is caused predominantly by homologous somatic recombination events, leading to the replacement of the Apc wild-type allele with a second ApcMin allele (22). We reasoned that in our transgenic model LOH could be accomplished by an inactivating transposon insertion, as opposed to duplication of the Min allele. To test this hypothesis, we performed PCR on DNA from tumors to amplify the region surrounding the Min mutation (T2860A). By sequencing the PCR amplicon, LOH can be ascertained in ApcMin mice by measuring the ratio of the T:A trace peak heights at the location of the Min mutation. In heterozygous tissue the T:A ratio is between 0.8 and 1.2, which is considered MOH, but in tissue that has lost the wild-type allele the ratio drops below 0.5 (Fig. S2). Ratios between 0.5–0.8 and >1.2 are considered uninformative, most likely caused by contamination from nontumor tissue. Of the 96 tumors tested, 47 gave informative results (Table S1). Of these 47 tumors, 32 had an identified transposon insertion in the Apc locus, and 15 did not. The majority (73%) of tumors lacking a transposon insertion in Apc had T:A ratios <0.5, indicating LOH probably caused by loss of the entire allele. In support of our hypothesis, 53% of the tumors that had a transposon insertion in the Apc locus had T:A ratios between 0.8 and 1.2, indicating maintenance of the wild-type Apc locus at the site of the Min mutation. This result suggests that in these tumors the wild-type Apc allele is inactivated by the transposon more frequently than by duplication of the Min allele.
Set of CIS Identified in ApcMin Mice Differs Significantly from Those Found in Apc Wild-Type Mice.
We compared the list of genes identified in this study with the 77 genes identified in the screen we performed on an Apc wild-type background (12). Surprisingly, only four genes were identified in both studies: Apc, nuclear receptor binding SET domain protein 1 (Nsd1), Sfi1 homolog, spindle assembly associated (Sfi1), and WW domain containing adaptor with coiled-coil (Wac). There are several reasons that could explain why the overlap between the two studies was low. First, the total number of genes that could contribute to tumor formation may be large enough that the size of these two studies is not sufficient to saturate the candidate genes. Second, because we use a statistical method to identify cancer genes, it is likely that transposon insertions contributed to carcinogenesis in some of the tumors, but the insertions did not occur at a rate high enough to qualify as a CIS. In support of this hypothesis, 70% of the loci identified as CIS in this study (23 of 33) also had one or more insertions in the same locus in the previous study (12). Third, the overlap may be small because selection pressure for specific genetic mutations in cells that already have an Apc mutation is biased toward a different set of cancer genes than in cells with a different initial mutation. Fourth, because of technical limitations, our method of amplifying and sequencing transposon insertions does not identify all transposon insertions, so a portion of driver mutations will not be identified. For example, analysis of replicate sequencing runs indicates that 20–40% of the PCR amplicons in a given library are not sequenced in a given GS FLX sequencing run (SI Materials and Methods and Table S2).
Relevance to Human Disease.
To determine the relevance of these findings to human cancers, we analyzed the regions of human orthology to the CIS loci and the orthologous human genes. Of the 30 candidate mouse genes associated with a CIS, 28 had human orthologs. We queried the literature for mutations and recurrent copy number changes in these genes in human CRC. Three of the genes, APC, NSD1, and phosphodiesterase 4D-interacting protein (PDE4DIP), are considered bona fide cancer genes based on the cancer gene census maintained by the Wellcome Trust Sanger Institute (23). Eight genes, APC, activating transcription factor-2 (ATF2), atlastin GTPase 2 (ATL2), casein kinase 1, alpha 1 (CSNK1A1), integrin, alpha M (ITGAM), programmed cell death 6-interacting protein (PDCD6IP), and WAC, have documented mutations in human cancers cataloged in the COSMIC database (24).
We found strong concordance between the CIS mouse loci and orthologous regions in the human genome showing recurrent chromosomal losses and gains in human CRC (25–32). Of the 33 identified CIS, 31 can be mapped to an orthologous human locus. Of these 31 candidate cancer loci, 24 are found in regions that commonly are lost or gained in human CRC (Table S3), including several of the CIS that are orthologous to human chromosomal arms 18q, 17p, 5q, and 4q. Interestingly, one CIS that has no annotated genes nearby (CIS No gene 16) is in an orthologous region (3q21–24) that is associated with CRC based on genome-wide linkage analyses (33, 34). To determine the significance of this overlap, we performed the analysis using randomly generated CIS lists and a single dataset of regions that are lost recurrently in human CRC (27). Roughly 250 genomic regions in this dataset were lost in >5% of the human samples tested, and 22 of the 31 CIS were located within these regions. In 10,000 simulations using equivalent-sized randomly generated CIS lists, we find an overlap of this magnitude <0.3% of the time. These results suggest that our SB screen may be capable of pinpointing the affected genes in these regions.
