Abstract
Defects in neuronal connectivity of the brain are well documented among schizophrenia patients. Although the schizophrenia susceptibility gene Disrupted-in-Schizophrenia 1 (DISC1) has been implicated in various neurodevelopmental processes, its role in regulating axonal connections remains elusive. Here, a heterologous DISC1 transgenic system in the relatively simple and well-characterized Caenorhabditis elegans motor neurons has been established to investigate whether DISC1 regulates axon guidance during development. Transgenic DISC1 in C. elegans motor neurons is enriched in the migrating growth cones and causes guidance defects of their growing axons. The abnormal axonal phenotypes induced by DISC1 are similar to those by gain-of-function rac genes. In vivo genetic interaction studies revealed that the UNC-73/TRIO-RAC-PAK signaling pathway is activated by ectopic DISC1 in C. elegans motor axons. Using in vitro GST pull-down and coimmunoprecipitation assays, we found that DISC1 binds specifically to the amino half of spectrin repeats of TRIO, thereby preventing TRIO's amino half of spectrin repeats from interacting with its first guanine nucleotide exchange factor (GEF) domain, GEF1, and facilitating the recruitment of RAC1 to TRIO. In cultured mammalian cells, RAC1 is activated by increased TRIO's GEF activity when DISC1 is present. These results together indicate that the TRIO-RAC-PAK signaling pathway can be exploited and modulated by DISC1 to regulate axonal connectivity in the developing brain.
Keywords: genetic model, rolipram
Schizophrenia is a neurodevelopmental disorder with genetic predispositions (1, 2). Although the etiology and neuropathology of schizophrenia are still elusive, functional and neuroanatomical studies from patients have documented various abnormalities in the diseased brain. In particular, defects in neuronal connectivity during development have been proposed as an important precipitating factor for schizophrenia, which is thought to be unmasked by other developmental events or environmental stressors later in life (3). It is therefore likely that some of the major schizophrenia susceptibility genes are important for regulating axonal connections during development.
In recent years, the effort to search for genes susceptible to schizophrenia has led to the identification of the Disrupted-in-Schizophrenia 1 (DISC1) gene, whose mutation is highly associated with schizophrenia and other major human mental diseases (4). DISC1 has roles in neuron proliferation, neuron migration, axon outgrowth, and synapse formation/maturation (5–8). In our previous studies, we identified an unexpected role of DISC1 in regulating the guidance of developing axons in the adult-born hippocampal dentate granule cells (5, 9). These effects expand the known functions of DISC1 but sheds little light on how DISC1 effects axon guidance.
To systemically study the role of DISC1 in axon guidance and to identify the signaling pathways that might be involved, we set out to establish a heterologous genetic system in the nematode Caenorhabditis elegans, in which no endogenous DISC1 homologous gene is identified (10). In this genetic model, DISC1 is accumulated in the growth cone, and transgenic animals exhibited axon guidance defects. Further genetic interaction studies showed that DISC1 regulates axon guidance through activation of RAC-PAK signaling pathways. Importantly, we confirmed this finding in mammalian cultured cells and demonstrated that DISC1 can interact with mammalian TRIO to activate the RAC-PAK signal pathway.
Results
Heterologous Mouse DISC1 (mDISC1) Induces Axon Guidance Defects in C. elegans.
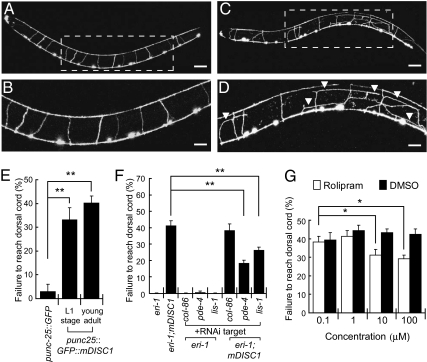
A GFP and mDISC1 fusion protein (GFP::mDISC1) was expressed in C. elegans D-type dorsal (DD) and ventral (VD) motor neurons under a GABAergic-specific unc-25 promoter. These neurons are located along the ventral midline of the worm body and project their commissural axons dorsally (Fig. 1 A and B). In these mDISC1 transgenic worms, some of the commissures failed to reach their dorsal destinations. We quantified the severity of axon guidance defect by calculating the percentage of commissures that failed to reach the dorsal cord at young adult stages. In mDISC1 transgenic animals, approximately 40% of commissures showed guidance defects, compared with less than 5% in control animals expressing GFP alone. (Fig. 1 C–E).
