Abstract
NMDA receptors are key regulators of synaptic plasticity, and their hypofunction is thought to contribute to the pathophysiology of CNS disorders. Furthermore, NMDA receptors participate in the formation, maintenance, and elimination of synapses. The consequences of NMDA receptor hypofunction on synapse biology were explored in a genetic mouse model, in which the levels of NMDA receptors are reduced to 10% of normal levels (i.e., NR1-knockdown mice). In these mice, synapse number is reduced in an age-dependent manner; reductions are observed at the postpubertal age of 6 wk, but normal at 2 wk of age. Efforts to uncover the biochemical underpinnings of this phenomenon reveal synapse-specific reductions in 14–3-3ε protein and in Disrupted in Schizophrenia-1 (DISC1), two schizophrenia susceptibility factors that have been implicated in the regulation of spine density. Subchronic administration of MK-801, an NMDA receptor antagonist, produces similar synaptic reductions in both spine density and DISC1, indicating that synaptic levels of DISC1 are regulated by NMDA receptor function. The synaptic reduction of DISC1 and 14–3-3ε is developmentally correlated with the age-dependent decrease in striatal spine density.
Keywords: glutamate, neurodevelopmental
A defining feature of neurons is their ability to alter the number and strength of synaptic connections with experience. At the cellular level, changes in synapse number, or postsynaptic spine density, occur with learning and memory formation (1) or exposure to psychoactive drugs (2), and in neurodevelopmental diseases including schizophrenia (3, 4), fragile-X mental retardation (5), and Rett syndrome (6). At the molecular level, NMDA-type glutamate receptors have long been appreciated for their role in the formation and maintenance of glutamatergic synapses (7), and as mediators of synaptic plasticity (8). Several studies have shown a positive correlation between NMDA receptor activity and spine density (9–12), with notable exceptions (13, 14). However, the molecular mechanisms by which NMDA receptors regulate spine density remain to be fully elucidated. In the case of disease states such as schizophrenia, a fuller understanding of this molecular machinery may point to new therapeutic strategies.
The striatum represents an ideal brain region in which to further explore the biochemical mechanisms by which NMDA receptors regulate spine density, because the vast majority of neurons (95%) within this brain structure are medium spiny neurons (MSNs), which have densely spinous dendrites, upon which glutamate and dopamine afferents converge (15, 16). This neuronal homogeneity allows for ex vivo biochemical preparation of synaptic proteins from a nearly homogenous neuronal substrate. MSNs are thought to be a principal site of action of antipsychotic drugs because they express the highest levels of D2 dopamine receptors (17). Furthermore, they participate in many of the cognitive and limbic behaviors that are altered in schizophrenia (17, 18).
We hypothesized that reduced NMDA receptor function would alter the biochemical composition of striatal MSN synapses, leading to signaling and structural changes in postsynaptic spines. By studying a genetic mouse model with a 90% reduction in NMDA receptors, we found that spine density was decreased in MSNs of the striatum, and this decrease was age-dependent. Taking an unbiased, proteomic approach to investigate synapse biochemistry in these mice, we found developmentally regulated, synapse-specific decreases in two proteins, 14–3-3ε and DISC1 (Disrupted in Schizophrenia-1). Subchronic treatment of WT mice with an NMDA receptor antagonist decreased spine density and also decreased synaptic protein levels of DISC1, suggesting that DISC1 protein in particular was regulated at the synapse by NMDA receptor transmission. The developmentally-regulated decrease in 14–3-3ε, DISC1, and synapse number occurred after adolescence, mirroring the onset of symptoms for certain CNS disorders in humans.
Results
NMDA Receptor Deficient Mice Show an Age-Dependent Deficit in Synaptic Spine Density.
To examine the synaptic consequences of NMDA receptor deficiency, we used genetically engineered mice with a knockdown in expression of the common subunit of NMDA receptors, NR1 (NR1-KD mice). These mice, although they do not recapitulate a human disease-causing mutation, do have a global reduction in functional NMDA receptors, and display behaviors similar to those induced by psychotomimetic doses of NMDA receptor antagonists (19). Furthermore, their abnormal behaviors (including hyperactivity, reduced sociability, reduced sensorimotor gating) are considered endophenotypes of schizophrenia and can be normalized to some extent by administration of antipsychotic agents (19–22).
