Abstract
Corticosteroids are potent modulators of human higher cognitive function. They are released in response to stress, and are thought to be involved in the modulation of cognitive function by inducing distinct rapid nongenomic, and slow genomic changes, affecting neural plasticity throughout the brain. However, their exact effects on the neural correlates of higher-order cognitive function as performed by the prefrontal cortex at the human brain system level remain to be elucidated. Here, we targeted these time-dependent effects of corticosteroids on prefrontal cortex processing in humans using a working memory (WM) paradigm during functional MRI scanning. Implementing a randomized, double-blind, placebo-controlled design, 72 young, healthy men received 10 mg hydrocortisone either 30 min (rapid corticosteroid effects) or 240 min (slow corticosteroid effects), or placebo before a numerical n-back task with differential load (0- to 3-back). Corticosteroids’ slow effects appeared to improve working memory performance and increased neuronal activity during WM performance in the dorsolateral prefrontal cortex depending on WM load, whereas no effects of corticosteroids’ rapid actions were observed. Thereby, the slow actions of corticosteroids seem to facilitate adequate higher-order cognitive functioning, which may support recovery in the aftermath of stress exposure.
Corticosteroids are key modulators of human cognition. They are released in response to stress as the end product of the hypothalamic–adrenal–pituitary (HPA) axis, and are known to readily cross the blood–brain barrier to affect brain processing (1). Corticosteroids ensure sufficient energy supply to challenged tissues and control the excitability of neuronal networks, and are thereby thought to support and regulate the stress response (2). The hormones exert their actions upon binding of the mineralocorticoid (MR) and glucocorticoid receptor (GR), abundantly expressed in the brain (3–5). Recent animal research has indicated that receptor-binding causes both immediate nongenomic effects (6) and slow, genomic effects that manifest themselves several hours after stress exposure (7, 8). By these distinct mechanisms, corticosteroids seem to influence neural plasticity in a time-dependent manner (9).
So far, most research on modulation of cognition has focused on medial temporal lobe structures, where corticosteroids have been shown to affect neuronal excitability, synaptic plasticity, and processes of memory retrieval and consolidation (10, 11). However, moderate to high levels of receptor expression in the prefrontal cortex (PFC) (5) make this structure susceptible to corticosteroid modulation as well. A current working hypothesis states that corticosteroids’ rapid nongenomic effects work in concert with the effects of catecholamines during the early phase of the stress response (9, 12), and thereby optimize rapid adaptive behavior by reallocating neural resources away from higher-order cognitive processing regions in the PFC to promote vigilance, instinctive behavior, and the encoding of the stressful experience into memory (13). Meanwhile, the corticosteroid-induced genomic cascade is initiated, which is hypothesized to restore PFC function in the aftermath of stress (13). Although findings from both animal (14, 15) and human literature (16, 17) provide initial evidence for corticosteroid modulation of PFC signaling, both the neural and functional consequences on higher-cognitive function and their time-dependency remain to be tested.
Here, we targeted both the rapid (putatively nongenomic) and slow (putatively genomic) effects of corticosteroids on PFC processing using a working memory (WM) paradigm during functional MRI (fMRI) in humans. WM refers to a system maintaining relevant information in a temporary buffer that is constantly updated to guide behavior (18). It is typically associated with the activation of a frontoparietal executive function network, including the dorsolateral prefrontal cortex (DLPFC) (18). Implementing a randomized, double-blind, placebo-controlled design, 72 young, healthy men received 10 mg hydrocortisone—known to mimic corticosteroid levels observed during moderate to severe stress—either 30 min (to target corticosteroid rapid effects) or 240 min (to assess corticosteroid slow effects) before a numerical n-back task. To investigate whether corticosteroid effects depend on task difficulty, we manipulated WM load using a 0-, 1-, 2-, and 3-back condition.
Results
Physiological and Psychological Measures.