Candidate Genes Regulate Proliferation of Human CRC Cell Lines.
We tested nine genes [CCR4-NOT transcription complex, subunit 1 (CNOT1), PDE4DIP, PDCD6IP, ATF2, SFI1, formin-binding protein 1-like (FNBP1L), myosin VB (MYO5B), sorting nexin 24 (SNX24), and stromal antigen 1 (STAG1)] for their effect on proliferation of the human CRC cell line SW480 by knocking down message levels using siRNA. We used the SW480 line because it has an APC gene-truncation mutation similar to the ApcMin mutation (35). Cells were transfected two times at 48-h intervals with siRNA targeting the human genes. Knockdown efficiency was at least 50% for all nine genes as measured by quantitative real-time PCR. Cell proliferation was measured using a tetrazolium-based colorimetric assay on days two and six after the second transfection. Depletion of five (CNOT1, PDE4DIP, PDCD6IP, ATF2, and SFI1) of the nine genes tested resulted in a significant decrease in cell viability compared with a control siRNA of at least 33% at day six after transfection (Table S4).
Discussion
Using a transposon-based forward genetic screen in mice, we identified 33 genomic loci that probably cooperate with a germline mutation in the Apc gene to cause intestinal tumorigenesis. The most frequently mutated locus was the Apc locus, a result that supports the hypothesis that there is strong selective pressure to lose the wild-type copy during tumor formation. SB insertions in Apc were found in 72 of 96 tumors (75%), suggesting that 75% of the tumors in ApcMin SB test mice undergo LOH at the Apc locus via SB insertional mutagenesis. This hypothesis is supported by sequencing of the region spanning the 1-bp Min mutation, which indicated that the majority of tumors containing SB insertions at the Apc locus maintained heterozygosity at the location of the T:A Min mutation. In contrast, the majority of tumors lacking a transposon insertion in Apc showed loss of the wild-type sequence at the Min mutation site. These results suggest that the majority of tumors underwent biallelic loss of Apc activity through transposon insertion or somatic recombination. However, it also is possible that in some cases activity was lost through other mechanisms of Apc inactivation or through transposon insertion substituting for loss of the wild-type allele.
Although LOH at the Apc locus is the rate-limiting event in tumor initiation in the ApcMin mouse and in familial and sporadic APC-deficient CRC, loss of APC probably is insufficient for the survival and growth of transformed cells into adenomas and, eventually, adenocarcinomas. SB-mutagenized animals showed increased polyp number but no evidence of adenocarcinoma or metastasis. Thus, it is likely that the CIS candidate genes identified in this SB screen contribute in a diverse fashion to initiation, establishment, and survival of adenomas. Moreover, depending on the complexity of the mechanism or pathway, the CIS candidates discovered in our screen, like the relatively large number of genes reported to be mutant in individual human CRC (3), might be expected to occur at a low frequency if mutations at any one of multiple genes in a complex pathway can contribute equally to tumorigenesis.
Aside from Apc, only a few of the remaining 28 known genes that we identified as CIS in our screen have been implicated directly in CRC development, although >90% are located in genomic regions that are lost or gained in human CRC. To eliminate false positives, we removed CIS that also were identified in a control dataset of unselected transposon insertions mapped in tail snips. However, it is possible that other tissue-specific hotspots could result in false positives. Nevertheless, the known functions of several of the CIS candidate genes make them plausible candidates for drivers of human CRC. For example, CNOT1 is a member of the Ccr4-Not complex, which is implicated in mRNA decay and transcriptional repression. In human cells, CNOT1 has been reported to be a repressor of nuclear receptor-mediated transcription (36). One target of CNOT1 repression appears to be estrogen receptor alpha (ERα), via interactions between CNOT1 and the ligand-binding domain of ERα. Inhibition of CNOT1 caused an increase in the expression of ERα target genes in breast cancer cells, and ERα has been shown to be a tumor suppressor gene in the intestinal tract (37); in particular, knockout of ERα in ApcMin mice caused a significant increase in intestinal tumorigenesis (38).
