Abstract
Several prominent hypotheses have been posed to explain the immense variability among plant species in defense against herbivores. A major concept in the evolutionary ecology of plant defenses is that tradeoffs of defense strategies are likely to generate and maintain species diversity. In particular, tradeoffs between constitutive and induced resistance and tradeoffs relating these strategies to growth and competitive ability have been predicted. We performed three independent experiments on 58 plant species from 15 different plant families to address these hypotheses in a phylogenetic framework. Because evolutionary tradeoffs may be altered by human-imposed artificial selection, we used 18 wild plant species and 40 cultivated garden-plant species. Across all 58 plant species, we demonstrate a tradeoff between constitutive and induced resistance, which was robust to accounting for phylogenetic history of the species. Moreover, the tradeoff was driven by wild species and was not evident for cultivated species. In addition, we demonstrate that more competitive species—but not fast growing ones—had lower constitutive but higher induced resistance. Thus, our multispecies experiments indicate that the competition–defense tradeoff holds for constitutive resistance and is complemented by a positive relationship of competitive ability with induced resistance. We conclude that the studied genetically determined tradeoffs are indeed likely to play an important role in shaping the high diversity observed among plant species in resistance against herbivores and in life history traits.
Keywords: comparative experimental study, phylogenetic corrections, plant defense theory, plant–insect interaction, Spodoptera littoralis
Given that green plants are the ultimate source of energy for most other organisms, it is not surprising that plants evolved a variety of resistance strategies, which can be constitutively expressed or induced after damage (1). Although herbivory selects for enhanced plant resistance, plants vary greatly in their resistance, both among species (2) and among genotypes within species (3). This indicates that being well defended may not always be the best strategy—most likely because allocation to resistance may physiologically constrain other investments (reviewed in refs. 4 and 5) and because constraints on resource allocation may produce negative genetic correlations between resistance mechanisms and other life-history traits (6–8). The current assumption in evolutionary ecology is that such tradeoffs contribute to the generation and maintenance of species diversity (8).
Several hypotheses on tradeoffs associated with constitutive and induced resistance have been formulated. Among the most fundamental ones is the long-predicted tradeoff between constitutive and induced resistance itself (6, 9). This hypothesis is derived from the idea that resistance is costly and that a species already well defended by high constitutive resistance will benefit only negligibly by further induced resistance (1, 10). However, general empirical evidence for this predicted tradeoff is still ambiguous (11), largely because most studies considered only single or very few species. Moreover, some studies reporting a tradeoff between constitutive and induced resistance were apparently compromised by spurious correlations due to including the same data for calculating constitutive and induced resistance (12). In addition, most studies that did not find such a tradeoff compared cultivated plant species or genotypes that had been subjected to artificial selection. In cultivated plants the selection pressures maintaining a tradeoff might be alleviated because nonnative cultivated plants might have escaped natural enemies (e.g., refs. 13 and 14), are artificially protected from enemies, or might have undergone artificial breeding that changed or broke up tradeoffs. As a consequence, those cultivated species might be relieved from evolutionary forces that shape tradeoffs in nature (15). However, to date no study has explicitly tested whether tradeoffs differ between cultivated and wild species.
Another fundamental hypothesis is the growth-rate or resource-availability hypothesis (16). It predicts that slow-growing plant species, which typically evolved in resource-limited environments, are less able to replace lost tissue than fast-growing plant species from more productive and competitive environments and should therefore invest in constitutive rather than induced resistance. As a corollary, induced resistance is expected to be higher in fast-growing and more competitive species. Several studies tested for correlations between constitutive resistance and growth, but to date only one study tested whether both constitutive and induced resistance are correlated with growth in the predicted manner (17). Moreover, although it is frequently assumed that fast growth is equivalent to high competitive ability (13), no study has explicitly tested whether competitive ability—rather than growth—of plant species trades off with constitutive resistance and whether competitive plants therefore might have evolved the strategy of induced resistance.
To address the generality of the relationships among constitutive and induced resistance, growth rate, and competitive ability, we took a comparative experimental approach. We performed three independent experiments to assess several important plant traits of 58 plant species from 15 different plant families. In the first experiment, we assessed constitutive and induced resistance to the bioassay caterpillar Spodoptera littoralis, whose feeding response, although possibly not representative for all herbivores, is considered to be a good approximation of plant resistance against generalist herbivores (17); in the second one growth rate; and in the third one competitive ability. To test whether evolutionarily important tradeoffs have disappeared in cultivated species, we compared cultivated horticultural plants (40 species) with wild plant species (18 species). Correcting for phylogenetic relatedness among species, we addressed the following specific questions. (i) Is there a tradeoff between constitutive and induced resistance among our study species, and if so, does it differ between wild and cultivated species? (ii) How are constitutive and induced resistance related to growth rate and competitive ability of wild and cultivated plant species?