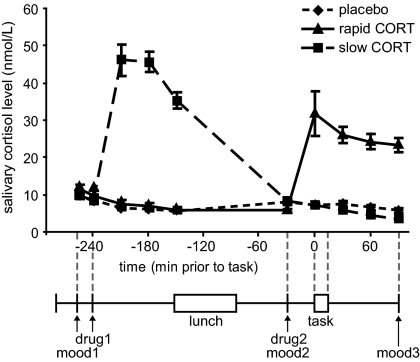
As expected, oral administration of 10 mg hydrocortisone increased salivary cortisol levels to those observed during moderate to severe stress (19) (Fig. 1), which was evidenced by a significant main effect of group [F(2,65) = 43.30, P < 0.001] and a time × group interaction [F(18,116) = 26.17, P < 0.001]. Increased levels were observed from 30 min postadministration onward in both hydrocortisone administration conditions, and the levels remained elevated for at least 90 min. As intended, treatment resulted in elevated cortisol levels during fMRI scanning in the rapid hydrocortisone condition, whereas the levels in the slow condition had already returned to baseline.
Fig. 1.
Experimental design and salivary cortisol curves. Participants received two capsules (drug1 and drug2) containing either 10 mg hydrocortisone (CORT) or placebo at different time points before the numerical n-back task (0/1/2/3-back). Hydrocortisone intake significantly elevated salivary cortisol levels in both hydrocortisone administration conditions to levels observed during moderate to severe stress. mood, Mood based on POMS questionnaire (20–22). Error bars represent SEM.
Postexperiment debriefing showed that participants were unable to identify the substance received. As expected, hydrocortisone administration did not affect autonomic measures of heart rate [main effect of drug: F(2,64) < 1] and heart rate variability [F(2,64) < 1, NS] (Table 1). Furthermore, drug administration did not affect mood as assessed three times during the experiment using the Profile of Mood States (POMS) questionnaire (20–22) (Table 1). Although significant reductions in levels of depression scores [Friedman's ANOVA; χ2(2) = 8.99, P = 0.011], anger scores [χ2(2) = 7.43, P = 0.024], vigor scores [χ2(2) = 79.05, P < 0.001], and tension scores [χ2(2) = 18.38, P < 0.001] were observed over the course of the experiment, and levels of fatigue [χ2(2) = 52.40, P < 0.001] increased, none of these factors was affected by drug administration. Hence, differences in brain activity found between drug conditions cannot readily be explained by any physiological or psychological side effects of drug administration.
Table 1.
Physiological and psychological measures
| Placebo | Rapid CORT | Slow CORT | |
| Mood state | |||
| Depression 1 (t = 30 min) | 0.26 (0.13) | 0.82 (0.37) | 0.65 (0.32) |
| 2 (t = 255 min) | 0.09 (0.06) | 0.64 (0.35) | 0.13 (0.07) |
| 3 (t = 375 min) | 0.04 (0.04) | 0.59 (0.24) | 0.13 (0.10) |
| Anger 1 (t = 30 min) | 0.61 (0.23) | 1.18 (0.40) | 1.00 (0.43) |
| 2 (t = 255 min) | 0.30 (0.19) | 0.45 (0.23) | 0.48 (0.20) |
| 3 (t = 375 min) | 0.22 (0.18) | 0.73 (0.29) | 0.87 (0.32) |
| Fatigue 1 (t = 30 min) | 1.17 (0.30) | 1.68 (0.50) | 2.70 (0.61) |
| 2 (t = 255 min) | 1.35 (0.44) | 1.55 (0.52) | 2.43 (0.56) |
| 3 (t = 375 min) | 3.52 (0.67) | 5.23 (0.69) | 4.22 (0.71) |
| Vigor 1 (t = 30 min) | 12.65(0.79) | 10.50 (0.77) | 11.70 (0.90) |
| 2 (t = 255 min) | 10.43 (0.68) | 8.73 (0.75) | 10.26 (0.96) |
| 3 (t = 375 min) | 7.57 (0.88) | 4.86 (0.82) | 7.13 (0.91) |
| Tension 1 (t = 30 min) | 1.00 (0.27) | 1.36 (0.29) | 1.30 (0.46) |
| 2 (t = 255 min) | 0.35 (0.13) | 1.09 (0.35) | 0.96 (0.30) |
| 3 (t = 375 min) | 0.26 (0.16) | 0.64 (0.20) | 0.17 (0.10) |
| Heart rate (beats/min) | 65.60 (1.96) | 67.04 (2.57) | 68.30 (2.41) |
| Heart rate variability (ms2) | 70.76 (4.95) | 62.71 (4.99) | 67.02 (6.60) |
Data are mean (SEM). CORT, hydrocortisone.