Fig. 1.
Expression of mDISC1 in C. elegans motor neurons causes axon guidance defects. (A–D) Confocal images of the commissural axons from DD and VD motor neurons at L4. In wild-type animals (A and B), all of the commissures reach the dorsal nerve cord. In mDISC1 transgenic animals (C and D), the commissures exhibit guidance defects (arrowheads). Boxed areas in A and C are magnified in B and D, respectively. (Scale bars: A and C, 20 μm; B and D, 10 μm.) (E) The adult mDISC1 animals have an average of 40% axon guidance defect. The L1 transgenic animals exhibit similar percentage of defects, indicating that the phenotype is caused by a defect in the guidance of developing axons rather than to abnormal regrowth from degenerated axons. (F) The axon guidance defects caused by expressing mDISC1 are suppressed by RNAi knockdown of C. elegans homologs of known mammalian DISC1-interacting molecules (n = 25–60). col-86, negative control gene. (G) Incubation of the mDISC1 animals with a PDE4-specific inhibitor, rolipram, suppresses the guidance defects (n = 60). Bars represent the SE. *P < 0.05, **P < 0.001 (Student's t test). In all pictures, anterior is to the left and dorsal is up.
Transgenic mDISC1 in C. elegans Motor Neurons Functions in a Similar Fashion as in Vertebrate Neurons.
To validate the use of heterologous mDISC1 in C. elegans, we first characterized its subcellular localization in motor neurons. In vertebrate neurons, DISC1 is known to be present in the growth cone (11). Similarly, in mDISC1 transgenic worms, GFP::mDISC1 was observed in migrating VD growth cones at larval stage 2 (L2) (75%, n = 20) (Fig. S1A) and in the tips of mature axons at L4 or young adult stage after the motor axons had already reached their final targets (Fig. S1B). The local accumulation of mDISC1 inside the growth cone was also observed in dissociated neurons from mDISC1 transgenic animals (Fig. S1C), suggesting that extrinsic factors are not involved in the mDISC1 localization. By testing serial deletion constructs of mDISC1, we found that such accumulation at axon tips was regulated by its C terminus (Fig. S1D), as has been suggested in vertebrate cells (4).
DISC1 is known to interact with more than a dozen vertebrate genes (4). Among these genes, homologs of two well-known mDISC1-interacting genes, pde-4 and lis-1, are present in the C. elegans motor neurons (12) (Fig. S2). To validate studying mDISC1-mediated signaling pathways in the C. elegans heterologous system, we tested whether the phenotype caused by ectopic mDISC1 was suppressed by knockdown of these two C. elegans genes. We found the axon guidance defects in mDISC1 transgenic animals were suppressed by RNAi knockdown of pde-4 (Fig. 1F). RNAi of lis-1 caused embryonic lethality as previously reported (12). However, some escapers that had milder RNAi effects showed significant suppression of the guidance defect (Fig. 1F). Together, these studies demonstrate mDISC1 proteins in the C. elegans motor neurons behave similarly to those in vertebrate neurons.
mDISC1 Causes Axon Guidance Defects and Ectopic Branching in C. elegans Motor Neurons by Activating the UNC-73-RAC-PAK Signaling Pathway.