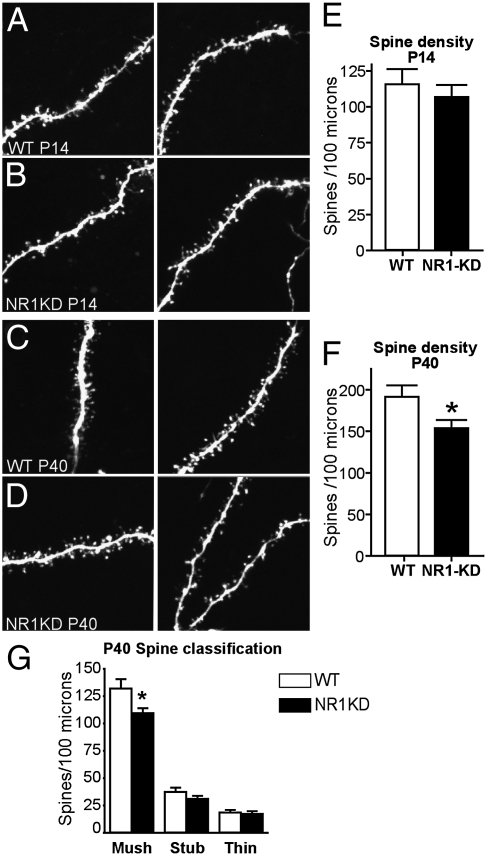
The integrity of synapses was investigated by measuring the density of postsynaptic spines from MSNs of the striatum. Spine density was reduced by 18% in 6-wk-old, postadolescent NR1-KD mice. This reduction was caused by a selective decrease in the number of mushroom-shaped spines, the morphology of which is thought to reflect mature synapses (Fig. 1).
Fig. 1.
NR1-KD mice show an age-dependent reduction in spine density of MSNs. Representative photomicrographs of dendritic shaft and spines taken from WT or NR1-KD mice at P14 (A and B) and P40 (C and D). Spine images were taken from acute slices by using patch-clamp cell labeling with Alexa Fluor 594 and two-photon laser scanning microscopy. (E) Quantification of spine density: combined counts of mushroom, thin, and stubby spines per 100 μm of dendrite (n = 6 neurons from three animals for each genotype; P > 0.05, two-tailed t test). (F) Quantification of spine density: combined counts of mushroom, thin, and stubby spines per 100 μm of dendrite (n = 8 neurons from three animals for each genotype; *P < 0.05, two-tailed t test). (G) Quantification of spine density in F for each of the three classes of spine morphology: mushroom shaped (Mush), stubby (Stub), and thin.
Reduced spine density could result from impaired synapse formation or maintenance. To address this question, we measured synapse number in juvenile mice at 2 wk of age. At this stage in development, when synaptic connections are forming, spine density is normal in NR1-KD mice (Fig. 1). Hence, in this model of NMDA receptor deficiency, reductions in synapse number are age-dependent and are more evident at a developmental period associated with synapse elimination (23).
Biochemical Reductions in 14–3-3ε and DISC1 Are Synapse-Specific.
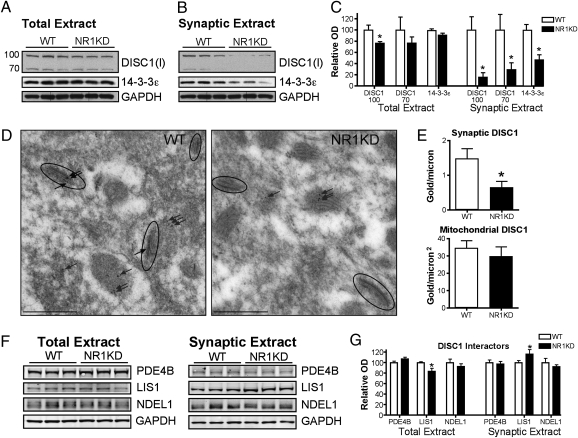
We pursued the molecular deficits underlying synaptic changes in NR1-KD mice through an unbiased proteomic approach to identify synaptic proteins that might be selectively changed in their level of expression. Synaptic fractions from striatal preparations of WT and NR1-KD mice were isolated by sucrose density gradient and used for 2D difference in gel electrophoresis (DIGE) and MS (Fig. S1). By using this approach we found that 14–3-3ε was reduced in synaptic fractions from NR1-KD striatum, whereas the total overall levels of this protein were unchanged (Fig. 2). Because the 2D-DIGE approach lacks the sensitivity to detect all protein species, we hypothesized that reduction of 14–3-3ε may point to additional changes in a molecular pathway.