Working Memory Performance.
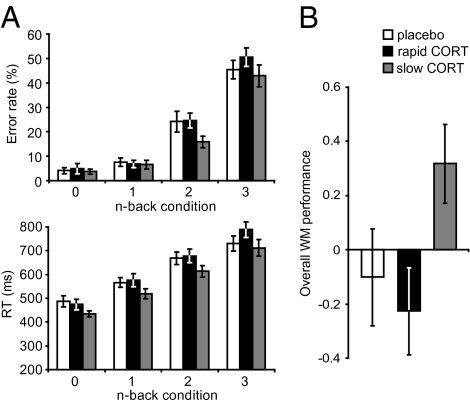
Separate ANOVAs for both performance measures of accuracy and reaction times (RTs) were conducted with WM load as within-subject factor, and drug condition as between-subject factor. There were robust effects of WM load on both accuracy [F(3,63) = 107.72, P < 0.001] and RTs [F(3,63) = 97.96, P < 0.001] (Fig. 2A). These analyses revealed no significant main effect of drug [accuracy: F(2,65) = 1.59, P = 0.212; RTs: F(2,65) = 1.98, P = 0.146] or a WM-load × drug interaction (accuracy and RTs: all F < 1) on both performance measures, although a tendency toward shorter RTs and improved accuracy could be observed for the slow hydrocortisone (CORT) group (Fig. 2A). Because performance on the n-back task can be regarded as a combined measure of both accuracy and RTs of responding (both assessing voluntary attention (23) and efficacy of information processing (24)), the two measures were combined to create one overall WM performance measure (25) (Materials and Methods). Analysis of this combined performance measure revealed that CORT administration indeed affected WM performance (F(2,42) = 3.25, P = 0.045). This main effect of drug was driven by an improved performance of the slow CORT group compared with the rapid CORT group [T(42) = 6.58, P = 0.014, Fig. 2B] and close to significant improvement compared with the placebo group [T(43) = 3.59, P = 0.065]. The rapid CORT and placebo groups did not differ on WM-performance [T(42) = 0.233, NS]. The observed effects seemed to be driven by drug effects at high WM load [2- and 3-back conditions; F(2,64) = 3.34, P = 0.042], as there was no significant difference between drug conditions at low WM load [0- and 1-back; F(2,64) = 1.68, P = 0.195]. However, the drug × load interaction failed to reach significance [F(2,64) < 1].
Fig. 2.
Behavioral performance in n-back task. (A) Mean error rates and RTs of the 0-, 1-, 2-, and 3-back conditions for the three drug conditions did not reveal any effects of hydrocortisone (CORT). (B) Combination of error rates and RTs into one overall WM performance measure revealed that the slow CORT group outperformed both other groups. Error bars represent SEM.
Brain Activation.
We first identified brain regions activated by performing the numerical n-back task by contrasting 3-, 2-, 1-back with 0-back conditions (collapsing across groups). As expected, the WM task activated an extended set of brain regions in the bilateral prefrontal cortex (including the DLPFC), bilateral inferior parietal cortex, inferior occipital lobe, cerebellum and other related regions (Table 2 and Fig. S1A). The opposite contrast, regions deactivated by WM processing, revealed the default mode network including the posterior cingulate cortex, the ventral medial PFC extending into the orbitofrontal cortex and the medial temporal lobe (Table 2 and Fig. S1B).
Table 2.