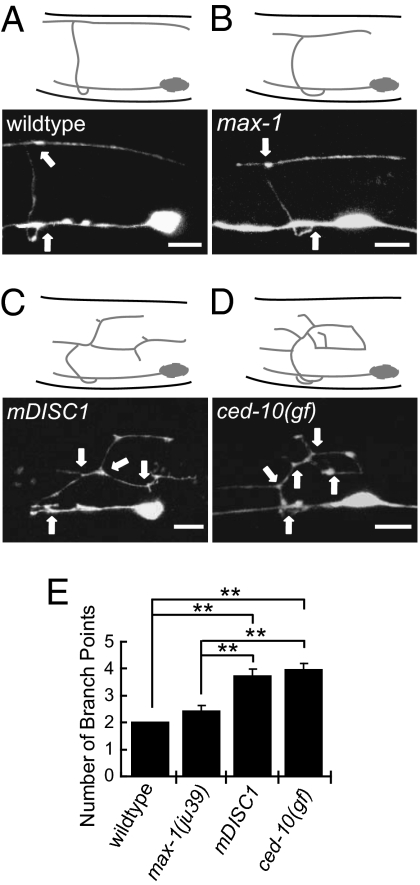
UNC-73-RAC-PAK signaling has previously been characterized in the guidance of C. elegans motor commissural axons (13). Interestingly, the C. elegans motor axons expressing the mDISC1 gene exhibited a unique branching defect very similar to those in the transgenic worms expressing gain-of-function (gf) rac genes (14) (Fig. 2). In wild-type worms, each DD or VD axon has two branch points: the first point is located at the start of the commissural branch that extends from the ventral axon process, and The second branch point is at the end of the commissure that branches into anteriorly and posteriorly extended processes (Fig. 2 A and E). The average branch points in many axon guidance mutants such as max-1 remain unchanged (Fig. 2 B and E). However, in both mDISC1 and rac(gf) transgenic animals, affected axons not only exhibited a similar abnormal branching pattern but also had significantly more branch points (Fig. 2 C–E). In addition to the branching phenotype, both mDISC1 and rac(gf) transgenic animals also displayed axon guidance defects with misguided commissures failing to reach the dorsal cord. Involvement of RAC signaling by mDISC1 was further supported by the observation that expression of dominant-negative (dn) rac genes in mDISC1 transgenic animals suppressed the axon guidance defects, but expression of wild-type rac genes enhanced the phenotype (Fig. 3B).
Fig. 2.
mDISC1 transgenic animals exhibit a similar ectopic branching phenotype seen in the transgenic animals expressing rac(gf) genes. (A) A confocal image of the representative wild-type DD motor neuron at L1 stage. The DD motor neuron first extends its short axon process along the ventral cord, from which the commissure branches out and migrates circumferentially to the dorsal side of the body. When the commissure hits the dorsal cord, it branches again and extends both anteriorly and posteriorly along the dorsal cord. The two branch points are indicated by arrows. (B) In max-1 mutant animals, although the commissural axons often fail to reach the dorsal cord, these axons exhibit two branch points as seen in the wild type (arrows). (C and D) The misguided DD axons in either mDISC1 (C) or rac(gf) (D) transgenic worms exhibit significantly more branch points (arrows). All images are taken from a single DD neuron at L1 stage. (Scale bar: 5 μm.) (E) Quantification of the branch points for each genetic background. The branch point is defined as a point with an additional neurite extending more than 1 μm. Bars represent the SE (n = 21). **P < 0.001 (Student's t test). In all pictures, anterior is to the left and dorsal is up.
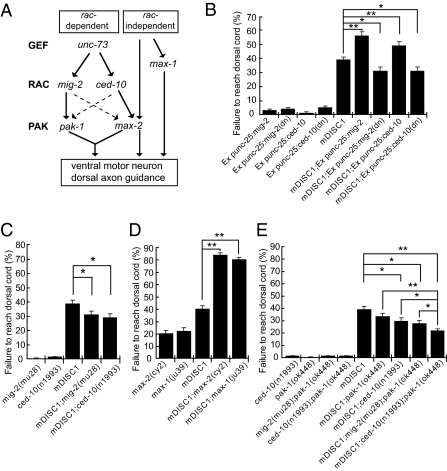
Fig. 3.
