Today science is in the age of biology and biology is in the age of genomics. Sequencing the entire genome of an organism, an enterprise that could not have been imagined barely 50 y ago, is being thought of as the first step toward a complete understanding of its biology. If I had been asked to recommend just two families of living organisms from which to pick the first two species for whole-genome sequencing, I would surely have suggested Hominidae (with ourselves) and Formicidae (with all ants). My choice of the ant family is easy to justify. The family Formicidae consists of approximately 14,000 species of ants, all of which exhibit advanced and sophisticated social life, not unlike our own in many respects and perhaps surpassing us in some ways. The ants live in colonies headed by one or a small number of fertile queens and large number (which can sometimes run into millions) of sterile workers, and display sophisticated division of labor and most impressive levels of communication and coordination among colony members (Fig 1). One of the many features of great interest is the vastly different phenotypes and lifespans of queens and workers, despite developing from the same genome. Ants have achieved spectacular ecological success and dominance, accounting for more than a third of all insect biomass and, along with termites, for more than 25% of all animal biomass in some tropical forests (1).
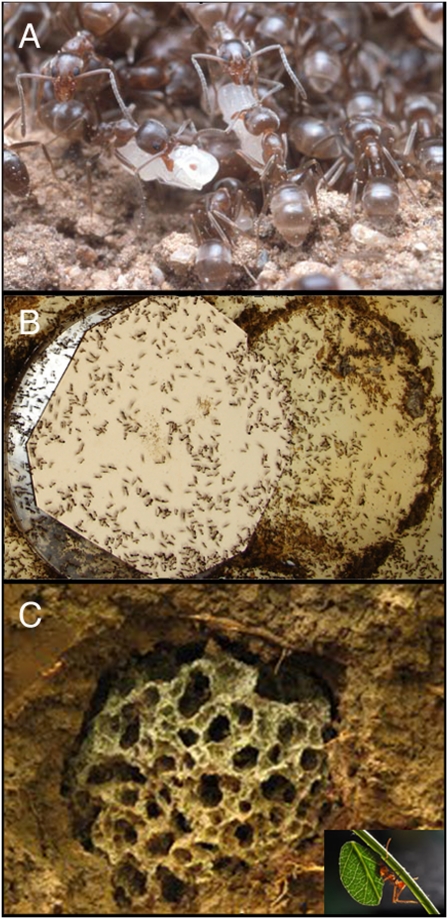
Fig. 1.
(A) A portion of the nest of the invasive Argentine ant L. humile showing workers tending brood (photo: Marc Dantzker). (B) A small portion of a laboratory colony of a red imported fire ant S. invicta (photo: Yannick Wurm). (C) An example of the fungus garden of the leaf-cutter ant A. cephalotes (photo: Jarrod Scott) with a single ant carrying a leaf also shown (Inset; photo: Alex Wild).
Whole-genome sequencing was, until recently, a relatively expensive and time-consuming affair, so many organisms had to wait in a queue for their turn. We humans had to wait until the year 2001 and the ants have had to wait until hundreds of other animals, plants, and microbes had been sequenced. However, fortunately, the wait is now over. The genomes of the invasive Argentine ant Linepithema humile (2), the red harvester ant Pogonomyrmex barbatus (3), and the fire ant Solenopsis invicta (4) are being simultaneously unveiled in PNAS. The genome sequences of two other ants, Camponotus floridanus and Harpegnathos saltator (5), were also published recently. In addition the genome sequence of the leaf-cutter ant Atta cephalotes will soon be published in another journal (6), taking the total to six. Thus, we are truly witnessing the birth of ant genomics, indeed, of comparative ant genomics. Actually, we now find ourselves in an even better situation because the honeybee genome has already been sequenced (7), taking the number of eusocial species to seven, and because three species of the parasitic wasp Nasonia have also been sequenced (8), we have 10 hymenopteran genome sequences for comparative study.
