Abstract
Alzheimer disease is a major cause of cognitive failure, and a pathogenically related but more subtle process accounts for many cases of mild memory symptoms in older humans. Insoluble fibrillar plaques of amyloid β-proteins (Aβ) and neurofibrillary deposits of hyperphosphorylated tau proteins are the diagnostic lesions of AD, but their temporal mechanistic relationship has long been debated. The recent recognition that small, diffusible oligomers may be the principal bioactive form of Aβ raises the key question of whether these are sufficient to initiate cytoskeletal change and neurite degeneration. A few studies have examined the effects of oligomers of synthetic Aβ peptides of one defined length at supraphysiological concentrations, but the existence of such assemblies in the AD brain is not established. Here, we isolated Aβ dimers, the most abundant form of soluble oligomer detectable in the human brain, from the cortices of typical AD subjects and found that at subnanomolar concentrations, they first induced hyperphosphorylation of tau at AD-relevant epitopes in hippocampal neurons and then disrupted the microtubule cytoskeleton and caused neuritic degeneration, all in the absence of amyloid fibrils. Application of pure, synthetic dimers confirmed the effects of the natural AD dimers, although the former were far less potent. Knocking down endogenous tau fully prevented the neuritic changes, whereas overexpressing human tau accelerated them. Coadministering Aβ N-terminal antibodies neutralized the cytoskeletal disruption. We conclude that natural dimers isolated from the AD brain are sufficient to potently induce AD-type tau phosphorylation and then neuritic dystrophy, but passive immunotherapy mitigates this.
Alzheimer disease (AD) and its harbinger, mild cognitive impairment–amnestic type, comprise the most prevalent late-life cognitive disorder in humans. The aging of the population in developed nations has led to predictions that the prevalence of Alzheimer-type dementia will rise substantially during the next few decades. Intensive research over almost 30 y has led to the hypothesis that progressive cerebral accumulation of the 42-residue amyloid β-protein (Aβ) may precipitate the synaptic dysfunction and cytoskeletal changes that underlie the symptoms of AD (1). Although insoluble amyloid plaques are one of the two neuropathological hallmarks of AD, recent studies suggest that these are in equilibrium with small, diffusible oligomers of Aβ that may serve as the principal synaptotoxic form of the protein (2).
A major unresolved question about AD pathogenesis is the relationship of Aβ deposits to the other cardinal lesion of the disease, the neurofibrillary tangle. These two lesions occur together in virtually all cases of AD, but whether Aβ build-up is directly responsible for the neurofibrillary degeneration of AD is the subject of debate. Specifically, the growing experimental evidence that key features of the AD phenotype, such as dendritic spine loss, altered hippocampal synaptic plasticity, and impaired memory can be triggered by Aβ oligomers (3–9)—including those isolated directly from patients’ brains (10)—raises the question of whether soluble Aβ oligomers are responsible by themselves for inducing altered tau phosphorylation, cytoskeletal change, and degeneration of neurites. Here, we address this central issue by isolating Aβ dimers, the major form of soluble oligomer that can be detected and isolated from human brain (10), from the cerebral cortex of typical AD cases and showing that they first induce tau phosphorylation at specific epitopes characteristic of AD in primary hippocampal neurons, and then produce cytoskeletal collapse and neuritic degeneration, but knock-down of endogenous tau fully prevents this phenotype. Two key advantages of our approach are: (i) it examines natural oligomers of the heterogeneous Aβ peptides that exist at low nanomolar concentrations in AD patients, and (ii) it uses a cell-culture system to apply in a systematic fashion biochemically fractionated and well-defined Aβ species, something not possible in in vivo mouse models, where a complex array of Aβ assembly forms coexist.
Results
Soluble Aβ Oligomers Isolated from the AD Cortex Induce Marked Cytoskeletal Abnormalities at Subnanomolar Concentrations in Primary Hippocampal Neurons.