Another gene identified in this study, Pdcd6ip (also known as ALG-2 interacting protein X, Alix) is involved in membrane trafficking and apoptosis (39). Pdcd6ip produces a protein that binds to the protein product of Pdcd6, a proapoptotic gene involved in T-cell receptor–, Fas-, and glucocorticoid-induced cell death (40). Pdcd6ip also can block down-regulation of the EGF receptor (EGFR), thereby having a positive effect on growth factor signaling (41). These contradictory roles could explain why loss of Pdcd6ip in the mouse tumors promoted growth (via the loss of the proapoptotic function) but the loss of Pdcd6ip in SW480 cells caused decreased proliferation (via increased down-regulation of EGFR). Further functional studies are required to elucidate the role of this adaptor protein.
In summary, our approach identified 30 genes that probably modify tumorigenesis in the ApcMin model of human CRC. Further functional analysis of these CIS candidate genes may provide insights into the etiology and treatment of human CRC, especially those cancers arising downstream of APC deficiency.
Materials and Methods
Detailed protocols are given in SI Materials and Methods.
Mice.
Mice containing ApcMin, Rosa26-LsL-SB11, Villin-Cre, and T2/Onc were reared using Institutional Animal Care and Use Committee-approved protocols. All mice were on an isogenic C57BL/6J background. Mice were monitored daily and killed and necropsied when moribund or after 120 d.
Histopathology and Immunohistochemistry.
Formalin-fixed tissues were embedded in paraffin, and standard techniques were used to stain tissue sections with H&E. Standard immunohistochemistry techniques were used to detect β-catenin.
Linker-Mediated PCR.
Linkers [described previously (42)] were ligated to NlaIII- (right-side) or BfaI- (left side) digested genomic DNA using T4 DNA ligase. A secondary digest (XhoI, right side; BamHI, left side) was performed to destroy concatamer-generated products. Primary and secondary PCR was performed using primers specific for linker and SB transposon sequences along with Fusion and barcode sequences. PCR amplicons were sequenced using the GS FLX (Roche).
Sequence Analysis.
Sequences were analyzed for the presence of the barcode, inverted repeat/direct repeat (IR/DR) sequences required for transposition, and linker sequences. Genomic sequence was blasted against the mouse genome using BLASTN at 95% stringency and requiring a single match. Of the 324,898 sequences analyzed, 53% could be uniquely mapped to the mouse genome. Sequences were removed if they were redundant, on the donor concatamer resident chromosome (Chr 1), in the En2 gene (because the En2 sequence is present in the transposon), and when a single TA dinucleotide contained multiple insertions from several tumors from multiple mice (because of the possibility of a PCR artifact). The remaining 30,088 nonredundant sequences were used to identify CIS. A CIS was defined by Monte Carlo simulations using a random dataset of 30,088 insertions.
Apc LOH Analysis.
To measure LOH for the ApcMin mutation, DNA was isolated from individual polyps, and PCR was performed using primers that flank the mutation (sense primer: CGGAGTAAGCAGAGACACAA; antisense primer: GGGAGGTATGAATGGCTGAT). The PCR product was purified using Qiagen 96 MinElute vacuum purification plates per the manufacturer's protocol and was sequenced using the sense primer as the sequencing primer. Trace peak heights at the location of the mutation were measured for each tumor, and the ratio of the T peak to the A peak was calculated.
Comparisons with Human Data.
Eight publicly available studies measuring DNA copy number in CRC compared with normal tissue were analyzed. Mutations in human tumors were examined using the Catalog of Somatic Mutations in Cancer database (24), and cancer gene status was based on the Census of Human Cancer Genes maintained by the Wellcome Trust Sanger Institute (23).
Knockdown of CIS Candidate Genes using siRNA in SW480 Cells.
SW480 cells were obtained from ATCC (catalog no. CCL-228) and were cultured under recommended conditions. Transient siRNA transfection was used to deplete expression of Min CIS genes.
Cell Viability Assay.
Viability of siRNA-treated SW480 cells was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Cell Viability Kit 1; Roche Applied Sciences).