Fig. 2.
NR1-KD mice have a synapse-specific depletion of 14–3-3ε and DISC1 proteins. (A) Western blot of total striatal extracts (25 μg) and (B) striatal synaptic plasma membrane fractions (15 μg protein). Monoclonal antibody to 14–3-3ε and polyclonal antibody to DISC1 (Invitrogen antibody, I) detects a 29-kDa isoform of 14–3-3ε and 100-kDa and 70-kDa isoforms of DISC1. (C) 14–3-3ε and DISC1 levels normalized to GAPDH demonstrate a synapse-specific decrease in 14–3-3ε, and for DISC1 a modest decrease in total extract, but substantial depletion of DISC1 in synaptic extracts (n = 6 for each genotype; *P < 0.05, two-tailed t test). (D) Representative photomicrographs of DISC1 labeling by postembedding immunogold transmission EM. DISC1 immunoreactivity is detected in pre- and postsynaptic membranes of asymmetric synapses of the striatum (circled synapse with arrows), and in mitochondria (uncircled arrows). (E) Quantification of DISC1 immunogold labeling on asymmetric synapses or within mitochondria (n = 3 animals for each genotype; *P < 0.05, two-tailed t test). (F) Western blot of total (25 μg) and synaptic (15 μg) striatal protein extracts to detect relative levels of PDE4B, LIS1, and NDEL1 from WT and NR1KD mice. (G) Levels normalized to GAPDH indicate no change in PDE4B or NDEL1, a modest decrease in total LIS1 levels, and an increase in synaptic LIS1 (n = 6 for each genotype; *P < 0.05, two-tailed t test).
The 14–3-3 proteins bind to phosphorylation sites on their target proteins and regulate their activity, stability, trafficking, and interactions (24). The genes encoding the 14–3-3 family of proteins, including 14–3-3ε (Ywhae), are associated with schizophrenia (25, 26), and message levels of several 14–3-3 genes are reduced in prefrontal cortex of postmortem schizophrenic brains (27). Furthermore, 14–3-3 proteins are known to interact with DISC1, placing them in a molecular pathway of schizophrenia pathogenesis (28, 29).
DISC1 is a scaffolding protein that is thought to coordinate other synaptic proteins (30–32), and indeed plays a key role in synaptic spine maintenance in association with the activation of NMDA receptors (33). Thus, we hypothesized that the results from the unbiased approach might reflect a possible defect of a molecular pathway involving DISC1 in the synapse.
We examined the molecular status of DISC1 in the synapse, and found that the pool of DISC1 in the synaptic plasma membrane fraction of striatum was also markedly diminished in NR1-KD mice (Fig. 2), whereas total DISC1 levels were less appreciably altered. Ultrastructure analysis with postembedding immunogold EM confirmed the synapse specificity of the reduction (Fig. 2). DISC1 was selectively reduced at asymmetric synapses in NR1-KD striatum, whereas the amount of mitochondrial DISC1 assessed from the same dendritic terminals was unchanged.
Reductions in DISC1 were most marked in the striatum, whereas more subtle reductions were observed in frontal cortex and hippocampus (Figs. S2 and S3). Within the striatum, synaptic reductions in protein levels of the DISC1 complex were limited to DISC1 and 14–3-3ε, and levels of other DISC1 interactors, such as LIS1, NDEL1, and PDE4B (30, 34), were unchanged or slightly elevated in synaptic preparations from NR1-KD mice (Fig. 2).
Pharmacological Blockade of NMDA Receptors Decreases Spine Density and Synaptic DISC1.