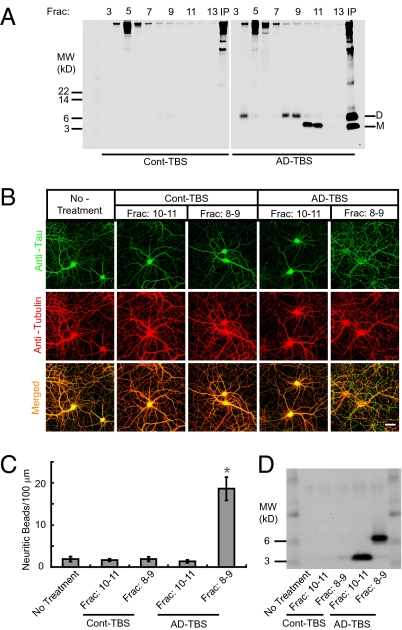
To determine whether soluble Aβ oligomers are sufficient to trigger neurofibrillary degeneration, even in the absence of amyloid plaques, we isolated Aβ dimers, the most abundant detectable form of soluble oligomer, from the AD cerebral cortex as recently described (10). These dimers are principally composed of Aβ42 (10). Soluble (Tris-buffered saline, TBS) extracts from cortical homogenates of humans diagnosed with clinically and neuropathologically typical AD (AD-TBS extracts) or age-matched non-AD subjects (Cont-TBS extracts) were immunoprecipitated with 3D6, a monoclonal antibody specific for the free N terminus (Asp-1) of human Aβ, and the immunoprecipitate was eluted with lithium dodecyl sulfate (LDS) and subjected to size exclusion chromatography (a method we refer to as IP-SEC). Aβ monomers and LDS-stable dimers could be separated and detected in the SEC fractions of the AD-TBS extracts but not in the corresponding fractions of Cont-TBS, as expected (Fig. 1A). Importantly, SDS-stable higher oligomers, including dodecamers, were not detectable by IP-SEC in AD-TBS extracts (Fig. 1A); accordingly, we focused on the dimers, cortical levels, of which were recently shown by others to correlate strongly with several features of the AD phenotype (Mini-Mental State Examination score; Blessed cognitive score; Braak score; synaptic protein levels) (11). We prepared primary cultures of hippocampal neurons from E18 rat embryos. After 18 d in culture, mature hippocampal neurons were exposed to either the monomer-rich or the dimer-rich SEC fractions of the AD-TBS extracts [or the corresponding fractions of Cont-TBS, which share other cortical proteins with the AD-TBS SEC fractions (Fig. 1A) but contain no Aβ] for 3 d, at which time the cultures were fixed for immunocytochemistry. Application of IP-SEC fractions 8–9 of the AD-TBS extract, which contain soluble, SDS-stable Aβ dimers but few or no monomers (Fig. 1A), induced a marked degeneration of neurites after 3 d of exposure, as indicated by extensively beaded, dystrophic neurites and an abnormal microtubule cytoskeleton revealed by Tubulin and tau immunocytochemistry (Fig. 1 B and C, and Fig. S1). In contrast, applications of the Aβ monomer-rich fractions (fractions 10–11) of AD-TBS from the same SEC run or else either of the corresponding SEC fractions (fractions 8–9, 10–11) of Cont-TBS induced no significant neuritic alteration (Fig. 1B and C). The application of the Aβ dimers to hippocampal neurons for 2 d did not induce significant neuritic degeneration, but at higher magnification we observed some slightly beaded neurites (Fig. S1), suggesting the initiation of a progressive neurite degeneration. Following the 3-d treatment, we were able to recover residual Aβ monomers or dimers by immunoprecipitating the conditioned media (Fig. 1D), thereby verifying the continuous exposure of the neurons to these soluble Aβ species. The concentration of human Aβ dimers in the final culture medium was around 0.5 nM, as determined by ELISA or quantitative Western blot, values similar to those observed for Aβ in the AD cortex (10). Although exposure to the Aβ dimers invariably disrupted the neuritic cytoskeleton, neuronal cell bodies generally survived the 3-d treatment, and there was no significant increase in the very low baseline levels of apoptotic cells in the cultures, as revealed by TUNEL staining (Fig. S2). The collapse of the cytoskeleton was Aβ-dependent, because prior immunodepletion of the dimer-rich SEC fractions by the Aβ antiserum AW7 fully prevented the effects of the dimers, whereas its preimmune serum was without effect (Fig. S3).
Fig. 1.
Cytoskeletal abnormalities induced in primary hippocampal neurons by soluble Aβ oligomers isolated by SEC from the AD cerebral cortex. (A) AD-TBS (Right) or Cont-TBS (Left) was immunoprecipitated with 3D6 (3 μg/mL), eluted with sample buffer containing 4% LDS, and subjected to SEC. SEC of the immunoprecipitaton of AD-TBS resolves Aβ dimers (fractions 8–9) from monomers (fractions 10–11), as detected by Western blot with 6E10 + 2G3 + 21F12. IP, 3D6 immunoprecipitates of the starting Cont-TBS or AD-TBS extracts used for this SEC. (B) Confocal images showing the tau (green) and Tubulin (red) immunoreactivities of the cytoskeleton of hippocampal neurons [days in vitro (DIV) 21] after 3-d treatment with Aβ monomers (SEC fractions 10–11) or Aβ dimers (SEC fractions 8–9) isolated from AD-TBS or the corresponding fractions from Cont-TBS. (Scale bar, 50 μm.) (C) Histograms represent the average number of tau-positive beads along 100-μm lengths of Tubulin-positive neurites under different conditions. Asterisk indicates data significantly different from those of neurons without treatment (P < 0.01 by Student t test). Error bars, SEM. (D) After a 3-d incubation on primary neurons, the conditioned media were immunoprecipitated with Aβ antiserum AW7. Aβ monomers and dimers were precipitated from medium that contained the reconstituted IP-SEC fractions of AD-TBS but not from that with Cont-TBS.