Results
Phylogenetic Signal.
We found a significant phylogenetic signal for relative growth rate (K = 0.453, n = 51, P = 0.002) and competitive ability (K = 0.289, n = 53, P = 0.044), indicating that part of their variation was predicted by evolutionary relationships among species. However, we found no significant phylogenetic signal for constitutive (K = 0.268, n = 58, P = 0.068) or induced resistance (K = 0.163, n = 58, P = 0.787).
Tradeoff Between Constitutive and Induced Resistance.
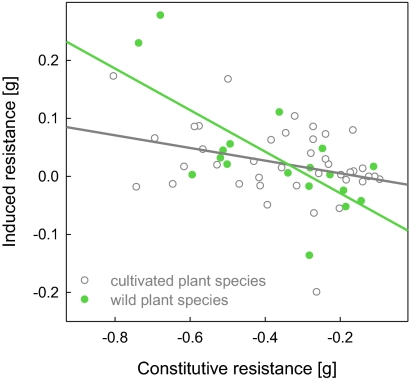
Across all 58 plant species, induced and constitutive resistance were negatively correlated with each other [correction for spurious correlation (12): observed correlation = −0.451, one-sided P = 0.031]. This tradeoff was also significant when we corrected for phylogeny (estimate ± SEM = −0.249 ± 0.058, P < 0.001), indicating the robustness of our result. Interestingly, this tradeoff was significant among wild plant species [correction for spurious correlation (12): observed correlation = −0.696, one-sided P = 0.016] but was not evident among cultivated plant species (observed correlation = −0.309, one-sided P = 0.208). This strongly suggests that the tradeoff has been lost for species under cultivation. This was also reflected in a significant interaction of species status (wild vs. cultivated) with constitutive resistance using induced resistance as dependent variable, both in a phylogenetically uncorrected (estimate = −0.249 ± 0.099, P = 0.015; Fig. 1 and Table S1) and a phylogenetically corrected analysis (estimate ± SEM = −0.288 ± 0.092, P = 0.003; Table S1).
Fig. 1.
Relationship between mean constitutive (−1 × adjusted final mass of caterpillars on undamaged plants) and induced resistance [−1 × (adjusted final mass of caterpillars on damaged plants − adjusted final mass of caterpillars on undamaged plants)] for the 18 wild and 40 cultivated plant species. Depicted are raw data points and a fitted line, separately for wild (green line) and cultivated (gray line) plant species.
Relationship of Resistance with Relative Growth Rate.
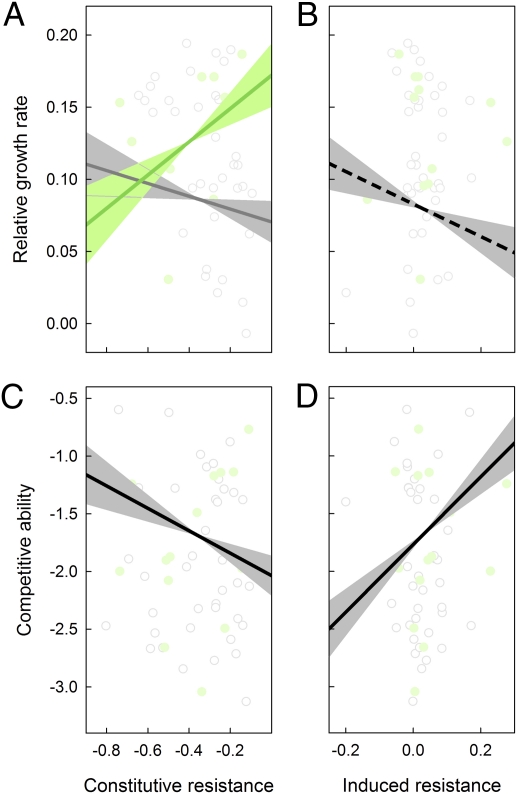
Cultivated species seemed to follow the predictions of the growth-rate hypothesis—species with higher constitutive resistance had lower growth rates—but this was not significant (accounting for phylogeny and analyzed separately for cultivated plants: estimate ± SEM = −0.043 ± 0.040, P = 0.289; Fig. 2 and Table S2). On the other hand, wild plant species with higher constitutive resistance tended to have higher instead of lower relative growth rates (accounting for phylogeny and analyzed separately for wild plants: estimate ± SEM = 0.113 ± 0.052, P = 0.051; Fig. 2 and Table S2). These different patterns for cultivated and wild plant species were reflected in a significant constitutive resistance × species status interaction using relative growth rate as dependent variable (accounting for phylogeny: estimate ± SEM = 0.162 ± 0.055, P = 0.005; Table S1). Consequently, across all species, constitutive resistance was not significantly related to relative growth rate (Table 1).