Peak voxel and corresponding F/T values of significantly activated clusters in main effects of working memory
| MNI coordinates |
|||||
| Region | BA | x | y | z | F/T value |
| Positive main effect of WM | |||||
| Extended activation cluster covering precentral gyrus, superior, middle PFC | L 6,8,10,32, 44–46,48 | −30 | 0 | 60 | 18.61*** |
| R 6,8,10,32, 44–46,48 | 30 | 2 | 58 | 19.27*** | |
| Supplementary motor area | L 32 | −4 | 16 | 46 | 21.57*** |
| Inferior parietal cortex | L 40 | −36 | −46 | 44 | 22.15*** |
| R 40 | 44 | −46 | 52 | 21.44*** | |
| Angular gyrus | R 40 | 38 | −54 | 52 | 21.06*** |
| Inferior temporal gyrus | R 37 | 56 | −54 | −12 | 9.21*** |
| Cerebellum, 9 | L | −10 | −58 | −52 | 4.91* |
| Calcarine | R 17 | 14 | −72 | 12 | 5.04* |
| L 17 | −10 | −98 | 0 | 6.48*** | |
| Inferior occipital lobe | R 18 | 30 | −92 | −6 | 8.49*** |
| L 18 | −26 | −94 | −8 | 8.06*** | |
| Negative main effect of WM | |||||
| Ventral medial PFC | L 10 | −4 | 60 | 18 | 17.62*** |
| L 10 | −4 | 56 | −4 | 18.95*** | |
| Rectus | L 11 | −2 | 44 | −16 | 19.01*** |
| Inferior orbitofrontal gyrus | L 47 | −32 | 34 | −14 | 17.23*** |
| Inferior temporal gyrus | L 21 | −56 | −4 | −26 | 14.74*** |
| Supramarginal/superior temporal gyrus | R 48 | 56 | −26 | 24 | 13.87*** |
| Fusiform gyrus/parahippocampal gyrus | L 37/20 | −26 | −42 | −10 | 14.78*** |
| R 37/20 | 30 | −32 | −16 | 12.72*** | |
| including hippocampus | L 37 | −30 | −32 | −12 | 10.78*** |
| R 20 | 28 | −20 | −16 | 10.92*** | |
| Middle temporal gyrus | L 21 | −64 | −44 | −4 | 6.46*** |
| R 21 | 64 | −2 | −20 | 13.05*** | |
| Posterior cingulate cortex | L 23 | −4 | −46 | 30 | 23.21*** |
| Precuneus | L 30 | −6 | −52 | 16 | 22.04*** |
| Angular gyrus | L 39 | −50 | −66 | 32 | 17.72*** |
| Cerebellum, 9 | R | 4 | −54 | −44 | 5.06* |
| Cerebellum, crus1/2 | R | 28 | −82 | −34 | 13.43*** |
| L | −28 | −82 | −34 | 6.71*** | |
All effects are analyzed using voxel-level statistics. WM, working memory; MNI, Montreal Neurological Institute; BA, Brodmann Area; R, right; L, left.
*P < 0.05 whole-brain corrected; **P < 0.01 whole-brain corrected; ***P < 0.001 whole-brain corrected.
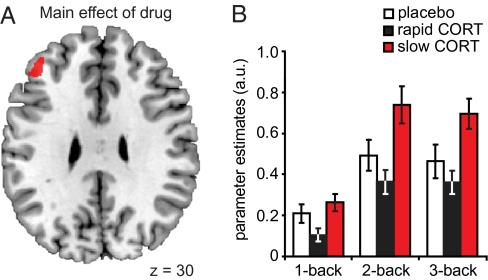
To examine how corticosteroids affect working memory processing over time, we first identified those brain regions the activity of which was modulated by any of the drug conditions. This analysis showed that the only significant effect of hydrocortisone was observed in the left DLPFC [(x = −40, y = 42, z = 32) F(2,194) = 11.52, Pcorrected = 0.030] (Fig. 3). We next extracted the data from this cluster to analyze whether the effects of hydrocortisone were moderated by WM load using orthogonal contrasts. First of all, the main effect of WM load was significant [F(2,63) = 4.83, P = 0.011] and showed that the DLPFC displayed greater activity with increasing load. More importantly, the effect of WM load on DLPFC activation was modulated by hydrocortisone administration [drug × WM-load interaction; F(4,128) = 2.56, P = 0.042]. Further analyses showed that this interaction was driven by more prominent drug effects at high compared with low WM loads [drug × WM-load (1- vs. 2-back); F(2,64) = 5.17, P = 0.008, drug × WM-load (one- vs. three-back); F(2,64) = 4.57, P = 0.014], whereas the drug effect between the high WM-load conditions (two- and three-back) did not differ significantly [F(2,64) < 1].