Importantly, when hippocampal neurons were cultured for 7 d, application of the soluble Aβ dimers did not induce a collapse of the cytoskeleton or other changes (Fig. S4A), in accord with evidence that the neurotoxicity of synthetic Aβ (at much higher concentrations) requires the maturation of neurons in vitro (12, 13). To explore the basis for this intriguing selectivity, we performed quantitative Western blotting of lysates from hippocampal neurons cultured for 7 or 18 d and found that in the 18-d neurons, the expression of mature tau (∼55 kDa) was significantly increased, but the phosphorylation of the tau kinase GSK3β at serine 9, which is a negative regulator of GSK3β activity (14), was decreased (Fig. S4B). These results suggest that the time-dependent maturation of tau and of GSK3β activity (the latter creates the AT8 phosphoepitope on tau; see below) is associated with the ability of Aβ dimers to induce neurite degeneration. However, more work is needed to identify additional changes underlying the selective vulnerability of older cultured neurons to Aβ oligomers.
Pure Synthetic Aβ Dimers Induce Cytoskeletal Disruption Similar to That Caused by Natural Dimers Isolated from the AD Brain.
In light of several studies describing the neurotoxic effects of synthetic Aβ aggregates or cell-secreted Aβ oligomers (3, 5–8, 15–17), we assessed the effects of such preparations on the neuritic cytoskeleton in an attempt to confirm the changes described above with natural dimers isolated from the AD cortex. We examined a synthetic human Aβ40 peptide in which serine 26 is mutated to cysteine (Aβ40 S26C), enabling the formation of stable, disulfide-bonded dimers under oxidizing conditions (10, 18). This pure dimer was sufficient to induce an increasing alteration of the microtubule cytoskeleton and neuritic architecture in a dose-dependent manner, with a strong effect similar to that of the AD-TBS extracts observed at 500-nM concentration (Fig. S5 A and B). A minor degree of neuritic beading could be observed at Aβ40 S26C dimer concentrations as low as 100 nM (Fig. S5B). Importantly, the synthetic dimers always required much higher concentrations (>100-fold) to induce cytoskeletal effects comparable to those of the natural dimers isolated from AD cortex.
Disruption of the Neuritic Cytoskeleton Induced by Soluble Aβ Oligomers Is Dependent on Tau Expression.
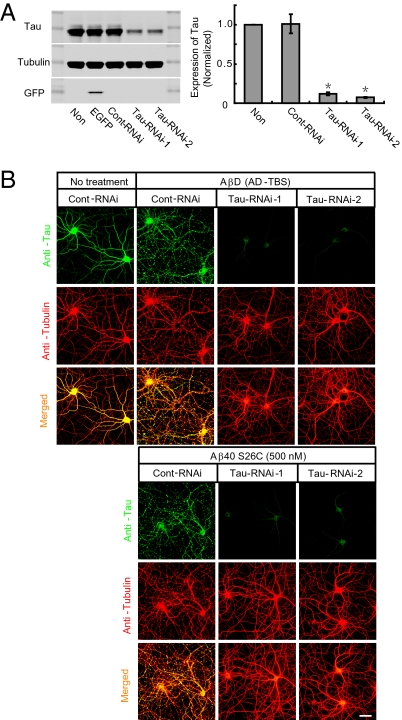
Neurofibrillary tangles and dystrophic neurites, the key cytopathological lesions of neurons in AD, contain abnormal filaments of hyperphosphorylated tau proteins (19–21). Numerous studies suggest that the tau protein is a mediator of the neuronal degeneration induced by supraphysiological concentrations of synthetic Aβ fibrils in vitro (8, 16, 22) and of the memory deficits of transgenic mice expressing mutant human amyloid precursor protein (APP) (23). Accordingly, we asked whether the neuritic cytokeletal disruption caused by natural Aβ oligomers in the absence of amyloid fibrils was dependent on tau expression. Two weeks after neurons were transduced with lentivirus encoding siRNA against tau, we detected a highly significant decrease (>85%) of tau expression compared with control siRNA or no transduction (Fig. 2A). When SEC fractions containing soluble Aβ dimers from AD-TBS or else pure synthetic Aβ40 S26C dimers (500 nM) were applied, these each induced neuronal cytoskeletal disruption in control siRNA-treated neurons, but had no significant effect on the tau knock-down neurons (Fig. 2B).
Fig. 2.
Disruption of the neuritic cytoskeleton by soluble Aβ oligomers is dependent on tau expression. (A) Representative Western blots showing the expression of endogenous tau in primary hippocampal neurons (DIV18) transduced with lentivirus encoding EGFP or scrambled RNAi (Cont-RNAi) or RNAi against rat tau (Tau-RNAi-1, Tau-RNAi-2). Western blotting of Tubulin or GFP served as a control. Histograms represent the average expression level of tau, normalized to values in parallel cultures without lentiviral transduction. Asterisks indicate data significantly different from those of neurons without transducton (P < 0.01 by paired Student t test). Data are from five independent experiments; error bars, SEM. (B) Confocal images showing the tau (green) and microtubule (red) cytoskeleton of primary hippocampal neurons (DIV21) transduced with lentivirus encoding Cont-RNAi or Tau-RNAi-1 or Tau-RNAi-2 after 3-d treatment with AβD from AD-TBS or pure Aβ40 S26C (500 nM). (Scale bar, 50 μm.)