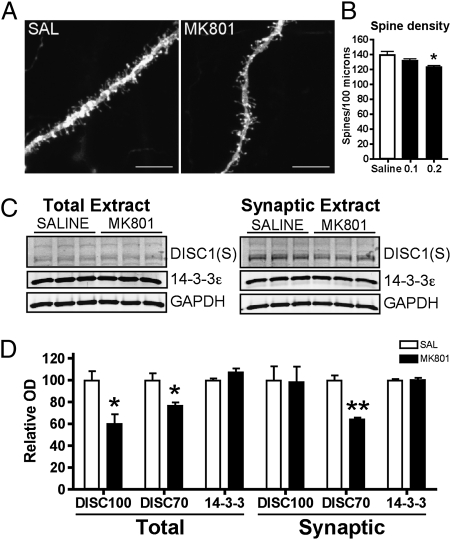
To confirm that suppression of NMDA receptor can decrease synapse number and synaptic DISC1, we pharmacologically treated WT mice subchronically with MK801, a highly selective, noncompetitive NMDA receptor antagonist. A dose of 0.2 mg/kg/h was delivered by s.c. osmotic minipump over a 1- or 2-wk period in adult mice (12 wk of age). This dose regimen does not induce toxicity (Fig. S4), but was shown to induce hyperactivity and disrupt social behaviors in WT mice to a similar extent as observed in untreated NR1-KD mice (Figs. S5 and S6). There was a dose-dependent reduction in spine density in MK801-treated mice (Fig. 3). In addition, MK801-treated mice displayed a 40% reduction in synaptic DISC1, supporting the concept that synaptic DISC1 is responsive to NMDA receptor-mediated neurotransmission (Fig. 3). Notably 14–3-3ε levels were not affected by MK801 administration, suggesting that DISC1 levels are more responsive than 14–3-3ε to transient reductions in NMDA receptor transmission, and may involve different regulatory mechanisms.
Fig. 3.
Subchronic treatment with MK-801 reduces synapse number and DISC1 protein in adult striatum. (A) Representative photomicrographs of dendritic shaft and spines from MNs of saline solution- and MK801-treated mice (0.2 mg/kg/h, 7 d). (Scale bar: 10 μm.) (B) Quantification of spine density per 100 μm of dendrite from mice treated with saline solution or 0.1 or 0.2 mg/kg/h MK801 (n = 6 neurons from three animals for each treatment; P = 0.051 two-tailed t test). (C) Total striatal protein extracts (25 μg) and synaptic plasma membrane extracts (15 μg) taken from mice treated with saline solution or a 14-d infusion of MK-801 delivered by osmotic minipump (0.2 mg/kg/h); Western blot detects 14–3-3ε and DISC1 (Santa Cruz, S). (D) Relative levels of DISC1 and 14–3-3ε normalized to GAPDH levels (n = 6 for each treatment group; *P < 0.05 and **P < 0.01, two-tailed t test).
Alterations in Synapse Biochemistry Are Age-Dependent.
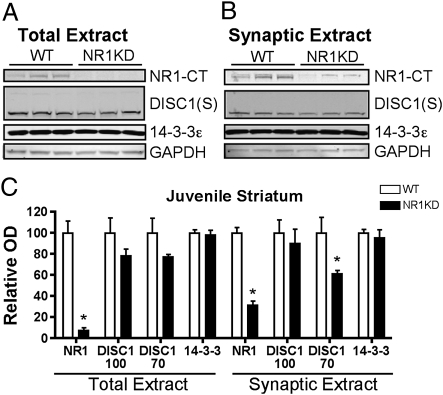
Because the reductions in striatal spine density showed an age-dependent phenotype, we asked whether the synaptic changes in 14–3-3ε and DISC1 were similarly age-related. In juvenile NR1-KD mice (aged 2 wk), there were no synaptic deficits in the levels of 14–3-3ε. DISC1 levels were only modestly decreased (Fig. 4), and the levels of PDE4B, LIS1, and NDEL1 were unchanged (Fig. S7). Although a reduction was seen in extracts from juvenile mice, the extent was not as substantial as that detected after adolescence (Fig. 2). Hence, both 14–3-3ε and DISC1 showed an age-dependent reduction in synaptic protein levels that was more evident in older mice.
Fig. 4.
Synaptic decreases in DISC1 are less substantial in juvenile NR1-KD mice. (A) Western blot of total protein extracts (25 μg) from WT and NR1-KD mice at P14, blotted for NR1 C-terminus, DISC1 (Santa Cruz, S), and 14–3-3ε. (B) Western blot of synaptic plasma membrane striatal extracts (15 μg) from the same experimental groups. (C) Relative levels of proteins normalized to GAPDH levels (n = 6 for each genotype; *P < 0.05, two-tailed t test).