Fig. 2.
Relationship between (A) constitutive resistance [g] and relative growth rate [gg−1d−1], (B) induced resistance [g] and relative growth rate, (C) constitutive resistance and competitive ability [g], and (D) induced resistance and competitive ability for the (A and B) 13 wild (green dots) and 38 cultivated (gray dots) and (C and D) 15 wild (green dots) and 38 cultivated (gray dots) plant species. Note that phylogenetically uncorrected (i.e., raw) data points are depicted, and a linear best fit from the phylogenetically corrected correlations using the GLS method, in A separately for wild (green line) and cultivated (gray line) plant species. Shaded areas around the lines indicate the estimated slope plus and minus 1 SE of the slope. Significant relationships are indicated by a solid line, marginal significant relationships by a dashed line.
Table 1.
Correlations between species traits assessed with GLS regression models
| Y variable | X variable | n | Coefficient ± SEM | Phylogenetic correction |
| Induced resistance | Constitutive resistance | 58 | −0.249 ± 0.058**** | No |
| Relative growth rate | Constitutive resistance | 51 | −0.006 ± 0.034 | Yes |
| Relative growth rate | Induced resistance | 51 | −0.113 ± 0.064* | Yes |
| Competitive ability | Constitutive resistance | 53 | −0.972 ± 0.465** | Yes |
| Competitive ability | Induced resistance | 53 | 2.930 ± 0.850*** | Yes |
Correlations are either raw or phylogenetically corrected, depending on the presence of a phylogenetic signal in at least one of the correlated variables. Shown are regression coefficients ± SEs (SEM).
*P < 0.1; **P < 0.05; ***P < 0.01; ****P < 0.001.
Across all study species, induced resistance was not positively, as expected, but slightly negatively correlated with relative growth rate (accounting for phylogeny; Fig. 2 and Table 1). This marginally significant correlation did not differ between wild and cultivated plant species (induced resistance × species status interaction using relative growth rate as dependent variable: estimate ± SEM = −0.123 ± 0.133, P = 0.362; Table S1). Overall, our results do not support the predictions of the growth-rate hypothesis.
Relationship of Resistance with Competitive Ability.
Although it is frequently assumed that fast growth is equivalent to competitive ability, relative growth rate was not significantly positively correlated with competitive ability across all study species (accounting for phylogeny: estimate ± SEM = −0.004 ± 0.009) but followed a humped relationship, with highest competitive ability at intermediate growth rates (accounting for phylogeny: estimate for the quadratic term of growth rate ± SEM = −0.263 ± 0.131, P = 0.049). This relationship did not differ between wild and cultivated plant species [nonsignificant (relative growth rate)2 × species status interaction using competitive ability as dependent variable: estimate ± SEM = −0.222 ± 0.333].
Species with higher competitive ability had significantly lower constitutive resistance and significantly higher induced resistance (accounting for phylogeny; Fig. 2 and Table 1). These relationships did not differ between wild and cultivated plant species (constitutive resistance × species status interaction using competitive ability as dependent variable: estimate ± SEM = −0.715 ± 0.834, P = 0.396; induced resistance × species status interaction using competitive ability as dependent variable: estimate ± SEM = 0.857 ± 1.796, P = 0.635; Table S1). These results indicate a genetically determined tradeoff between competitive ability and constitutive resistance, which for good competitors is counterbalanced by their high ability to induce resistance.
Discussion
Tradeoff Between Constitutive and Induced Resistance.
Different types and strategies of resistance, large variation among species in the degree of resistance, and their potential consequences for the evolution of other plant traits have inspired biologists for decades. We demonstrated the long-predicted tradeoff between constitutive and induced resistance (6, 9) using a large taxonomically diverse number of herbaceous plant species. As hypothesized, the tradeoff between constitutive and induced resistance was significant among wild species but not among cultivated species. Concordantly, most previous studies that did not find a tradeoff between constitutive and induced resistance used genotypes or varieties of cultivated crop species (6, 18–21). The presence of the tradeoff in wild plant species and its absence in cultivated plant species indicates that evolutionary rather than physiological forces cause the tradeoff in nature. This clearly calls for using wild species when addressing natural evolutionary patterns in life-history traits and shows the advantage of using cultivated plant species as controls.
Two previous studies found a positive, instead of a negative, correlation between components of constitutive and induced resistance (2, 22). Possibly, this apparent contradiction with our results reflects that those studies looked at single defensive compounds rather than at an integrative measure of plant resistance obtained, for example, by using generalist bioassay caterpillars. This explanation would be in line with recent claims that, although the univariate tradeoff model may not hold for individual elements of plant defense, there might be tradeoffs between so-called defense syndromes (alternative strategies) (2, 23, 24). We conclude that constitutive and induced resistance, both consisting of a combination of various, possibly even positively covarying, resistance traits, represent two alternative adaptive strategies that tradeoff with each other.