mDISC1 in C. elegans motor neurons interacts genetically with RAC-PAK signaling pathways to regulate axon guidance. (A) Schematic diagram summarizing the known RAC-dependent and RAC-independent signaling pathways in developing C. elegans motor neurons (13). The RAC-dependent pathway involves unc-73 (a GEF gene), two functional redundant rac genes, ced-10 and mig-2, and two downstream pak genes, pak-1 and max-2. The RAC-independent pathway is simplified by showing a completely RAC-independent gene, max-1. Note that the max-2 gene also has a RAC-independent function. Solid arrows indicate direct activation, and dashed arrows indicate weaker activation. (B) The severity of axon guidance defects in mDISC1 transgenic animals is significantly suppressed by ced-10(dn) or mig-2(dn), whereas expression of wild-type ced-10 or mig-2 enhances the axon guidance defects of mDISC1 transgenic animals. (C) Single mutants of ced-10 or mig-2 suppress the axon guidance defects of mDISC1 transgenic animals. (D) Axon guidance defects in mDISC1 transgenic animals are greatly enhanced by loss of genes that can transduce signals independent of the RAC signaling pathway. (E) Double mutants of pak-1;ced-10 suppress the defects more significantly than either pak-1 or ced-10 single mutants. They also suppress the defects more significantly than pak-1;mig-2 double mutants, consistent with the preferential uses of the rac-pak genes in the motor axons. In all diagrams, bars represent the SE (n = 32–62). *P < 0.05, **P < 0.001 (Student's t test).
As summarized in Fig. 3A, the two rac genes, ced-10 and mig-2, function redundantly in motor axons. Although single mutants of rac genes cause no axon guidance defects, double rac mutants exhibit severe axon guidance defects. On the other hand, the roles of the two pak genes, pak-1 and max-2, are slightly different: MAX-2 has RAC-dependent and RAC-independent roles, but PAK-1 functions completely in the RAC signaling pathway, which is redundant to MAX-2 (Fig. 3A). Consistent with the rac(dn) experiments, single mutants of mig-2 or ced-10 partially but significantly suppressed the axon guidance defects (Fig. 3C). It is important to note that mutants with severe axon guidance defects would preclude us from performing genetic suppression experiments. We therefore were unable to test double mutants such as ced-10;mig-2 that, by themselves, showed severe axon guidance defects (13, 14). However, double mutants of pak-1;ced-10, which did not exhibit axon guidance defects by themselves (13), suppressed the defects of mDISC1 transgenic animals more significantly than either pak-1 or ced-10 single mutants (Fig. 3E). Our previous genetic studies have shown that, in RAC-PAK signaling, PAK-1 is the preferential effector for MIG-2, whereas MAX-2 functions preferentially downstream of CED-10 (Fig. 3A) (13). Consistent with this finding, pak-1;ced-10 double mutant was a stronger suppressor than pak-1;mig-2 double mutant in transgenic mDISC1 background (Fig. 3E).
We reasoned that, if activation of RAC signaling by ectopic mDISC1 is a mechanism by which axon guidance is affected, mutants of genes that transduce signals by RAC-independent pathways would be predicted to enhance the axon guidance defects in mDISC1 transgenic animals. Indeed, consistent with its additional RAC-independent role, max-2 single mutant enhanced the defects (Fig. 3D). In addition, the axon guidance defects of mDISC1 transgenic animals were enhanced by the mutation of a RAC-independent gene, max-1 (Fig. 3D). Collectively, these data not only support mDISC1 activation of RAC-PAK signaling but also suggest mDISC1 acts as an upstream regulator of the RAC-PAK signaling.
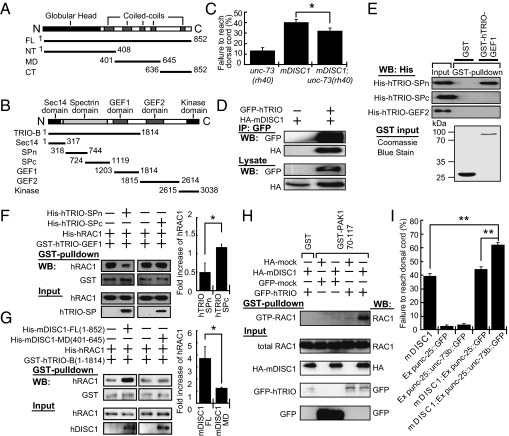
Given that UNC-73 is the main guanine nucleotide exchange factor (GEF) activating the RAC-PAK signaling in C. elegans motor axons (15, 16), we investigated whether DISC1 activates RAC-PAK signaling through unc-73. The first GEF domain, GEF1, of UNC-73 is specific for activating RAC, which is both necessary and sufficient for the function of UNC-73 in axon guidance (15). The axon guidance defects of the mDISC1 transgenic worms were suppressed by a hypomorphic unc-73 allele, rh40, which has a missense mutation in its GEF1 domain (Fig. 4C). Thus, in heterologous C. elegans motor axons, mDISC1 is likely to activate RAC-PAK signaling through UNC-73.