Supplementary Material
Acknowledgments
Pauline Jackson, Jerica Burchard, and Annette Rod provided technical assistance for the gene expression analyses of CIS genes. We thank the following University of Minnesota Masonic Cancer Center Cores: Biostatistics and Informatics Shared Resource, Comparative Pathology, and Mouse Genetics Laboratory. We also thank the Minnesota Supercomputing Institute and the BioMedical Genomics Center. Research was funded by American Cancer Society postdoctoral fellowship PF-06-282-01-MGO (to T.K.S.), a National Cancer Institute Pathway to Independence Award 1K99CA151672-01 (to T.K.S.), and by National Institutes of Health Grants R01CA113636-01A1 (to D.A.L.) and R01 CA134759-01A1 (to D.A.L. and R.T.C.). A University of Minnesota Academic Health Center Faculty Development Grant provided additional funding (to D.A.L. and R.T.C.). B.L.T.’s research is supported in part by a fellowship from the Annette Boman Women's Fellowship program. B.L.T. and L.Z. were recipients of a research support award from the University of Minnesota Duluth chapter of Sigma Xi.
Footnotes
Conflict of interest statement: D.A.L. is a cofounder of, and has an equity interest in, Discovery Genomics Inc. (DGI), a biotechnology company that is pursuing SB technology for human gene therapy. No resources or personnel from DGI were involved in this work. The University of Minnesota has filed a patent related to the work described in this paper. All other authors state no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018012108/-/DCSupplemental.
References
- 1.Grady WM, Markowitz SD. Genetic and epigenetic alterations in colon cancer. Annu Rev Genomics Hum Genet. 2002;3:101–128. doi: 10.1146/annurev.genom.3.022502.103043. [DOI] [PubMed] [Google Scholar]
- 2.Takayama T, Miyanishi K, Hayashi T, Sato Y, Niitsu Y. Colorectal cancer: Genetics of development and metastasis. J Gastroenterol. 2006;41:185–192. doi: 10.1007/s00535-006-1801-6. [DOI] [PubMed] [Google Scholar]
- 3.Wood LD, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 4.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 5.Su LK, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 6.Fodde R. The APC gene in colorectal cancer. Eur J Cancer. 2002;38:867–871. doi: 10.1016/s0959-8049(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich WF, et al. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 8.Cormier RT, et al. Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nat Genet. 1997;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- 9.Levy DB, et al. Inactivation of both APC alleles in human and mouse tumors. Cancer Res. 1994;54:5953–5958. [PubMed] [Google Scholar]
- 10.Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994;54:5947–5952. [PubMed] [Google Scholar]
- 11.Haigis KM, Dove WF. A Robertsonian translocation suppresses a somatic recombination pathway to loss of heterozygosity. Nat Genet. 2003;33:33–39. doi: 10.1038/ng1055. [DOI] [PubMed] [Google Scholar]
- 12.Starr TK, et al. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science. 2009;323:1747–1750. doi: 10.1126/science.1163040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell SM, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 14.Collier LS, Carlson CM, Ravimohan S, Dupuy AJ, Largaespada DA. Cancer gene discovery using Sleeping Beauty transposon-based somatic mutagenesis in the mouse. Nature. 2005;436:272–276. doi: 10.1038/nature03681. [DOI] [PubMed] [Google Scholar]
- 15.Zambrowicz BP, et al. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuy AJ, et al. A modified sleeping beauty transposon system that can be used to model a wide variety of human cancers in mice. Cancer Res. 2009;69:8150–8156. doi: 10.1158/0008-5472.CAN-09-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.el Marjou F, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 18.McAlpine CA, Barak Y, Matise I, Cormier RT. Intestinal-specific PPARgamma deficiency enhances tumorigenesis in ApcMin/+ mice. Int J Cancer. 2006;119:2339–2346. doi: 10.1002/ijc.22115. [DOI] [PubMed] [Google Scholar]
- 19.Fodde R, Smits R. Disease model: Familial adenomatous polyposis. Trends Mol Med. 2001;7:369–373. doi: 10.1016/s1471-4914(01)02050-0. [DOI] [PubMed] [Google Scholar]