Constitutive and Induced Resistance in Relation to Relative Growth Rate.
Several previous studies reported a tradeoff between constitutive resistance and growth rate, which supports the growth-rate or resource-availability hypothesis (e.g., refs. 16, 17, and 25), and its universality has been claimed (26). Cultivated plant species in our study showed a trend to follow the predictions of the growth-rate hypothesis, with slow-growing species having more constitutive resistance than fast-growing species. Interestingly, however, we found the opposite for wild plant species. Moreover, rather than a positive association between induced resistance and relative growth rate, we found a marginally significant negative one. Our results therefore indicate that there is no universal support for a tradeoff between constitutive resistance and growth rate or for a corresponding positive association between induced resistance and growth rate.
It has been suggested that the tradeoff between growth and constitutive resistance should not hold if resistance is provided by low-cost, qualitative compounds (27). In addition, it is assumed that fast-growing species, if they have any antiherbivore resistance at all, accumulate low concentrations of highly potent, qualitative compounds (e.g., alkaloids, cardenolides, glucosinolates), which are mainly effective against generalist herbivores, such as our bioassay caterpillar. Slow-growing species instead are assumed to accumulate mainly quantitative compounds (e.g., lignin, condensed tannins), which reduce the digestibility of the plants for generalist and specialist herbivores (28). Therefore, one hypothesis might be that in natural habitats, to be able to grow and defend, there is selection in fast-growing plants toward qualitative rather than quantitative compounds. In cultivated garden plants, which during their breeding programs are frequently protected from herbivores and usually do not experience nutrient deficiency, selection for qualitative compounds might be relaxed. The relation of growth and resistance in those plant species might rather follow physiological constraints, as predicted by the growth-rate hypothesis. For these reasons, our results are in line with recent suggestions that fast growth and constitutive resistance are not necessarily alternative strategies (27, 29, 30).
Surprisingly few studies assessed the association between induced resistance and growth rate across multiple species. Karban and Baldwin (1) reported in a review that slow-growing species frequently have the ability to induce resistance, but that it tends to be stronger in species categorized as fast growers. In a comparative experiment with 18 species (17), plant species from a low-resource and stressful environment produced less biomass and had lower induced but higher constitutive resistance than plant species from a more productive habitat, as predicted by the growth-rate hypothesis. The discrepancy of our results with the one described (17) could reflect that the latter did not measure growth rate explicitly but measured biomass production. Furthermore, it could be that under certain habitat conditions, tolerance to herbivory rather than resistance might be the optimal strategy. In a modeling approach, Ito and Sakai (31) showed that this is likely to be the case, when the probability and effect of herbivory is consistently low.
Although, concordant with our findings, resistance against a generalist herbivore seems to not necessarily constrain growth, there is plenty of evidence that resistance is generally costly for plants (4, 5, 32). Therefore, although not constraining growth, it is very likely that resistance is constraining important life-history traits other than growth. Because plants in nature hardly ever grow alone, and—as demonstrated by the humped relationship between competitive ability and growth in our experiments—the fastest-growing plant species are not necessarily the best competitors, competitive ability might be more likely than growth to shape resistance-deployment strategies of plants.
Constitutive and Induced Resistance in Relation to Competitive Ability.
Competition and herbivory are among the most important ecological forces affecting plant evolution (9). It is assumed that increased constitutive resistance compromises the competitive ability of plants (33), resulting in a competition–constitutive resistance tradeoff. Compared with constitutive resistance, induced resistance might be more valuable for plants faced with strong competition: in the absence of enemies more resources can be allocated to competitive ability, because the plant only induces defense when attacked. Consequently, plants can be competitive and well defended at the same time, when necessary. Indeed, among both wild and cultivated plant species, the more competitive ones had lower constitutive resistance and higher abilities to induce resistance.
A positive relationship between induced resistance and competitive ability has been addressed in very few previous studies. Cipollini (34) found that mutants of Arabidopsis thaliana lacking the ability to induce resistance are poorer competitors than the wild type, and he suggested that pathways essential to produce induced resistance also mediate some plant responses to competitors. Kong et al. (35) demonstrated that herbivore-inducible compounds in Ageratum conyzoides can possess allelopathic potential and directly suppress the growth of neighboring plants. We did not aim at disentangling whether more-competitive plant species are better competitors because (i) they induce resistance and directly or indirectly affect their neighbors, as described above (34, 35), or (ii) because low constitutive resistance combined with the ability to induce resistance is selectively favored in competitive environments on the basis of cost-effectiveness. However, our study shows not only that the competition–defense tradeoff is holding for constitutive resistance, but also that it is complemented by higher induced resistance of more competitive species. This seems to be an often-overlooked valuable strategy, especially of species in resource-rich competitive environments.