The developmental trajectory of these protein and synapse deficits showed that the decrease in synaptic DISC1 preceded the reduction in spine density in NR1-KD mice compared with controls. Spine density was normal in NR1-KD mice at 2 wk of age; however, at this stage in development, the reduction in DISC1 protein was already evident, albeit modest (25% reduction; Figs. 1 and 4). At later ages, the reduction in DISC1 protein was robust (50%–80% reduction), with an accompanying decrease in spine density (Figs. 1 and 2). This phenomenon was not caused by developmental changes in the mutation's effect on NR1 protein, because juvenile NR1-KD mice had reductions in NR1 subunit levels that were 10% to 20% of WT levels (Fig. 4), which was similar to the reduction observed in adult NR1-KD striatum (20).
Discussion
By using NR1-KD mice as a model of impaired NMDA receptor function, we discovered age-dependent changes in synapse number and in two proteins that are implicated in schizophrenia and are reported to regulate spine density, 14–3-3ε and DISC1.
NR1-KD mice have global reductions in the levels of NMDA receptors caused by a hypomorphic mutation of Grin1. The nature of the mutation is particularly valuable for understanding the role NMDA receptors play in the developing brain, in contrast to acute pharmacological models of NMDA receptor antagonism. Furthermore, as they have a cis-acting mutation, the NR1-KD mice have reductions in NMDA receptors that correlate with the normal temporal expression of Grin1 through development, allowing perturbations of NMDA signaling during prenatal and postnatal development.
Our observation of decreased striatal spine density in NR1-KD mice is consistent with other studies in hippocampal and cortical neurons that have shown a positive correlation between NMDA receptor activity and spine density (9–12). However, there are studies in which cell-specific genetic deletion of NMDA receptors does not result in reduced spine density (13, 14). Furthermore, acute NMDA receptor antagonism with ketamine has recently been shown to increase spine density, at least transiently, in the cortex (35). In the reports of cell-specific, genetic deletion of NMDA receptors, temporal differences in the developmental stage of Cre expression may explain the variable effects on synapse number (10, 11, 13, 14). With pharmacological studies, it is conceivable that transient and sustained NMDA receptor blockade have opposite effects on synapse function and ultimately on spine density. As in the genetic models, it is important to note the developmental stage at which NMDA receptor antagonism is induced. In the present study, subchronic MK801 was delivered in adult mice, aged 12 wk; at this stage of development, synapse number is fairly stable. It is possible that the degree of DISC1 reduction, or reduction in spine density, might have been greater if NMDA receptor signaling were perturbed during early postnatal development, as was the case in the NR1-KD model.
In examining the molecular underpinnings of reduced synaptic number, our study provides the initial evidence for a role for NMDA receptors in regulating synaptic levels of DISC1 and its interactor 14–3-3ε. The molecular interactions of both DISC1 and 14–3-3ε are numerous, yet both proteins have been reproducibly implicated in the processes regulating neurite outgrowth and spine density (26, 31, 33, 36, 37). 14–3-3ε binds phosphorylated NDEL1 and serves as a positive regulator of NDEL1 function (37), and active NDEL1 is required for neurite outgrowth (38). Furthermore, DISC1 itself is reported to regulate Rac1-mediated spine remodeling through its synaptic interaction with kalirin, and this pathway is sensitive to NMDA receptor activation (33), suggesting that DISC1 functional activity is downstream of NMDA receptor signaling. In the present study, we demonstrate that protein levels of DISC1 and synapse numbers are also regulated by NMDA receptor signaling. This was supported not only in NR1-KD mice, but also by pharmacological blockade of NMDA receptors with MK801 in WT mice, at a dose that induces schizophrenia-like endophenotypes. Importantly, these findings form a link between those schizophrenia susceptibility factors that reduce NMDA receptor activity or function and synaptic DISC1 biology.