Fig. 4.
In mammalian systems, DISC1 binds to TRIO and activates RAC1 signaling. (A) Schematic diagram summarizing mDISC1 constructs used to generate His-tagged recombinants. mDISC1 contains an N-terminal globular head domain and multiple coiled-coil domains in the C terminus. The numbers indicate the beginning and ending amino acids for each construct. (B) Schematic diagram summarizing hTRIO constructs used to generate GST-tagged recombinants. hTRIO consists of a Sec14 homolog domain, a spectrin repeats (SP) domain, two GEF domains (GEF1 and GEF2), and a kinase domain. (C) Axon guidance defects in mDISC1 transgenic worms are significantly suppressed by rh40, a hypomorphic mutant allele of unc-73/trio (n = 50–69). (D) mDISC1 and hTRIO are coimmunoprecipitated as a protein complex when expressed in COS cells. (E) The SPn of hTRIO, but not the SPc or the GEF2 domain, binds to its GEF1 as shown in the GST pull-down assays. The loading inputs are 5% of the total purified His-tagged proteins. (F) hTRIO-SPn specifically inhibits the binding of human RAC1 (hRAC1) to hTRIO-GEF1 domain in the GST pull-down assays. The ratios of hRAC1 pulled down by hTRIO-GEF1 in the presence of hTRIO-SPn or hTRIO-SPc were quantified and are shown in the histogram (n = 3). The ratio is indicated as fold increase relative to that without adding hTRIO-SP proteins. (G) hTRIO pulls down more hRAC1 in the presence of mDISC1 full-length (mDISC1-FL) but not a control mDISC1 fragment (mDISC1-MD) that does not bind to hTRIO. The ratios of hRAC1 pull-down were quantified as in F (n = 3). (H) The RAC1 activities in COS cells are significantly increased when hTRIO is coexpressed with mDISC1. The activity of RAC1 was assayed by the amount of GTP-RAC1 pulled-down by GST-PAK1 70–117 (45). (I) Expression of unc-73b significantly enhances the motor axon guidance defects in mDISC1 transgenic animals (n = 54–60). In all diagrams, bars represent the SE. *P < 0.05, **P < 0.001 (Student's t test).
mDISC1 Activates RAC Signaling by Direct Binding with TRIO/UNC-73 in Mammalian Cells.
To validate and further investigate the interactions between mDISC1 and UNC-73-RAC-PAK signaling in mammalian cells, we used mammalian homologous proteins to study the molecular interactions. We found that DISC1 coimmunoprecipitated with TRIO, an UNC-73 mammalian homolog (Fig. 4D), as independently reported in a previous yeast two-hybrid study (17). TRIO is a large Dbl family protein, whose structure consists of two GEF domains: the RAC1-specific first GEF (GEF1) and the RHO-specific second GEF (GEF2) (Fig. 4B). Domain mapping indicated that N-terminal globular domain of mDISC1 bound to the amino half of spectrin repeats (SPn) and the GEF2 of human TRIO (hTRIO) (Fig. S3). Because the GEF2 domain is dispensable for its function in guiding commissural motor axons in C. elegans (15), we focused on the interaction of DISC1 with TRIO-SPn and asked how such an interaction affected RAC-PAK signaling.