Conclusion
The advance in doing phylogenetically corrected comparative multispecies experiments enables testing for general tradeoffs in plants. In related fields, such an approach should be used analogously to better understand the generality of tradeoffs among further life-history traits. Our results, that constitutive and induced resistance are alternative adaptive strategies and that competitive ability rather than growth rate of plants is tightly linked to resistance against herbivores, provide insights into general patterns of defense-deployment strategies. Because differences in such strategies among species are genetically determined, we conclude that the studied tradeoffs are indeed likely to play an important role in shaping the high diversity observed among plant species in resistance against herbivores and in life-history traits.
Methods
Plant Species.
We obtained seeds of 77 herbaceous plant species from 15 different plant families (2–11 species per family). Thirtyone of the species are wild plant species native to Switzerland, and the remaining 46 species are exotic plants used as ornamental garden plants in Switzerland. To avoid confounding effects of the wild and cultivated status of species with taxonomy, we had both wild and cultivated species in almost all families. Because we wanted wild plant species to be representative of wild genotypes, we obtained them from Swiss seed suppliers providing these species for restoration projects and ecological compensation areas (UFA Samen, Wyss Samen und Pflanzen, and Samen-Steffen). Seeds of cultivated species were instead obtained mainly from nurseries (B & T World Seeds, Thompson & Morgan, and Wyss Samen und Pflanzen) and are likely to have undergone intense breeding. In three experiments, we assessed, respectively, herbivore resistance, growth rate, and competitive ability of all plant species. Because of low germination rates for some species, we used 51–58 of the 77 species in these experiments (Table S3). Most of the species with low germination rates were wild plant species, which led to an imbalance between the number of wild and cultivated plant species.
Assessment of Constitutive and Induced Resistance to Herbivory.
Herbivore species.
As bioassay herbivore for the assessment of constitutive and induced resistance, we used larvae of the Egyptian cotton leafworm Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae), which are known to feed on plants of at least 40 plant families (36). The extreme polyphagy of S. littoralis makes it an excellent bioassay species for comparing leaf palatability across plant species from different families (e.g., refs. 37–40), and its feeding response and performance are used as integrative and functionally relevant measures of plant resistance (15). Another advantage of S. littoralis for the present study is that it is native to (sub)tropical Africa and therefore unlikely to share a coevolutionary history with any of our study species. Therefore, it is unlikely that S. littoralis would have had an a priori biased preference for wild or cultivated species. Caterpillars for our experiments originated from a laboratory stock (Institute of Cell Biology, Bern, Switzerland) bred on a bean-based artificial diet to avoid adaptation of the insects to any of our study species.
Experimental design.
In August 2008, seeds of our plant species were germinated in trays filled with potting soil. For 18 wild and 40 cultivated species with sufficient numbers of seedlings (Table S3), we planted 10 seedlings into 1-L pots with potting soil (totaling 580 plants) and placed them in a greenhouse (15 °C night, 28 °C day, and a constant day length of 14 h). All plants were watered as needed. Because the species did not germinate simultaneously, the experiment was performed over a period of 4 mo until all 58 species had been tested. Confamiliar wild and cultivated species were tested at the same time.
After 8 wk of growth, we enclosed all 580 plants individually into perforated polyester bags. To induce possible resistance mechanisms in half of the plants of each species, we allowed two third- to fourth-instar caterpillars (hereafter called induction caterpillars) of S. littoralis to feed on these plants for 1 d. Directly after removal of the induction caterpillars, we added one third-instar larva of S. littoralis as bioassay caterpillar to each plant—both to the induced and noninduced plants—and allowed them to feed for 5 d. We assessed the increase in biomass of the bioassay caterpillars by recording fresh mass of the caterpillars before and after feeding (41).
For each plant species the effect of induction on final caterpillar mass was assessed by means of an analysis of covariation with type I sums of squares after considering effects of initial caterpillar mass as covariate (42, 43). The resulting adjusted final mass therefore represents caterpillar growth during the experiment. As a measure of constitutive resistance, we used mean adjusted final mass of caterpillars feeding on undamaged plants per plant species. Induced resistance was assessed as adjusted mass of caterpillars feeding on damaged plants minus the adjusted mass of caterpillars feeding on nondamaged plants (see refs. 12 and 40). Because large increases in caterpillar mass indicate low resistance of the plants, we multiplied caterpillar mass and the differences in caterpillar mass, respectively, with −1 for easier visualization (i.e., high values correspond to high resistance).