The Argentine ant L. humile is remarkable in many ways, especially because of its successful invasion from South America to every Mediterranean-type climate, including most of Europe and North America (9), and even more so because it appears to form mega-supercolonies ranging over hundreds of thousands of kilometers (10). The harvester ant P. barbatus is a rather famous granivore, being a favorite model to study variations in social organization (11), mechanisms of caste determination (12), and the organization of labor (13). The fire ant S. invicta, introduced from South America, has spread across the United States and has become one of the most serious pests threatening agriculture and human life and defying most extermination efforts (14). C. floridanus, found in the southeastern United States, is perhaps the most nondescript of the lot but it is good to have to compare with the others, especially because of its well organized, monogynous colonies with only two worker castes (15). H. saltator is rather special, a jumping ant from India whose workers can copulate with males from their own colonies and contribute to egg-laying, alongside the queens, as gamergates (i.e., married workers) (16). Finally, A. cephalotes is another “star” as ants go, being an extremely serious pest of agriculture in the Neotropics, a status achieved as a result of its habit of harvesting leaves and using them to cultivate fungal gardens—a 50-million-year-old form of ant agriculture (17).
With the publication of these six ant genomes, we have thus obtained a total of approximately 1.5 billion base pairs’ worth of new data. What can we do with this massive amount of data? Are the data worth the time, effort, and money that went into their collection? The answers to these questions should not be taken as obviously being in the affirmative, but should be examined very carefully. In addition to the actual genome sequences, most of these articles provide basic information such as genome size, expected number of genes, transcriptome sequences, and information about duplicated genes, missing genes, and transposable elements. Each article makes preliminary comparisons with some of the other related genomes to point out similarities and differences. Each article also lists several pleasing results. For example, the Argentine ant genome has expansions and/or abundance of gustatory, odorant receptor, cytochrome P450, royal jelly protein, and methylation-related genes and a paucity of immune genes. The harvester ant genome shows expansion of chemoreception and cytochrome P450 genes. The fire ant genome has multiple copies of vitellogenin, hundreds of olfactory receptors, and an expansion of lipid-processing genes. The Camponotus and Harpegnathos genomes reveal many ant-specific genes (as opposed to other hymenopteran genes) and have already revealed interesting caste-specific differences in gene expression. The Atta genome shows reduction in genes related to nutrition acquisition and digestion. The honeybee genome shows a paucity of genes related to immunity and detoxification, an excess of genes for chemoreception and pollen and nectar utilization, and an interesting diversification of genes coding for royal jelly proteins. Although sometimes claimed to be tests of a priori predictions, these pleasing features should only be considered as being “not inconsistent” with what one might expect on the basis of prior knowledge of the biology, behavior, and evolution of social insects, especially the high fecundity of their queens, great significance of chemical communication in their lives, and high levels of social hygiene. In short, there are no surprises, at least so far.
Is this all we learn? Certainly not. The facts gleaned and described in the first article reporting the sequence of an organism's genome should really be thought of as no more than a postcard sent home by a visitor giving first impressions of a city—say, New York—after spending just a day. The longer the visitor stays, the more he learns, and if he stays for years, he may write books about the architecture, the art and fashion scene, the crime scene, the ethnic composition of the city, and so on. We should expect something similar from those who will continue to study these genome sequences for many years to come. Even a more complete annotation should permit a more detailed comparison of the available sequences and provide greater insights into the biology of these fascinating organisms. The sequencing of more ant and social insect genomes, which is sure to follow, should add exponentially
With the publication of these six ant genomes, we have thus obtained a total of approximately 1.5 billion base pairs' worth of new data.
to the success of this enterprise. The addition of every new genome sequence to our toolkit should also add significantly to our ability to apply the tools of genomics to species whose genomes have not been sequenced. Gene hunting and gene expression studies, which can now be applied ever more widely to sequenced as well as unsequenced species, should help unravel the underlying genetic architecture of many ecologically and socially interesting traits (18). However, is that enough? A creative scholar studying the city of New York for many years should also attempt to explain why the city has come to be what it is, why it has this architecture and not that, why the art and fashion scene is different from that of Paris, why some areas are safe and some are not, and why the city has this ethnic composition and not another—à la Jared Diamond (19, 20).