First, in a series of in vitro pull-down assays, we found that the RAC1-specific TRIO-GEF1 specifically bound to TRIO-SPn but not to the carboxyl half of spectrin repeats (SPc) or TRIO-GEF2 (Fig. 4E). Second, the binding between TRIO-GEF1 and its effector RAC1 was significantly compromised in the presence of exogenous TRIO-SPn (Fig. 4F). These results indicate that the recruitment of RAC1 to TRIO's GEF1 domain is inhibited by the binding of its SPn. Next, we investigated the effect of mDISC1 on the interaction between RAC1 and hTRIO-B, a mammalian equivalent to the C. elegans unc-73b isoform that is sufficient to rescue the axon guidance defect caused by unc-73 mutants (15). In vitro pull-down experiments showed that mDISC1 itself did not bind RAC1 (Fig. S4), but hTRIO-B recruited more RAC1 in the presence of mDISC1 (Fig. 4G).
To confirm the in vitro biochemical results, a RAC1 activity assay was performed in COS cells. As shown in Fig. 4H, active GTP-bound RAC1 was dramatically increased in the presence of both DISC1 and TRIO. In addition, when unc-73b was expressed in wild-type C. elegans motor neurons, only very mild axon guidance defect was observed. However, expression of unc-73b in the motor neurons of mDISC1 transgenic animals strongly enhanced the axon guidance defects (Fig. 4I). Together, we conclude that DISC1 can interact with TRIO's SPn, which results in activation of UNC-73/TRIO-RAC-PAK signaling to induce axon guidance defects.
Discussion
A heterologous DISC1 genetic model has been established in C. elegans motor neurons. Using this system, we studied how DISC1 is involved in the signaling pathways of axon guidance. We showed that DISC1 can activate the RAC-PAK signaling pathway via interacting with UNC-73/TRIO in C. elegans motor axons. Importantly, this DISC1-mediated signaling pathway is phylogenetically conserved. In mammalian cultured cells, DISC1 directly interacts with the SPn domain of TRIO. This interaction facilitates the recruitment of RAC1 to the GEF1 domain of TRIO and results in activation of RAC1 activity.
C. elegans is a relatively simple genetic system for investigating molecular signaling pathways. By establishing the mDISC1 transgenic animal, we were able to dissect the molecular interactions in detail. We found that, in C. elegans motor neurons, DISC1 interacts with a GEF, UNC-73/TRIO, and activates the RAC signaling to regulate axon guidance. TRIO's role in axon guidance has been well studied, and its functions are phylogenetically conserved (15, 18, 19). Thus, our results were readily applicable to the mammalian system. Indeed, we demonstrated similar interactions of DISC1 with the TRIO-RAC signaling in mammalian cultured cells. TRIO is a Dbl family protein, which has two distinct GEF domains (20). Our analysis suggests an inhibitory control of GEF1 activity by the SPn domain of TRIO. Consistent with these findings, the N terminus of TRIO has been shown to act as a dominant-negative inhibitor of TRIO's activity (21), and similar inhibitory mechanisms have been reported in other Dbl family proteins (22, 23). Thus, despite its heterologous nature, the established mDISC1 genetic system has proven useful for studying DISC1’s functions.
Neuronal dysconnection has been demonstrated in the brains of schizophrenic patients (24, 25). However, the causes of such abnormalities are still unknown. Recent evidence has begun to show that schizophrenia susceptibility genes are essential for axonal connectivity during development. For example, neuregulin, a well known schizophrenia susceptibility gene, is required for thalamocortical projections in mice (26, 27). Our studies in mouse hippocampi and in C. elegans motor neurons together also show that DISC1 plays a role in regulating axonal connections (9). Because DISC1 is involved in essentially all aspects of neurodevelopment (4–7, 11), and because more than a dozen cytosolic proteins are reported to interact with DISC1 (6, 8, 28–30), it is likely that DISC1 acts as a scaffold protein inside neurons, recruiting various signaling components to exert its specific function at different developmental stages. It is therefore not surprising that, in contrast to our results, DISC1 has recently been reported to interact with another RAC1-GEF, Kalirin-7, and to inhibit the RAC1 signaling during dendritic spine morphogenesis (31). Despite the differences, these studies together suggest that mutations of genes involved in the RAC-PAK signaling pathway might cause schizophrenia or other related mental illnesses. Indeed, abnormal expressions or mutations of genes in this RAC-PAK pathway have been reported in human genomic studies of patients with psychotic symptoms (32–34).