In schizophrenia, changes in dendritic complexity and spine density have been detected in the cerebral cortex (3, 4). In the NR1-KD mice, our cursory assessment of cortical synaptic biochemistry did not reveal substantial changes in these two proteins. The striatum-selective reduction in synaptic DISC1 in NR1-KD mice suggests that (i) MSNs may be particularly responsive to alterations in NMDA receptor signaling or (ii) our experimental conditions were not sensitive enough to detect cell-specific changes in the cerebral cortex. For example, heterogeneous subsets of cortical cells may mask pyramidal neuron-specific changes in synaptic DISC1. Nonetheless, our findings are in line with the proposed differential cellular roles of DISC1 in various brain regions and cell types (39).
The delayed emergence of synaptic and biochemical deficits in postadolescent and adult NR1-KD mice may point to a differential reliance on NMDA receptors during synapse formation, synapse elimination, and synapse maintenance (12). The deficit in NMDA receptor levels did not prevent synapse formation in the developing brain of NR1-KD mice, but was sufficient to reduce spine density after adolescence, suggesting a greater necessity for intact NMDA receptor levels during synapse elimination or maintenance. Several recent studies illustrate how the cellular and behavioral consequences of developmental perturbations in neuron function may not become evident until adulthood (40–43). Collectively, these studies and the present one demonstrate how genetic factors may act in an age-dependent fashion to produce adult-onset symptoms of CNS conditions associated with aberrant synaptic function. Furthermore, they highlight the relevance of multiple neuron types and brain regions, including the striatum, in the expression of cellular and behavioral endophenotypes associated with these conditions.
Materials and Methods
Animals.
The hypomorphic mutation in Grin1 to generate NR1 knockdown in NR1-KD mice was achieved by targeted insertion of a neomycin cassette into intron 17 as described (19). Experimental mice were generated from intercross of congenic C57BL/6J NR1-KD heterozygotes with congenic 129X1Sv/J NR1-KD heterozygotes. This breeding strategy was required to generate NR1-KD mice at Mendelian ratios on a defined genetic background, as C57BL/6J congenics had a high mortality rate. Mice were housed with a 12-h light/dark cycle (0700 hours to 1900 hours). Animal husbandry and experimentation was in accordance with Duke University Medical Center Institutional Animal Care and Use Committee approval and National Institutes of Health Guidelines for Care and Use of Animals.
Subcellular Fractionation of Protein Extracts.
Preparation of synaptic plasma membrane extracts was performed according to the method described previously (44, 45), and all steps were performed at 4 °C in the presence of protease inhibitors (Complete tablets with EDTA; Roche). Mice were killed by cervical dislocation, and striatum was rapidly dissected on ice. Pooled tissues from three to four mice were homogenized in 0.32M sucrose, 4 mM Hepes, pH 7.4, with a motor-driven Teflon/glass homogenizer. Total protein extract was reserved, and the remaining sample was centrifuged at 900 × g to remove nuclei. The supernatant was washed twice by centrifugation at 10,000 × g and resuspension in 0.32 M sucrose, and membranes in the resulting pellet were lysed by osmotic shock in water. These membranes were separated on a discontinuous sucrose gradient (1.2 M, 1.0 M, 0.8 M; 4 mM Hepes) by ultracentrifugation at 200,000 × g, and membranes at the 1.2-M interphase were collected as synaptic plasma membranes.
Western Blot.
Protein extracts were resolved on 10% polyacrylamide gels and transferred to nitrocellulose membranes. Primary antibody incubations were performed overnight at 4 °C, and the following antibodies were used: rabbit anti-DISC1 (Mid; 1:250; Invitrogen), goat anti-DISC1 (1:500; Santa Cruz), sheep anti-PDE4B (1:3,000; gift from Miles Houslay, University of Glasgow, Glasgow, Scotland), rabbit anti-LIS1 (1:500; Abcam), rat anti-NDEL1 (1:100; gift from A. Kamiya, Johns Hopkins University, Baltimore, MD), mouse anti–NR1-CT (1:1,000; Upstate), mouse anti-GAPDH (1:3,000; Abcam). See Fig. S8 for validation of DISC1 antibodies in F1 genetic background. For all antibodies, 5% milk/Tris-buffered saline–Tween-20 (TBST) was used for blocking and antibody steps, with the exception of anti-GAPDH, which required 5% BSA/TBST. Species-appropriate secondary antibodies were conjugated to HRP (Pierce) or infrared dye (Rockland Immunochemicals), and detected by enhanced chemiluminescence (Pierce) or Li-Cor Odyssey infrared imaging. Quantification of immunoblot labeling was performed with ImageJ64 (National Institutes of Health). Protein levels were normalized to GAPDH and expressed as relative optical density, and two-tailed t test was used to determine statistical significance using Excel software.