Acceleration of the Neuritotoxic Effect of Aβ Oligomers by Expressing Human Tau.
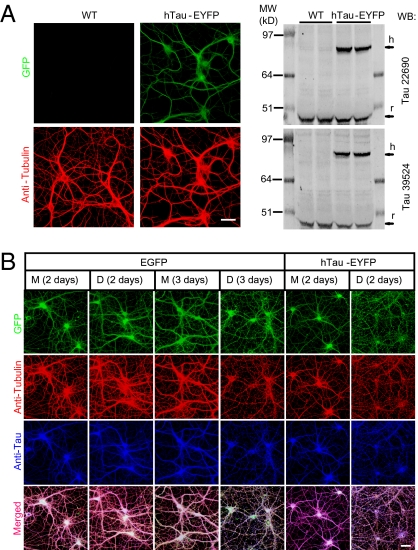
A recent analysis of transgenic mice expressing human tau revealed deficits in neural plasticity and memory, suggesting that the presence of human tau is sufficient to impair certain synaptic and cognitive functions (24). Our results above show that natural Aβ dimer-induced neuritic disruption is dependent on endogenous rodent tau. We asked whether expression of human tau in rat neurons enhances the neurotoxic effect of Aβ dimers. We transduced hippocampal neurons with lentivirus encoding the longest splice form of human tau fused to EYFP (hTau-EYFP), or just EGFP as a control. One week after transduction, we documented the expression of human tau by both blotting (Fig. 3A, Right) and immunostaining (Fig. 3A, Left). After treatment with soluble dimers isolated by SEC from AD-TBS, the neurites of neurons (> DIV 18) expressing human tau were substantially disrupted after only 2 d of exposure, at which time point the neurites of treated neurons expressing EGFP or not transduced were still morphologically intact (Fig. 3B and Fig. S1), as in all our previous experiments. Aβ monomers from the same SEC run had no discernable effect at 2 or 3 d of exposure (Fig. 3B).
Fig. 3.
The neuritotoxic effect of soluble Aβ oligomers is accelerated by expressing human tau. (A) Confocal images (Left) and Western blotting (Right) show the expression of hTau-EYFP in primary hippocampal neurons (DIV7) after transduction with lentivirus encoding hTau-EYFP. (B) Confocal images show the GFP fluorescence (green), microtubule (red) and total tau (blue) cytoskeleton of hippocampal neurons (DIV20 or DIV21) transduced with lentivirus encoding EGFP or hTau-EYFP after 2 or 3 d treatment with AβM or AβD from AD-TBS. (Scale bars, 50 μm.)
Soluble Aβ Oligomers Alter the Phosphorylation of Tau at AD-Relevant Epitopes.
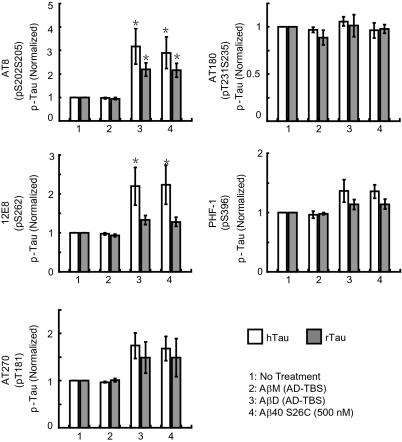
During AD pathogenesis, tau undergoes abnormal hyperphosphorylation that contributes to neurodegeneration (1, 8, 25, 26). The phosphorylation states of several epitopes within tau are increased in transgenic mice coexpressing mutant human APP, presenilin, and tau (27). Because we found above that tau plays a permissive role in the cytoskeletal alteration induced by soluble Aβ oligomers, it became important to elucidate which phosphoepitopes on tau may be altered by the natural oligomers. We transduced rat hippocampal neurons with EGFP or hTau-EYFP. After being cultured for 18 to 19 d, neurons were exposed to SEC-isolated human Aβ dimers for just 1 d, and the phosphorylation state of both the exogenous human and endogenous rat tau proteins was assayed by quantitative Western blotting with epitope-specific antibodies (the phosphorylated bands were normalized to the respective total tau signal in the same neurons) (Fig. 4 and Fig. S6). The cultures were also examined by immunocytochemistry (Fig. S7). Soluble Aβ dimers isolated from AD-TBS and pure Aβ40 S26C dimers each induced substantial increases in tau phosphorylation at Ser202/Ser205 (AT8 epitope) and at Ser262 (12E8 epitope), a moderate increase at Thr181 (AT270 epitope), and no significant changes at Ser231/Thr235 (AT180 epitope) or Ser396 (PHF-1 epitope). Identical application of the Aβ dimers to hippocampal neurons cultured for just 8 d did not induce any increase in tau phosphorylation at the AT8 or 12E8 epitopes (Fig. S6), in accord with the lack of neuritic effects at this age (see above). As an important control, Aβ monomers from the same SEC run of the same AD-TBS extracts did not significantly alter tau phosphorylation. As a positive control for the phosphoepitope quantification, treatment of the neuronal cultures with the phosphatase inhibitor, okadaic acid (200 nM, 2 h), consistently increased phosphorylation levels (Fig. S6). Immunodepleting Aβ from the dimer-rich SEC fractions before their application prevented the hyperphosphorylation of tau at the AT8 and 12E8 epitopes, indicating that Aβ dimers were necessary for the effect (Fig. S8). It is of interest that the oligomer-induced increases in phosphorylation showed some difference between human and rat tau; in the same neurons, human tau phosphorylation was more sensitive to the application of Aβ oligomers, so that the degree of increase at the AT8, 12E8, and AT270 epitopes of human tau (all known to be hyperphosphorylated in AD neurons) was greater than those of rat tau (Fig. 4).