Several transgenic C. elegans lines have been successfully established to study human neurodegenerative disorders (35–40). Such heterologous genetic models have proven to be very useful for understanding disease pathogenesis and developing therapeutic strategy. For instance, the heterologous model for polyglutamine aggregation in C. elegans has demonstrated that chronic expression of aggregation-prone proteins can disrupt the homeostasis of protein folding and cause pleiotropic cytotoxicity (35). The establishment of this transgenic DISC1 model expands the repertoire of C. elegans disease models to study human neurodevelopmental disorders. One important application of disease models in C. elegans is to identify drug targets or screen for new drugs (41–43). Recently, a large-scale small-molecule screen in C. elegans has successfully isolated a calcium channel blocker. Subsequent suppressor genetic screens also identify the α1-subunit of the L-type calcium channel as a potential drug target (44). Interestingly, when the mDISC1 transgenic animals were incubated with rolipram, a well-known phosphodiesterase 4 (PDE4)–specific inhibitor with anti-psychotic effects, the axon guidance defects were apparently suppressed (Fig. 1G). Thus, this established mDISC1 genetic system could be used in the future to screen for small molecules or chemicals with potential therapeutic effects on DISC1-related abnormalities.
Materials and Methods
Phenotypic Analysis.
The axon guidance defects were quantified by calculating the percentage of commissures from DD and VD motor neurons that failed to reach the dorsal cord within a single animal. For branching phenotype, the branch point was defined as a point with an additional neurite extending more than 1 μm in length. Multiple comparisons were performed with the two-tailed Student's t test and a Benjamini and Hochberg correction. In all figures, statistically significant differences are indicated by asterisks.
Descriptions of C. elegans strains, constructs, in vitro binding assay, RAC1 activity assay, and other general techniques are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the International C. elegans Gene Knockout Consortium for strains. We are grateful to Yuji Kohara, Erik Lundquist, Ken-Ichi Ogura, Yoshio Goshima, Hongjun Song, Gary Ruvkun, Andy Fire, and Anne Debant for materials. We thank Mark Lucanic, Noelle L'Etoile, Ting-Wen Cheng, Damien O'Halloran, Scott Hamilton, Bi-Tzeng Juang, Karl Murray, Hai-Gwo Hwu, Chih-Min Liu, members of the L'Etoile and Cheng laboratories, and members of the “Super Worm Group” at the University of California, Davis, for comments and advice. We also thank Wei-Wen Liu, Kimberly Zhou, and Abraham Noorbakhsh for technical help. This work was supported in part by a startup fund and a health system grant from the University of California, Davis (to H.-J.C.), and a grant from the National Taiwan University Hospital (to P.-H.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018128108/-/DCSupplemental.
References
- 1.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 2.Ross CA, Margolis RL, Reading SAJ, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DA, Lieberman JA. Catching up on schizophrenia: Natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 4.Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 5.Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao Y, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamiya A, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 8.Ozeki Y, et al. Disrupted-in-Schizophrenia-1 (DISC-1): Mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci USA. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faulkner RL, et al. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci USA. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bord L, et al. Primate disrupted-in-schizophrenia-1 (DISC1): High divergence of a gene for major mental illnesses in recent evolutionary history. Neurosci Res. 2006;56:286–293. doi: 10.1016/j.neures.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi K, et al. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8:685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- 12.Dawe AL, Caldwell KA, Harris PM, Morris NR, Caldwell GA. Evolutionarily conserved nuclear migration genes required for early embryonic development in Caenorhabditis elegans. Dev Genes Evol. 2001;211:434–441. doi: 10.1007/s004270100176. [DOI] [PubMed] [Google Scholar]
- 13.Lucanic M, Kiley M, Ashcroft N, L'Etoile N, Cheng H-J. The Caenorhabditis elegans P21-activated kinases are differentially required for UNC-6/netrin-mediated commissural motor axon guidance. Development. 2006;133:4549–4559. doi: 10.1242/dev.02648. [DOI] [PubMed] [Google Scholar]