Dendritic Spine Imaging and Measurement.
Acute striatal slices (350 μm thick) were prepared from postnatal day (P) 14 and P40 mutant and WT littermate mice by using an ice-cold cutting solution containing (in mM):110 choline chloride, 25 NaHCO3, 25 D-glucose, 11.6 sodium ascorbate, 7 MgSO4, 3.1 sodium pyruvate, 2.5 KCl, 1.25 NaH2PO4, and 0.5 CaCl2. Slices were incubated in gassed (95% O2/5% CO2) artificial CSF [containing (in mM): 127 NaCl, 25 NaHCO3, 25 D-glucose, 2.5 KCl, 1.0 MgCl2, 2.0 CaCl2, and 1.25 NaH2PO4] at 34 °C for 45 to 60 min and then at room temperature until used. Experiments were performed at room temperature (25 °C) in artificial CSF (flowing at 4–6 mL/min). Whole-cell patch electrodes (4–5 MΩ) contained (in mM) 130 KMeSO3, 10 Hepes, 10 sodium phosphocreatine, 3 ascorbate, 4 MgCl2, 4 Na2-ATP, 0.4 Na-GTP, 0.2 EGTA, and 0.04 Alexa 594. MSNs were first identified by cell soma shape using a 60× phase-contrast objective, and by membrane resting potential (−80 mV) once patched.
Imaging of patched MSNs was achieved with a custom-built two-photon laser scanning microscope. Fluorescence was detected in epifluorescence mode by using photomultiplier tubes (R3896; Hamamatsu Photonics). Image acquisition and data analysis was controlled by custom software (Matlab; Mathworks) (46).
Alternately, spine density was determined by diolistic labeling of perfusion fixed tissue. Mice were anesthetized with isoflurane and perfusion-fixed with 4% paraformaldehyde, pH 7.4. Coronal sections (100 μm) of striatum were obtained by vibratome and neurons were randomly labeled using DiI-coated, 1-μm gold particles delivered with a BioRad Gene Gun (47). One hour after labeling, sections were mounted on a glass slide and imaged by confocal microscopy. MSNs were identified by location and morphology within the striatum. Spine density was determined using Nikon Elements software.
Subchronic MK-801 Infusion.
Microosmotic pumps (model 1002; Alzet) were prepared to deliver a constant infusion of saline solution or 0.1 or 0.2 mg/kg/h of MK801 (Sigma) dissolved in saline solution. WT male animals aged 12 wk were used for the study, and were derived from the same breeding stock as described earlier (F1 hybrid mice). Animals were anesthetized with isoflurane and minipumps were implanted s.c. at the dorsal scruff of the neck. Seven to 14 d after implantation, animals were tested for locomotor and social behavior (SI Materials and Methods), and euthanized for biochemistry or spine density measurement.
Methods for 2D gel electrophoresis and in-gel analysis, DISC1 immunofluorescence, GFAP immunofluorescence, immunogold EM, and locomotor and social behavior studies are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We gratefully acknowledge the gift of antibody reagents from Dr. Miles Houslay (PDE4B), Dr. Qi Wang (D440 DISC1), and Dr. Atsushi Kamiya (NDEL1). We thank Dr. Sara Miller and Phillip Christopher of the Duke Electron Microscopy Service for experimental advice and tissue processing, Dr. Tanya Daigle for expert advice, Katherine Harley and Wendy Horsfall for technical assistance, and Sam Ngu for assistance with gold particle quantification. In addition, we thank Drs. Nicholas Brandon, Raul Gainetdinov, and Akiko Hayashi-Takagi for helpful discussion. This work was supported in part by National Institute on Drug Abuse Grant K01DA017703 (to A.J.R.); National Institute of Mental Health (NIMH) Grants R01MH079201, R01MH073853, and U19MH082441 (to M.G.C.); NIMH Grant R01MH080047 (to R.Y.); NIMH Grants R01MH069853, R01MH085226, R01MH088753, and MH084018 (Silvo O. Conte Center Grant; to A. Sawa); National Institute of Neurological Disorders and Stroke Grant R01NS068410 (to R.Y.); a National Alliance for Research in Schizophrenia and Affective Disorders Young Investigator Award (to A.J.R.); a National Alliance for Research in Schizophrenia and Affective Disorders Staglin Award (to A. Sawa); the Stanley Foundation (A. Sawa); the Howard Hughes Medical Institute (R.Y.); the Children's Tumor Foundation (A.F.O.); and the Portuguese Foundation for Science and Technology (A.F.O.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012621108/-/DCSupplemental.