Assessment of Relative Growth Rate.
To determine relative growth rate, we germinated species as before, in May 2008. Because of low germination rates of some species, this experiment included 51 of the 58 species for which we had assessed resistance to herbivory (13 wild and 38 cultivated species; Table S3). Because species did not germinate simultaneously, they differed in starting times. Two weeks after germination, we harvested six offspring per plant species and determined total dry weight as initial size measure. For each species, we then transplanted six additional offspring individually into 1-L pots filled with a 1:1 mixture of sand and plain field soil. Six weeks after germination of each species, we harvested the plants and again determined total dry weight. From these data, we calculated the relative growth rate (RGR) of each species (44) as:
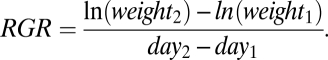 |
Assessment of Competitive Ability.
For the determination of competitive ability, it was important that the experiment started simultaneously for all species. Therefore, we germinated the plant species sequentially between March and June 2009, starting with the plant species having the longest and ending with the ones having the shortest time to germination. Because of low germination rates for some species, this experiment included 53 of the 58 species for which we had assessed resistance to herbivory (15 wild and 38 cultivated species; Table S3). For each species, we then transplanted 16 seedlings into 1-L pots filled with a 1:1 mixture of sand and plain field soil. Around eight of the 16 seedlings, we planted a circle of 10 competitors consisting of two seedlings of each of five native plant species that are frequently dominant in Swiss grasslands managed at low intensity (Holcus lanatus, Lolium perenne, Plantago lanceolata, Poa pratensis, and Trifolium repens, obtained from UFA Samen). Two weeks after transplanting, all pots were transferred from the greenhouse to an adjacent common garden (Muri near Bern, Switzerland) and randomly assigned to eight blocks, with the restriction that each species-by-treatment combination was represented once in each block. Plants were watered when needed.
At the end of the experiment, at the beginning of September 2009, we determined above-ground dry weight of all target plants growing without (Mcontrol) and with competitors (Mcompetition). As an index of competitive ability, we calculated the log-response ratio for each species (45) as:  .
.
Statistical Analysis.
When analyzing characteristics of different species, it is necessary to consider their phylogenetic relationship, because more closely related species are likely to be phenotypically more similar than others. Therefore, we constructed a phylogenetic tree of all our species (SI Methods and Fig. S1). We tested for a phylogenetic signal for each measured variable using K statistics on a random walk model of phenotypic evolution (46).
Raw and phylogenetically corrected correlations were estimated among species traits. We used generalized least squares (GLS), implemented in R (47) with and without correcting for phylogeny (Table S4), because this allowed us to consider multiple factors and interaction models. The GLS method codes the phylogeny as a variance–covariance matrix to account for the relatedness among species. Because this method corrects the dependent variable for phylogeny, we always used the variable with the phylogenetic signal as dependent variable (48). To test whether relationships between two variables differed between wild and cultivated plant species, we used the independent variable as a covariate and included its interaction term with the factor “species status” (wild vs. cultivated). We corrected for phylogeny if at least one of the analyzed variables had a significant phylogenetic signal and used raw (i.e., uncorrected) correlations when variables had no phylogenetic signal. We also did separate phylogenetically corrected analyses for wild and cultivated species. The results corroborate the ones of the combined analyses and are presented in the supporting information (Table S2).
Because data used for estimating constitutive resistance were also used for calculating induced resistance, constitutive and induced resistance are mathematically not independent from each other, which may cause a spurious correlation (12). To account for this, we used a permutation test implemented in MATLAB by Morris et al. (12). The test is based on a modified Monte-Carlo procedure that also takes sampling variation due to limited sample size and measurement error from environmental and genetic differences into account. To test whether a possible tradeoff between induced and constitutive resistance is differently pronounced for wild and cultivated plant species, we also did this analysis separately for wild and cultivated species. Ideally, one would correct simultaneously for phylogeny and spurious correlation, but we are not aware of any current technique able to do so (2).
Supplementary Material
Acknowledgments
We thank Bill Morris for conducting the statistical tests for the tradeoff between constitutive and induced resistance; Christopher Ball for assistance in the greenhouse; Beatrice Lanzrein for providing eggs of S. littoralis; Enrico Rezende for helpful comments on the phylogenetic analysis of the data; and Sergio Rasmann, Anurag Agrawal, the editor, and two anonymous reviewers for helpful comments on the paper. This study was funded by Swiss National Science Foundation Grant 3100A0-117722.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016508108/-/DCSupplemental.