Fig. 4.
Alteration of the phosphorylation state of tau at AD-relevant epitopes by soluble Aβ oligomers. Primary hippocampal neurons (DIV19) transduced with lentivirus encoding hTau-EYFP were treated under different conditions, as indicated in the key. Histograms represent the average levels of phosphorylation of human (h) or rat (r) tau at specific epitopes (AT8: Ser202/Ser205; 12E8: Ser262; AT270: Thr181; AT180: Ser231/Thr235; PHF-1: Ser396), normalized to the values in parallel cultures without treatment. Data are means of three independent experiments. Asterisks indicate data significantly different from those of neurons without treatment (P < 0.05 by paired Student t test). Error bars, SEM.
Specific Immunological Neutralization of the Cytoskeletal Alterations Induced by Human Aβ Dimers.
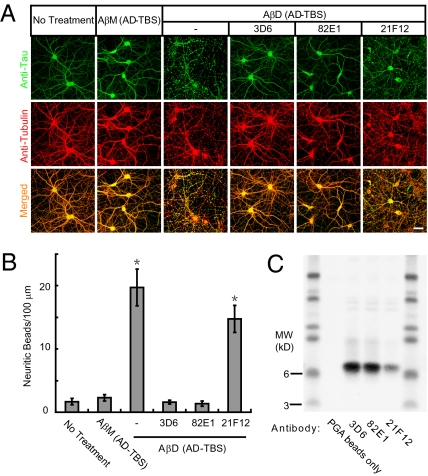
Immunotherapy against Aβ by both active (vaccination) and passive (antibody infusion) approaches has reached advanced clinical testing in AD patients. In light of all of the results above, we asked whether monoclonal antibodies directed at specific Aβ epitopes could modify the cytoskeletal alteration and neuritic degeneration caused by soluble dimers from the human (AD) brain. We tested three monoclonal Aβ antibodies: 3D6 to the free Asp-1 N terminus (a humanized version of which is in Phase 3 human trials); 82E1, another Asp-1 specific N-terminal antibody; and 21F12 to the free Ile-42 C terminus of Aβ42. Each antibody was coadministered (final concentration, 3 μg/mL) with the dimer-rich SEC fraction to mature (≥18 DIV) primary hippocampal cultures. As before, monomer-rich SEC fractions from the same chromatography served as a negative control. We found that when either 3D6 or 82E1 was present with the soluble dimers, no significant alteration of neuritic architecture and tau and Tubulin immunostaining were observed (Fig. 5 A and B). In contrast, 21F12 had no significant neutralizing effect (Fig. 5 A and B). This relative efficiency of neutralization was further confirmed by pull-down of Aβ dimers by protein G agarose (PGA) beads from the conditioned media of the antibody-treated neurons. Substantially larger amounts of Aβ dimers were pulled down by 3D6 and 82E1 than by 21F12, whereas PGA beads applied in the absence of antibodies pulled down no dimers (Fig. 5C).
Fig. 5.
Immunological neutralization of the cytoskeletal alterations induced by human Aβ dimers. (A) Confocal images showing the tau (green) and microtubule (red) cytoskeleton of primary hippocampal neurons (DIV21) after 3 d treatment with AβM or AβD from AD-TBS with or without monoclonal antibodies (3D6, 82E1, or 21F12) against human Aβ (each at 3 μg/mL). (Scale bar, 50 μm.) (B) Histograms represent the average number of tau-positive beads along 100-μm lengths of Tubulin-positive neurites under different conditions. Asterisks indicate data significantly different from those of neurons without treatment (P < 0.01 by Student t test). Error bars, SEM. (C) Pull-down of the Aβ dimers by PGA beads added to the conditioned media of the neurons after the 3-d treatment, as detected by Western blot with 6E10 + 2G3 + 21F12.