References
- 1.Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KW, et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garey LJ, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- 6.Chapleau CA, et al. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol Dis. 2009;35:219–233. doi: 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 8.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 9.Das S, et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- 10.Brigman JL, et al. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ultanir SK, et al. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci USA. 2007;104:19553–19558. doi: 10.1073/pnas.0704031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts AC, et al. Downregulation of NR3A-containing NMDARs is required for synapse maturation and memory consolidation. Neuron. 2009;63:342–356. doi: 10.1016/j.neuron.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rampon C, et al. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 14.Dang MT, et al. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci USA. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 16.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Kellendonk C, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 20.Ramsey AJ. NR1 knockdown mice as a representative model of the glutamate hypothesis of schizophrenia. Prog Brain Res. 2009;179:51–58. doi: 10.1016/S0079-6123(09)17906-2. [DOI] [PubMed] [Google Scholar]
- 21.Duncan GE, et al. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Halene TB, et al. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav. 2009;8:661–675. doi: 10.1111/j.1601-183X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 24.Aitken A. 14-3-3 proteins: A historic overview. Semin Cancer Biol. 2006;16:162–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Wong AH, et al. Identification of candidate genes for psychosis in rat models, and possible association between schizophrenia and the 14-3-3eta gene. Mol Psychiatry. 2003;8:156–166. doi: 10.1038/sj.mp.4001237. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda M, et al. Identification of YWHAE, a gene encoding 14-3-3epsilon, as a possible susceptibility gene for schizophrenia. Hum Mol Genet. 2008;17:3212–3222. doi: 10.1093/hmg/ddn217. [DOI] [PubMed] [Google Scholar]
- 27.Middleton FA, Peng L, Lewis DA, Levitt P, Mirnics K. Altered expression of 14-3-3 genes in the prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology. 2005;30:974–983. doi: 10.1038/sj.npp.1300674. [DOI] [PubMed] [Google Scholar]
- 28.Taya S, et al. DISC1 regulates the transport of the NUDEL/LIS1/14-3-3epsilon complex through kinesin-1. J Neurosci. 2007;27:15–26. doi: 10.1523/JNEUROSCI.3826-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozeki Y, et al. Disrupted-in-Schizophrenia-1 (DISC-1): Mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci USA. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camargo LM, et al. Disrupted in Schizophrenia 1 Interactome: Evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- 31.Brandon NJ, et al. Understanding the role of DISC1 in psychiatric disease and during normal development. J Neurosci. 2009;29:12768–12775. doi: 10.1523/JNEUROSCI.3355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, et al. The psychiatric disease risk factors DISC1 and TNIK interact to regulate synapse composition and function. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.87. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi-Takagi A, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millar JK, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 35.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kvajo M, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci USA. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyo-oka K, et al. 14-3-3epsilon is important for neuronal migration by binding to NUDEL: A molecular explanation for Miller-Dieker syndrome. Nat Genet. 2003;34:274–285. doi: 10.1038/ng1169. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi MA, et al. Assessing the role of endooligopeptidase activity of Ndel1 (nuclear-distribution gene E homolog like-1) in neurite outgrowth. Mol Cell Neurosci. 2010;44:353–361. doi: 10.1016/j.mcn.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 40.Cahill ME, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci USA. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belforte JE, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niwa M, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayhan Y, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: Evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2010 doi: 10.1038/mp.2009.144. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cotman CW, Taylor D. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol. 1972;55:696–711. doi: 10.1083/jcb.55.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 46.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: Flexible software for operating laser scanning microscopes. Biomed Eng Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–225. doi: 10.1016/s0896-6273(00)00031-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.