- 14.Struckhoff EC, Lundquist EA. The actin-binding protein UNC-115 is an effector of Rac signaling during axon pathfinding in C. elegans. Development. 2003;130:693–704. doi: 10.1242/dev.00300. [DOI] [PubMed] [Google Scholar]
- 15.Steven R, et al. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92:785–795. doi: 10.1016/s0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y-C, Cheng T-W, Lee M-C, Weng N-Y. Distinct rac activation pathways control Caenorhabditis elegans cell migration and axon outgrowth. Dev Biol. 2002;250:145–155. doi: 10.1006/dbio.2002.0785. [DOI] [PubMed] [Google Scholar]
- 17.Camargo LM, et al. Disrupted in Schizophrenia 1 Interactome: Evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- 18.Newsome TP, et al. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–294. doi: 10.1016/s0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 19.Briançon-Marjollet A, et al. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol Cell Biol. 2008;28:2314–2323. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bateman J, Van Vactor D. The Trio family of guanine-nucleotide-exchange factors: Regulators of axon guidance. J Cell Sci. 2001;114:1973–1980. doi: 10.1242/jcs.114.11.1973. [DOI] [PubMed] [Google Scholar]
- 21.Estrach S, et al. The human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr Biol. 2002;12:307–312. doi: 10.1016/s0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 22.Rossman KL, Der CJ, Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 23.Yu B, et al. Structural and energetic mechanisms of cooperative autoinhibition and activation of Vav1. Cell. 2010;140:246–256. doi: 10.1016/j.cell.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Woo R-S, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Bendito G, et al. Tangential neuronal migration controls axon guidance: A role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millar JK, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 29.Brandon NJ, et al. Disrupted in Schizophrenia 1 and Nudel form a neurodevelopmentally regulated protein complex: Implications for schizophrenia and other major neurological disorders. Mol Cell Neurosci. 2004;25:42–55. doi: 10.1016/j.mcn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Singh KK, et al. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi-Takagi A, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rejeb I, et al. A novel splice mutation in PAK3 gene underlying mental retardation with neuropsychiatric features. Eur J Hum Genet. 2008;16:1358–1363. doi: 10.1038/ejhg.2008.103. [DOI] [PubMed] [Google Scholar]
- 33.Allen KM, et al. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- 34.Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- 35.Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- 36.Faber PW, Alter JR, MacDonald ME, Hart AC. Polyglutamine-mediated dysfunction and apoptotic death of a Caenorhabditis elegans sensory neuron. Proc Natl Acad Sci USA. 1999;96:179–184. doi: 10.1073/pnas.96.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faber PW, Voisine C, King DC, Bates EA, Hart AC. Glutamine/proline-rich PQE-1 proteins protect Caenorhabditis elegans neurons from huntingtin polyglutamine neurotoxicity. Proc Natl Acad Sci USA. 2002;99:17131–17136. doi: 10.1073/pnas.262544899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamamichi S, et al. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson's disease model. Proc Natl Acad Sci USA. 2008;105:728–733. doi: 10.1073/pnas.0711018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraemer BC, et al. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci USA. 2003;100:9980–9985. doi: 10.1073/pnas.1533448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Link CD. Expression of human β-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giacomotto J, et al. Evaluation of the therapeutic potential of carbonic anhydrase inhibitors in two animal models of dystrophin deficient muscular dystrophy. Hum Mol Genet. 2009;18:4089–4101. doi: 10.1093/hmg/ddp358. [DOI] [PubMed] [Google Scholar]
- 42.Kaminsky R, et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- 43.Kwok TCY, et al. A genetic screen for dihydropyridine (DHP)-resistant worms reveals new residues required for DHP-blockage of mammalian calcium channels. PLoS Genet. 2008;4:e1000067. doi: 10.1371/journal.pgen.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwok TCY, et al. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006;441:91–95. doi: 10.1038/nature04657. [DOI] [PubMed] [Google Scholar]
- 45.Zahir N, et al. Autocrine laminin-5 ligates α6β4 integrin and activates RAC and NFκB to mediate anchorage-independent survival of mammary tumors. J Cell Biol. 2003;163:1397–1407. doi: 10.1083/jcb.200302023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.