Discussion
Understanding the relationship of the two pathognomonic changes of AD, amyloid accumulation and neurofibrillary degeneration, represents an ongoing goal of research on the disease. Here, we show that natural oligomers, principally dimers, isolated directly from the cortex of typical, late-onset AD patients are sufficient to induce tau hyperphosphorylation at AD-relevant epitopes and then disrupt the microtubule cytoskeleton and cause neuritic dystrophy. Soluble dimers from the human brain appear to have a conformation that is highly potent in inducing these neuronal changes, as concentrations in the subnanomolar range caused cytoskeletal collapse, whereas levels of pure synthetic dimers at least two orders of magnitude higher were needed to produce otherwise indistinguishable effects in the same experiments.
Numerous controls confirmed that the cytoskeletal changes we report are attributable specifically to human Aβ dimers [the most abundant form of soluble oligomers recoverable from AD cortex (10, 11, 28)]: (i) monomers from the same size-exclusion chromatography at equal or higher amounts had no effect; (ii) corresponding SEC fractions from age-matched control brains lacking Aβ were negative; (iii) the SDS-stable dimers were recoverable from the media at the end of the treatment, proving they were present throughout the exposures that resulted in alteration of the tau cytoskeleton; (iv) pure, synthetic Aβ dimers produced closely similar effects to AD-brain derived dimers (albeit at much higher concentrations); (v) immunodepletion of natural Aβ dimers from the SEC fractions precluded any subsequent neuritic injury; and (vi) coadministering highly specific monoclonal antibodies to the N terminus but not the C terminus of Aβ prevented the effects.
Intracerebral injection of synthetic Aβ fibrils into mice transgenic for mutant human tau can induce tau hyperphosphorylation and local neurofibrillary changes (29). Moreover, the coexpression of mutant human APP and mutant human tau leads to enhanced neuronal tau accumulation and dystrophic neurites in double-transgenic mice (30). Another mouse line transgenic for both mutant human APP and tau undergoes tau alterations that can be temporarily reversed by microinjecting anti-Aβ antibodies (31). Conversely, the crossing of tau knock-out mice to APP transgenic mice ameliorates the memory deficits that occur in the latter (23, 32), demonstrating the importance of endogenous tau in mediating adverse responses to Aβ. These and other mouse studies support a pathogenic relationship between Aβ accumulation and tau-mediated neuronal alteration, but in such in vivo models, one is unable to specify which assembly form of Aβ is responsible for any neuronal changes, as abundant monomers, dimers, higher oligomers, and amyloid fibrils exist in complex mixtures simultaneously. To specify which forms of Aβ can induce tau alteration and cytoskeletal degeneration, one must use a cell-culture system, which allows the controlled application of biochemically defined Aβ species that can be recovered and confirmed immediately after the exposure. To our knowledge, the present study is unique in providing evidence that natural Aβ oligomers, and specifically dimers isolated from the AD brain, are sufficient to induce tau hyperphosphorylation at AD-relevant epitopes, microtubule disruption, and neuritic degeneration, thus directly linking the accumulation of soluble oligomers per se to neurofibrillary degeneration.
The neuronal effects we describe clearly depend on the expression of tau: they were prevented by knocking down endogenous tau and were accelerated by expressing wild-type human tau. Our combined use of mature (≥18 DIV) cultures, biochemically isolated and defined human Aβ species, and an array of epitope-specific tau antibodies strongly supports the concept that soluble Aβ oligomers can induce AD-type cytoskeletal impairment in the absence of amyloid plaques. This result does not mean that plaques play no role in the fibrillary degeneration of neurons and their processes, as there is clear evidence that peri-plaque Aβ assemblies (type unspecified) are associated with local dendritic spine loss (33) and neuritic dystrophy in AD brains (34). Indeed, the presence of bioactive dimers within amyloid plaque cores (10) suggests that plaques serve as local reservoirs of small oligomers that can diffuse away from them and cause surrounding neuritic/synaptic injury.
Our observation on the potential of different monoclonal antibodies to neutralize oligomer effects on the tau cytoskeleton has relevance in light of current advanced trials of passive immunotherapy in AD. We find that two distinct antibodies to the free Asp-1 of Aβ, one of which (in humanized form) is in Phase 3 trials (35), are more potent in preventing the effects of soluble dimers from the AD cortex on the tau cytoskeleton than is a C-terminal specific antibody. This result suggests that endogenous dimers and other oligomers with an exposed N terminus (Asp-1) have a conformation that is particularly able to induce neurofibrillary degeneration (this article) and synapse loss (10). Active immunization with synthetic Aβ induces principally an N-terminal region antibody response in humans (36), and Phase 2 trials of a vaccine comprising an N-terminal Aβ fragment are underway. An earlier vaccine trial using full-length Aβ was halted prematurely because of occurrence of a self-limited meningoencephalitis in 6% of the 300 recipients (36). Nonetheless, those recipients having Aβ antibody responses showed less subsequent decline on some tests of verbal memory and an apparent decrease in CSF phospho-tau levels (36). A postmortem follow-up of a small subset of recipients from the Phase 1 trial of this full-length Aβ peptide vaccine, which suggested that some subjects could undergo marked clearance of Aβ plaques but still die with advanced dementia (37), is inconclusive, as it documented only two such subjects from a trial that originally included 80, and residual levels of Aβ oligomers in the brains were not assessed.