References
- 1.Karban R, Baldwin IT. Induced Responses to Herbivory. Chicago: Univ of Chicago; 1997. [Google Scholar]
- 2.Rasmann S, Agrawal AA, Cook SC, Erwin AC. Cardenolides, induced responses, and interactions between above- and belowground herbivores of milkweed (Asclepias spp.) Ecology. 2009;90:2393–2404. doi: 10.1890/08-1895.1. [DOI] [PubMed] [Google Scholar]
- 3.Han KP, Lincoln DE. The evolution of carbon allocation to plant secondary metabolites—a genetic analysis of cost in Diplacus aurantiacus. Evolution. 1994;48:1550–1563. doi: 10.1111/j.1558-5646.1994.tb02195.x. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson J, Purrington CB. Surveying patterns in the cost of resistance in plants. Am Nat. 1996;148:536–558. [Google Scholar]
- 5.Strauss SY, Rudgers JA, Lau JA, Irwin RE. Direct and ecological costs of resistance to herbivory. Trends Ecol. 2002;17:278–285. [Google Scholar]
- 6.Brody A, Karban R. Lack of a tradeoff between constitutive and induced defenses among varieties of cotton. Oikos. 1992;65:301–306. [Google Scholar]
- 7.Koricheva J, Nykänen H, Gianoli E. Meta-analysis of trade-offs among plant antiherbivore defenses: Are plants jack-of-all-trades, masters of all? Am Nat. 2004;163:64–75. doi: 10.1086/382601. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal AA, Conner JK, Rasmann S. In: Evolution After Darwin: The First 150 Years. Bell MA, Eanes WF, Futuyma DJ, Levinton JS, editors. Sunderland, MA: Sinauer Associates; 2010. pp. 242–268. [Google Scholar]
- 9.Herms DA, Mattson WJ. The dilemma of plants—to grow or defend. Q Rev Biol. 1992;67:283–335. [Google Scholar]
- 10.Mattson WJ, Lawrence RK, Haak RA, Herms DA, Charles P. In: Mechanisms of Woody Plant Defenses Against Insects. Search for Patterns. Mattson WJ, Levieux J, Bernard-Dagan C, editors. New York: Springer; 1988. pp. 3–38. [Google Scholar]
- 11.Lankau RA, Kliebenstein DJ. Competition, herbivory and genetics interact to determine the accumulation and fitness consequences of a defence metabolite. J Ecol. 2009;97:78–88. [Google Scholar]
- 12.Morris WF, Traw MB, Bergelson J. On testing for a tradeoff between constitutive and induced resistance. Oikos. 2006;112:102–110. [Google Scholar]
- 13.Blossey B, Nötzold R. Evolution of increased competitive ability in invasive non-indigenous plants: A hypothesis. J Ecol. 1995;83:887–889. [Google Scholar]
- 14.Chun YJ, van Kleunen M, Dawson W. The role of enemy release, tolerance and resistance in plant invasions: Linking damage to performance. Ecol Lett. 2010;13:937–946. doi: 10.1111/j.1461-0248.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 15.Leimu R, Koricheva J. A meta-analysis of trade-offs between plant tolerance and resistance to herbivores: Combining the evidence from ecological and agricultural studies. Oikos. 2006;112:1–9. [Google Scholar]
- 16.Coley PD, Bryant JP, Chapin FS., 3rd Resource availability and plant antiherbivore defense. Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- 17.Van Zandt PA. Plant defense, growth, and habitat: A comparative assessment of constitutive and induced resistance. Ecology. 2007;88:1984–1993. doi: 10.1890/06-1329.1. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal AA, Gorski PM, Tallamy DW. Polymorphism in plant defense against herbivory: Constitutive and induced resistance in Cucumis sativum. J Chem Ecol. 1999;25:2285–2304. [Google Scholar]
- 19.Havill NP, Raffa KF. Effects of elicitation treatment and genotypic variation on induced resistance in Populus: Impacts on gypsy moth (Lepidoptera: Lymantriidae) development and feeding behavior. Oecologia. 1999;120:295–303. doi: 10.1007/s004420050861. [DOI] [PubMed] [Google Scholar]
- 20.English-Loeb G, Karban R, Walker MA. Genotypic variation in constitutive and induced resistance in grapes against spider mite (Acari: Tetranychidae) herbivores. Environ Entomol. 1998;27:297–304. [Google Scholar]
- 21.Underwood N, Morris W, Gross K, Lockwood JR., III Induced resistance to Mexican bean beetles in soybean: Variation among genotypes and lack of correlation with constitutive resistance. Oecologia. 2000;122:83–89. doi: 10.1007/PL00008839. [DOI] [PubMed] [Google Scholar]
- 22.Siemens DH, Mitchell-Olds T. Evolution of pest-induced defenses in Brassica plants: Tests of theory. Ecology. 1998;79:632–646. [Google Scholar]