Fig. 3.
Main effect of drug on WM-related brain activity in the DLPFC. (A) Analysis of the main effect of drug revealed that corticosteroids specifically modulated activity in a prefrontal region. (B) Data extraction from this activation cluster revealed both a main effect of WM load, and a drug × WM-load interaction, caused by greater drug effects at higher WM load. This interaction in the DLPFC was caused by the slow effects of corticosteroids increasing DLPFC activity under high WM load. Error bars represent SEM. For visualization purposes, the statistical parametric map is thresholded at P < 0.001, uncorrected.
To determine which of the drug conditions induced these DLPFC effects, we continued with pairwise follow-up tests among the three drug conditions. These analyses revealed that the observed drug × WM-load interaction effect in the DLPFC was caused by the slow effects of corticosteroids under high WM-load. A history of corticosteroid elevation apparently induced increased high WM-load processing in the DLPFC compared with both placebo [F(1,43) = 6.31, P = 0.016] and the rapid corticosteroid conditions [F(1,42) = 12.82, P = 0.001]. Current elevation in corticosteroid level had no such effect on DLPFC activation [rapid CORT vs. placebo: F(1,42) = 1.24, NS].
Discussion
Here we targeted both the rapid (presumably nongenomic) and slow (presumably genomic) effects of corticosteroids on prefrontal working memory processing. Results revealed time-differential effects for corticosteroids’ actions, with their slow effects increasing WM-related activation of the DLPFC and thereby improving WM performance, whereas corticosteroids’ rapid effects did not induce any observable effect.
Previous work in animals has provided initial evidence that corticosteroids, in addition to their well-established slow genomic effects, also exert rapid nongenomic effects (9). The hormones have been shown to rapidly affect neuronal plasticity by binding to membrane mineralocorticoid receptors (MR), leading to a change in glutamate release (6). At the same time, a corticosteroid-induced genomic cascade is initiated by the binding of primarily intracellular glucocorticoid receptors (GRs) that, upon binding, translocate to the nucleus, where they function as transcription factors to modulate the expression of more than 200 genes (26). In the medial temporal lobe, these rapid and slow actions of corticosteroids were shown to have fundamentally distinct consequences in that they either enhanced or inhibited neuronal plasticity, respectively (6–8). Here, we aimed to dissociate these two effects on the human PFC experimentally by administrating 10 mg of hydrocortisone at either 30 or 240 min before the WM task. The timing of the rapid corticosteroid condition was based on a previous study in our laboratory revealing an elevation in human salivary cortisol levels from 30 min onward (27), and most prominent rapid, quickly reversible effects with corticosteroids administered directly to hippocampal slices in rodents (6). The genomic effects of corticosteroids, on the other hand, generally do not start earlier than 3 h after exposure to high corticosteroid levels (28, 29), and these effects last for hours (28, 30). Thus, administration of hydrocortisone at 30 min before scanning probably caused sufficiently high levels of the hormone in the brain to evoke rapid nongenomic effects, whereas this delay was too short to allow development of gene-mediated events. Conversely, when hydrocortisone was applied at 240 min before testing, hormone levels were so low (similar to baseline) during the behavioral task that nongenomic actions are not likely to happen, yet allowed enough time for the gene-mediated actions to occur.