Will something like this happen with the ant genomes? We should hope so because promises are being made. For example, the Camponotus–Harpegnathos article claims to help “provide experimental avenues to address long-standing hypotheses on the relationships among epigenetics, neurobiology, and behavior, as well as life-span regulation” (5); the Argentine ant article promises that “these tools will likely find widespread application and produce tangible benefits for agriculture, societies and ecosystems” and “will be productive avenues for future research that explores the basis of eusociality, and the cause and consequences of biological invasions” (2); the fire ant article claims to provide “the foundation for future evolutionary, biomedical, sociogenetic, and pest-management studies…” (4); and the Nasonia article claims that the genomic data “will ultimately provide tools and knowledge for further increasing the utility of parasitoids as pest insect-control agents” (8). Can these promises ever be met? I think the answer is yes. Will these promises be met in the foreseeable future? Probably, but not unless we consciously encourage diverse approaches in the study of social (and nonsocial) hymenopteran insects, ensure adequate opportunities for researchers continuing to use classical methods, and thus work hard to make the new and spectacular genomic resources of real utility available to those who are not investigating genomics themselves. In other words, we must take care not to make genomics so “fashionable” that there is no one left to apply genomics to the real problems that we sought to tackle in the first place. If we succeed in this, I believe genomics can indeed revolutionize the study of ant and social insect biology.
Footnotes
References
- 1.Wilson EO. Success and Dominance in Ecosystems: The Case of the Social Insects. Nordbünte, Germany: Ecology Institute; 1990. [Google Scholar]
- 2.Smith CD, et al. Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile) Proc Natl Acad Sci USA. 2011;108:5673–5678. doi: 10.1073/pnas.1008617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith CR, et al. Draft genome of the red harvester ant Pogonomyrmex barbatus. Proc Natl Acad Sci USA. 2011;108:5667–5672. doi: 10.1073/pnas.1007901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wurm Y, et al. The genome of the fire ant Solenopsis invicta. Proc Natl Acad Sci USA. 2011;108:5679–5684. doi: 10.1073/pnas.1009690108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonasio R, et al. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science. 2010;329:1068–1071. doi: 10.1126/science.1192428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suen G, et al. The genome sequence of the leaf-cutter ant Atta cephalotes reveals insights into its obligate symbiotic lifestyle. PLoS Genet. 2011;7:e1002007. doi: 10.1371/journal.pgen.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honeybee Genome Sequencing Consortium Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werren JH, et al. Nasonia Genome Working Group Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2010;327:343–348. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suarez AV, Holway DA, Case TJ. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc Natl Acad Sci USA. 2001;98:1095–1100. doi: 10.1073/pnas.98.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Wilgenburg E, Torres CW, Tsutsui ND. The global expansion of a single ant supercolony. Evolutionary Applications. 2010;3:136–143. doi: 10.1111/j.1752-4571.2009.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RA. Seed-harvester ants (Hymenoptera: Formicidae) of North America: An overview of ecology and biogeography. Sociobiology. 2000;36:83–122. [Google Scholar]
- 12.Helms Cahan S, et al. Extreme genetic differences between queens and workers in hybridizing Pogonomyrmex harvester ants. Proc Biol Sci. 2002;269:1871–1877. doi: 10.1098/rspb.2002.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon DM. Ants at Work: How an Insect Society Is Organized. New York: Free Press; 1999. [Google Scholar]
- 14.Tschinkel WR. The Fire Ants. Cambridge, MA: Harvard Univ Press; 2006. [Google Scholar]
- 15.Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990. [Google Scholar]
- 16.Peeters C, Liebig J, Hölldobler B. Sexual reproduction by both queens and workers in the ponerine ant Harpegnathos saltator. Insectes Soc. 2000;47:325–332. [Google Scholar]
- 17.Mueller UG, et al. The evolution of agriculture in insects. Annu Rev Ecol Evol Syst. 2005;36:563–595. [Google Scholar]
- 18.Thomas MA, Klaper R. Genomics for the ecological toolbox. Trends Ecol Evol. 2004;19:439–445. doi: 10.1016/j.tree.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Diamond J. Guns, Germs, and Steel—The Fates of Human Societies. New York: W.W. Norton; 1997. [Google Scholar]
- 20.Diamond J. Natural Experiments of History. Cambridge, MA: Harvard Univ Press; 2010. [Google Scholar]