Tau has been found to be phosphorylated at over 30 serine/threonine residues in the human brain (38, 39), and approximately half of these are canonical sites for proline-directed protein kinases, including certain members of the MAP kinase, cyclin-dependent kinase and glycogen synthase kinase 3 (GSK3) families (26, 40). On cultured hippocampal neurons, synthetic Aβ oligomers (sometimes called ADDLs) or AD brain extracts have been shown to induce tau phosphorylation at several epitopes (41). A recent study showed that synthetic ADDLs can induce missorting of tau into dendrites, tau phosphorylation, and disruption of microtubules (8), but this work used 5-μM concentrations of the synthetic ADDLs compared with the subnanomolar levels of natural human brain dimers used here. The mechanism by which diffusible extracellular oligomers of Aβ bind to neurons and lead to increased activity of select kinases that phosphorylate tau at some but not other epitopes remains unclear. Ittner et al. reported that tau protein, in addition to its principally axonal locus, is sorted in small amounts to dendrites and that this helps mediate the postsynaptic targeting of the src kinase Fyn, substrates of which are certain NMDA receptors (32). The authors postulated that Aβ-mediated enhanced targeting of tau to dendrites could alter this normal function. However, there are numerous different ways in which an interaction between Aβ oligomers and tau could occur in AD brains (42), and clarifying precisely how this occurs is the next major step for the approach we report here. In this regard, we consistently observed that hippocampal neurons cultured for ≤7 d were resistant to the cytoskeletal injury induced by the Aβ dimers, suggesting that signaling programs that develop in more mature neurons are required for expression of this phenotype. In their analyses of APP-only transgenic mice, Roberson et al. (23) saw no change in phosphoepitopes of endogenous murine tau at age 4 to 6 mo but did see phopshotau-positive punctae in periplaque dystrophic neurites at >20 mo. Here, we observed altered phosphorylation of endogenous rat tau (albeit less robustly than transfected human tau). Numerous APP transgenic mouse lines that do not also express human tau show neuritic dystrophy but no AD-type neurofibrillary tangles, suggesting that human tau may be necessary for full-blown tangle formation per se.
Our findings with natural dimers isolated directly from AD patients, coupled with the wide availability of postmortem brain tissue from AD and non-AD subjects, recommends the use of endogenous oligomers isolated from the human cortex (10, 43), as the most biologically relevant approach to learn how Aβ oligomers alter tau phosphorylation and cytoskeletal function. Various aggregated forms of synthetic Aβ designated ADDLs (3) or protofibrils (44, 45) and generated from high concentrations of a single, defined Aβ peptide, have not been proven to occur as such in the human brain, whereas heterogeneous dimers, trimers (10, 11, 28), slightly larger low-n oligomers (11), and dodecamers (28) have. Deciphering the mechanisms of these natural oligomers will require purifying them to homogeneity from the AD cortex, labeling them, and exposing primary neurons or brain slices to them to identify in unbiased fashion their molecular targets. We hypothesize that these targets are likely to be plasma membrane lipids (which would be expected to avidly bind the highly hydrophobic oligomers) rather than the hydrophilic ectodomains of protein receptors. Oligomer binding could perturb the fine structure of the lipid bilayer, and this could lead to secondary biophysical effects on the structure and function of various transmembrane receptors (e.g., the NMDA, AMPA, insulin, and α7-nicotinic receptors implicated to date) that may then contribute to the profound changes in the tau cytoskeleton documented here.
Materials and Methods
See SI Materials and Methods for detailed descriptions.
Human Brain Sample Preparation.
Frozen human cerebral cortices were provided by C. Lemere (Brigham and Women's Hospital/Harvard Medical School) or M. Frosch (Massachusetts General Hospital/Harvard Medical School) under Institutional Review Board-approved human studies protocols and by M. Farrell (Beaumont Hospital, Dublin, Ireland) in accord with local Ethics Committee guidelines and Ethical Review Committee/Institutional Review Board approval. Samples of temporal or frontal cortex containing white and gray matter were weighed. Freshly prepared, ice-cold TBS consisting of 20 mM Tris-HCl, 150 mM NaCl, pH 7.4, was added to the frozen cortex at 4:1 (TBS volume:brain wet weight) and homogenized with 25 strokes at a setting of 10 on a mechanical Dounce homogenizer. The homogenate was spun at 175,000 × g in a TLA100.2 rotor on a Beckman TL 100. The supernatant (called TBS extract) was aliquoted and stored at −80 °C.