Under conditions of acute stress, working memory is generally impaired (31–33), whereas neuronal firing and long-term potentiation in the PFC are known to be decreased (34–36). These effects are at least partly caused by the stress-related hormones norepinephrine and dopamine, which are known to impair prefrontal cortex function in higher doses (37). They subserve the initial fight-or-flight response by prioritizing rapid instinctive behavior (as mediated by, e.g., the amygdala) and emotional memory encoding (38, 39) over complex, higher-order cognitive functions as performed by the prefrontal cortex (37, 40). Because previous studies have shown that the rapid effects of corticosteroids act in concert with (and to amplify) the effects of catecholamines on long term memory (9, 12), we hypothesized impaired WM performance in the rapid CORT condition. However, we did not observe any rapid, nongenomic effects of corticosteroids on either WM performance or DLPFC activation. Previous studies of corticosteroid modulation of working memory performance show rather conflicting results on this topic. Studies have reported no effects on WM performance (41, 42), corticosteroid-induced improvements in both humans (43) and animals (44), as well as impairments (45) depending on concurrent sympathetic activation (31) or WM load (46). The latter findings suggest that fast actions of corticosteroids indeed have effects additive to those of noradrenergic activation in WM impairment. Work in rodents has shown that this concurrent noradrenergic activity of the amygdala actually is essential for corticosteroid-induced impaired WM to occur (47). In line with this, a recent human study of the effects of norepinephrine and corticosteroids on the neural correlates of memory formation showed that, specifically, the administration of both hormones caused a strong deactivation in the prefrontal cortex, whereas no such effects were observed when only corticosteroids were administered (17). Here we used different levels of difficulty (WM load), which presumably triggered different levels of arousal, but did not observe any rapid modulatory effects of hydrocortisone on WM performance or DLPFC processing. However, the levels of emotional arousal reached due to this manipulation most likely did not reach arousal levels observed under conditions of stress. Therefore, this issue of potentially interacting rapid corticosteroid and noradrenergic effects on PFC functioning remains open for future research. Regardless, our results show that corticosteroids by themselves do not modulate WM performance or WM-related DLPFC activity in a rapid nongenomic manner.
Corticosteroids’ slow, genomic effects on the other hand have often been seen as essential for adaptation and restoration of homeostasis following situations of acute stress (48). Here we provide a unique demonstration that exactly these delayed effects of corticosteroids boost WM processing. This effect was strongest at high WM load when cognitive demand is highest. Our findings of enhanced WM by corticosteroids are supported by two recent rodent studies in which the administration of corticosterone in the prefrontal cortex was shown to enhance glutamatergic transmission in PFC pyramidal neurons by increasing surface levels of NMDA- and AMPA-receptor subunits (44, 49). Moreover, one of these studies (44) showed that stress improved performance on a WM task 4 h later, but not immediately. Both this increase in glutamatergic transmission and improved behavioral performance were abolished by the administration of a selective GR antagonist, pointing toward the involvement of this receptor. Because the rapid stimulatory nongenomic effects of corticosteroids are thought to be mediated by corticosteroid binding of membrane MRs (6), this observed corticosteroid-induced WM improvement most likely involves a genomic mechanism. These findings in animals, together with the time-delay implemented for assessing the slow corticosteroid effects in this study, suggest that the observed improvement in WM-processing is mediated via a GR-dependent genomic mechanism. However, administration of a GR antagonist would be necessary to explicitly test this hypothesis in humans. Although extremely interesting and necessary for future understanding of corticosteroid effects, the realization of such an experiment is currently prohibited for practical reasons, as no selective GR antagonist has yet been registered for human use. Mifeprestone (RU-486) is the only compound commercially available (50); however, it is known to cross the blood–brain barrier only at very high concentrations (51) and, more importantly, to also act as a very potent progesterone receptor antagonist (52), which might cause many unwanted side effects. Future studies are therefore necessary to elucidate the exact underlying mechanism of the observed potentiation of WM processing. Nevertheless, we here show that, specifically, corticosteroids’ slow actions boost WM processing in the DLPFC, which are likely mediated via a GR-dependent genomic mechanism.
Obviously, several limitations of this study should be mentioned. First of all, the behavioral effects observed in this study were not very strong. Although trends were seen in absolute measures of reaction time and error rate, these trends failed to reach significance. Only the combination of both measures revealed an indication for enhanced performance in the slow corticosteroid group. However, because both measures contribute to behavioral performance in their own distinct ways (23, 24), we think that this combination is actually warranted. The combination of error rates and reaction times is often used to determine the speed–accuracy tradeoff displayed by participants. This speed–accuracy tradeoff refers to the fact that there is usually a tradeoff between these two measures, with either short reaction times causing many errors, or longer reaction times reducing the number of errors (53). Here however, we observed both faster and more accurate responses by participants in the slow CORT group compared with the other groups, so instead of a shift in tradeoff, we found additive effects both pointing toward improved performance.