- 23.Agrawal AA. Macroevolution of plant defense strategies. Trends Ecol Evol. 2007;22:103–109. doi: 10.1016/j.tree.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal AA, Fishbein M. Plant defense syndromes. Ecology. 2006;87(7 Suppl):S132–S149. doi: 10.1890/0012-9658(2006)87[132:pds]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Coley PD. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol Monogr. 1983;53:209–233. [Google Scholar]
- 26.Fine PVA, et al. The growth-defense trade-off and habitat specialization by plants in Amazonian forests. Ecology. 2006;87(7 Suppl):S150–S162. doi: 10.1890/0012-9658(2006)87[150:tgtahs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Valverde PL, Fornoni J, Núñez-Farfán J. Evolutionary ecology of Datura stramonium: Equal plant fitness benefits of growth and resistance against herbivory. J Evol Biol. 2003;16:127–137. doi: 10.1046/j.1420-9101.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- 28.Lambers H, Poorter H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv Ecol Res. 1992;23:187–261. [Google Scholar]
- 29.De Jong TJ, van der Meijden E. In the correlation between allocation to defence and regrowth in plants. Oikos. 2000;88:503–508. [Google Scholar]
- 30.Muola A, Mutikainen P, Laukkanen L, Lilley M, Leimu R. Genetic variation in herbivore resistance and tolerance: The role of plant life-history stage and type of damage. J Evol Biol. 2010;23:2185–2196. doi: 10.1111/j.1420-9101.2010.02077.x. [DOI] [PubMed] [Google Scholar]
- 31.Ito K, Sakai S. Optimal defense strategy against herbivory in plants: Conditions selecting for induced defense, constitutive defense, and no-defense. J Theor Biol. 2009;260:453–459. doi: 10.1016/j.jtbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Zangerl AR, Rutledge CE. Probability of attack and patterns of constitutive and induced defense: A test of optimal defense theory. Am Nat. 1996;147:599–608. [Google Scholar]
- 33.Cipollini DF, Bergelson J. Interspecific competition affects growth and herbivore damage of Brassica napus in the field. Plant Ecol. 2001;162:227–231. [Google Scholar]
- 34.Cipollini DF. Does competition magnify the fitness costs of induced response in Arabidopsis thaliana? A manipulative approach. Oecologia. 2002;131:514–520. doi: 10.1007/s00442-002-0909-5. [DOI] [PubMed] [Google Scholar]
- 35.Kong C, Hu F, Xu X. Allelopathic potential and chemical constituents of volatiles from Ageratum conyzoides under stress. J Chem Ecol. 2002;28:1173–1182. doi: 10.1023/a:1016229616845. [DOI] [PubMed] [Google Scholar]
- 36.Brown ES, Dewhurst CF. The genus Spodoptera (Lepidoptera, Noctuidae) in Africa and the Near East. Bull Entomol Res. 1975;65:221–262. [Google Scholar]
- 37.Hendriks RJJ, de Boer NJ, van Groenendael JM. Comparing the preferences of three herbivore species with resistance traits of 15 perennial dicots: The effects of phylogenetic constraints. Plant Ecol. 1999;143:141–152. [Google Scholar]
- 38.Schädler M, Roeder M, Brandl R, Matthies D. Interacting effects of elevated CO2, nutrient availability and plant species on a generalist invertebrate herbivore. Glob Change Biol. 2007;13:1005–1015. [Google Scholar]
- 39.Kempel A, Brandl R, Schädler M. Symbiotic soil microorganisms as players in aboveground plant-herbivore interactions—the role of rhizobia. Oikos. 2009;118:634–640. [Google Scholar]
- 40.Kempel A, Schmidt AK, Brandl R, Schädler M. Support from the underground: Induced plant resistance depends on arbuscular mycorrhizal fungi. Funct Ecol. 2010;24:293–300. [Google Scholar]
- 41.Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR. The carbon-nutrient balance hypothesis: Its rise and fall. Ecol Lett. 2001;4:86–95. [Google Scholar]
- 42.Raubenheimer D, Simpson SJ. Analysis of covariance: An alternative to nutritional indices. Entomol Exp Appl. 1992;62:221–231. [Google Scholar]
- 43.Horton DR, Redak RA. Further comments on analysis of covariance in insect dietary studies. Entomol Exp Appl. 1993;69:263–275. [Google Scholar]
- 44.Gibson DJ. Methods in Comparative Plant Population Ecology. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 45.Weigelt A, Jolliffe P. Indices of plant competition. J Ecol. 2003;91:707–720. [Google Scholar]
- 46.Blomberg SP, Garland T, Jr, Ives AR. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 47.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 48.Revell LJ. Phylogenetic signal and linear regression on species data. Method Ecol Evol. 2010;1:319–329. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.