Immunoprecipitation/Western Blot Analysis of Aβ.
We used an immunoprecipitation/Western blot protocol described previously (10, 15) to detect Aβ in the TBS extracts or neuronal culture medium.
Supplementary Material
Acknowledgments
We thank George Bloom for generously providing the pCMV-hTau-EYFP construct, Peter Seubert for the gift of 3D6 and 21F12 anitbodies, and Ganesh Shankar and other members of the D.J.S. laboratory for helpful discussions. This work was supported by National Institutes of Health Grants AG027443 and AG006173 (to D.J.S.).
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017033108/-/DCSupplemental.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 3.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodart JC, et al. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 5.Gong Y, et al. Alzheimer's disease-affected brain: Presence of oligomeric A β ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klyubin I, et al. Amyloid β protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 7.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zempel H, Thies E, Mandelkow E, Mandelkow E-MA. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tackenberg C, Brandt R. Divergent pathways mediate spine alterations and cell death induced by amyloid-β, wild-type tau, and R406W tau. J Neurosci. 2009;29:14439–14450. doi: 10.1523/JNEUROSCI.3590-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar GM, et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pham E, et al. Progressive accumulation of amyloid-β oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277:3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira A, Lu Q, Orecchio L, Kosik KS. Selective phosphorylation of adult tau isoforms in mature hippocampal neurons exposed to fibrillar A β. Mol Cell Neurosci. 1997;9:220–234. doi: 10.1006/mcne.1997.0615. [DOI] [PubMed] [Google Scholar]
- 13.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 β by phosphorylation: New kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh DM, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 16.King ME, et al. Tau-dependent microtubule disassembly initiated by prefibrillar β-amyloid. J Cell Biol. 2006;175:541–546. doi: 10.1083/jcb.200605187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabrese B, et al. Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-β protein. Mol Cell Neurosci. 2007;35:183–193. doi: 10.1016/j.mcn.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Nuallain B, et al. Amyloid β-protein dimers rapidly form stable synaptotoxic protofibrils. J Neurosci. 2010;30:14411–14419. doi: 10.1523/JNEUROSCI.3537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barghorn S, Mandelkow E. Toward a unified scheme for the aggregation of tau into Alzheimer paired helical filaments. Biochemistry. 2002;41:14885–14896. doi: 10.1021/bi026469j. [DOI] [PubMed] [Google Scholar]
- 20.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 21.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 22.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proc Natl Acad Sci USA. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberson ED, et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 24.Polydoro M, Acker CM, Duff K, Castillo PE, Davies P. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci. 2009;29:10741–10749. doi: 10.1523/JNEUROSCI.1065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joachim CL, Morris JH, Selkoe DJ, Kosik KS. Tau epitopes are incorporated into a range of lesions in Alzheimer's disease. J Neuropathol Exp Neurol. 1987;46:611–622. doi: 10.1097/00005072-198711000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Liu SJ, et al. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J Neurochem. 2003;87:1333–1344. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- 27.Oddo S, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 28.Lesne SE, et al. Distinct brain Aβ oligomers are associated with different stages of Alzhermer's disease. Society for Neuroscience Annual Meeting. 2009 Available at http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=267ba17b-f976-4e8d-a42f-eb5418d055d2&cKey=10f6e43e-2e53-405d-a16c-7bb46afc407e&mKey=%7b081F7976-E4CD-4F3D-A0AF-E8387992A658%7d. [Google Scholar]
- 29.Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 30.Lewis J, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 31.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FMA. β immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:1–20. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Ittner LM, et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 33.Koffie RM, et al. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowles RB, et al. Plaque-induced neurite abnormalities: Implications for disruption of neural networks in Alzheimer's disease. Proc Natl Acad Sci USA. 1999;96:5274–5279. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salloway S, et al. Bapineuzumab 201 Clinical Trial Investigators A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilman S, et al. AN1792(QS-21)-201 Study Team Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 37.Holmes C, et al. Long-term effects of Abeta42 immunisation in Alzheimer's disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 38.Drewes G, et al. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem. 1995;270:7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- 39.Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer's disease brain using nanoelectrospray mass spectrometry. J Neurochem. 1998;71:2465–2476. doi: 10.1046/j.1471-4159.1998.71062465.x. [DOI] [PubMed] [Google Scholar]
- 40.Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23:307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- 41.De Felice FG, et al. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by A β oligomers. Neurobiol Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ittner LM, Götz J. Amyloid-β and tau—A toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 43.Li S, et al. Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid β-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 45.Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr Observation of metastable Abeta amyloid protofibrils by atomic force microscopy. Chem Biol. 1997;4:119–125. doi: 10.1016/s1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.