- 20.Mueller PR, Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989;246:780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- 21.Keng VW, et al. A conditional transposon-based insertional mutagenesis screen for genes associated with mouse hepatocellular carcinoma. Nat Biotechnol. 2009;27:264–274. doi: 10.1038/nbt.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haigis KM, Caya JG, Reichelderfer M, Dove WF. Intestinal adenomas can develop with a stable karyotype and stable microsatellites. Proc Natl Acad Sci USA. 2002;99:8927–8931. doi: 10.1073/pnas.132275099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Futreal PA, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forbes S, et al. COSMIC 2005. Br J Cancer. 2006;94:318–322. doi: 10.1038/sj.bjc.6602928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelstein B, et al. Allelotype of colorectal carcinomas. Science. 1989;244:207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- 26.Ried T, et al. Comparative genomic hybridization reveals a specific pattern of chromosomal gains and losses during the genesis of colorectal tumors. Genes Chromosomes Cancer. 1996;15:234–245. doi: 10.1002/(SICI)1098-2264(199604)15:4<234::AID-GCC5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Nakao K, et al. High-resolution analysis of DNA copy number alterations in colorectal cancer by array-based comparative genomic hybridization. Carcinogenesis. 2004;25:1345–1357. doi: 10.1093/carcin/bgh134. [DOI] [PubMed] [Google Scholar]
- 28.Habermann JK, et al. Stage-specific alterations of the genome, transcriptome, and proteome during colorectal carcinogenesis. Genes Chromosomes Cancer. 2007;46:10–26. doi: 10.1002/gcc.20382. [DOI] [PubMed] [Google Scholar]
- 29.Lassmann S, et al. Array CGH identifies distinct DNA copy number profiles of oncogenes and tumor suppressor genes in chromosomal- and microsatellite-unstable sporadic colorectal carcinomas. J Mol Med. 2007;85:293–304. doi: 10.1007/s00109-006-0126-5. [DOI] [PubMed] [Google Scholar]
- 30.Shih IM, et al. Evidence that genetic instability occurs at an early stage of colorectal tumorigenesis. Cancer Res. 2001;61:818–822. [PubMed] [Google Scholar]
- 31.Derks S, et al. Integrated analysis of chromosomal, microsatellite and epigenetic instability in colorectal cancer identifies specific associations between promoter methylation of pivotal tumour suppressor and DNA repair genes and specific chromosomal alterations. Carcinogenesis. 2008;29:434–439. doi: 10.1093/carcin/bgm270. [DOI] [PubMed] [Google Scholar]
- 32.Kurashina K, et al. Chromosome copy number analysis in screening for prognosis-related genomic regions in colorectal carcinoma. Cancer Sci. 2008;99:1835–1840. doi: 10.1111/j.1349-7006.2008.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemp Z, et al. ColoRectal tumour Gene Identification (CoRGI) Study Consortium. Evidence for a colorectal cancer susceptibility locus on chromosome 3q21-q24 from a high-density SNP genome-wide linkage scan. Hum Mol Genet. 2006;15:2903–2910. doi: 10.1093/hmg/ddl231. [DOI] [PubMed] [Google Scholar]
- 34.Neklason DW, et al. Common familial colorectal cancer linked to chromosome 7q31: A genome-wide analysis. Cancer Res. 2008;68:8993–8997. doi: 10.1158/0008-5472.CAN-08-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishisho I, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 36.Winkler GS, Mulder KW, Bardwell VJ, Kalkhoven E, Timmers HT. Human Ccr4-Not complex is a ligand-dependent repressor of nuclear receptor-mediated transcription. EMBO J. 2006;25:3089–3099. doi: 10.1038/sj.emboj.7601194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho NL, Javid SH, Carothers AM, Redston M, Bertagnolli MM. Estrogen receptors alpha and beta are inhibitory modifiers of Apc-dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res. 2007;67:2366–2372. doi: 10.1158/0008-5472.CAN-06-3026. [DOI] [PubMed] [Google Scholar]
- 38.Cleveland AG, et al. Disruption of estrogen receptor signaling enhances intestinal neoplasia in Apc(Min/+) mice. Carcinogenesis. 2009;30:1581–1590. doi: 10.1093/carcin/bgp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odorizzi G. The multiple personalities of Alix. J Cell Sci. 2006;119:3025–3032. doi: 10.1242/jcs.03072. [DOI] [PubMed] [Google Scholar]
- 40.Vito P, Lacanà E, D'Adamio L. Interfering with apoptosis: Ca(2+)-binding protein ALG-2 and Alzheimer's disease gene ALG-3. Science. 1996;271:521–525. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt MH, et al. Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol Cell Biol. 2004;24:8981–8993. doi: 10.1128/MCB.24.20.8981-8993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.