It cannot be excluded that the lack of a strong behavioral effect is partly caused by the relatively low number of subjects in our fMRI study; this number is obviously lower than for less laborious psychopharmacological studies. Behavioral output is dependent on a multitude of factors (e.g., intelligence or motivation), and the variation in WM performance within each group is therefore quite substantial. For this reason, effects with rather small effect sizes, such as observed here, are not easily detected in behavior, certainly with the between-subjects comparison that was used. Regardless, we found significant brain effects that were in line with the behavioral effect, providing corroborative evidence. A second explanation for the rather weak behavioral effect might be that the dose of hydrocortisone administered was too low to induce stronger effects. We used 10 mg hydrocortisone in this study, because this dose is known to increase salivary cortisol levels to physiological levels observed under conditions of moderate to severe stress (19, 54, 55). Moreover, previous studies using a similar dose reported on the successful induction of corticosteroid effects on declarative memory (54, 55), which has been shown to be less sensitive to corticosteroid modulation than working memory (46). However, several studies reporting on corticosteroid effects on human cognition have used higher doses of hydrocortisone (17, 56, 57), and use of such a higher dose might possibly have induced stronger behavioral effects.
Another limitation of this study is that we investigated men only, which limits the generalization of the obtained results to women. Women are known to display HPA axis reactivity different from that of men and to exhibit smaller and more variable responses to stress (58), which appear to depend on the phase of the menstrual cycle and use of hormonal contraceptives (59). Although sex differences are important to consider, this issue was beyond the scope of this initial study, which is why we opted to recruit the population with the most stable response to corticosteroids, and excluded women from participation.
Finally, this pharmacological study obviously is not an exact copy of naturally occurring circumstances. Real-life cortisol release in response to stress is accompanied by the release of many other neuromodulators, such as norepinephrine, corticotropin-releasing hormone, dopamine, and serotonin (60). Mere administration of hydrocortisone lacks the interaction with these modulators, but does reveal a cleaner mechanistic account for the pure corticosteroid effect, which was the aim of this study.
Regardless of these potential limitations, the present results reveal two major findings. First, this study provides clear evidence for the existence of time-dependent effects of corticosteroids on human brain processing. The importance of this timing factor, although widely acknowledged in animal literature (61), has so far been neglected in human studies on corticosteroid effects. The majority of previous studies tested for corticosteroid effects ∼1 h after hydrocortisone administration (62–64), most probably resulting in a mix of corticosteroids’ rapid, nongenomic and slow, genomic effects. Our data suggests that future research on corticosteroids, along with the understanding of their effects, would greatly benefit from the incorporation of this crucial timing factor in experimental designs. Second, corticosteroids’ slow effects were shown to augment DLPFC processing and to facilitate WM performance. Because previous research has indicated that working memory and prefrontal processing are impaired under conditions of acute stress by the rapid actions of catecholamines (37), we speculate that these slow corticosteroid effects may counteract these changes and help the brain to recover in the aftermath of stress. Thereby, they may serve a highly adaptive function in normalizing brain processing when stress has subsided.
Materials and Methods
A more detailed description of the methods applied can be found in SI Materials and Methods.
Participants.
Seventy-two young (age range, 18–29 years; median, 21 years), right-handed, healthy male volunteers gave written informed consent to participate in the study. Individuals with any history of or current psychiatric, neurological, or endocrine disorders, or receiving any medication that affects central nervous system or endocrine systems, were excluded from participation. In addition, four participants were excluded from analyses because they displayed either abnormal basal salivary cortisol levels (>3 SDs above mean; one participant), or showed no elevation in salivary cortisol level in response to hydrocortisone intake, resulting in 23 men in the placebo group, 23 in the slow CORT group, and 22 in the rapid CORT group. The study was executed in accordance with the Declaration of Helsinki and approved by the local ethics committee (CMO region Arnhem-Nijmegen, The Netherlands).
Procedure.
Experiment.
To reduce the impact of diurnal variation in cortisol levels, all testing was performed in the afternoon, between 1200 hours (± 30 min) and 1800 hours (± 30 min), when hormone levels are relatively stable. Upon arrival, participants received an information brochure about the procedure, gave informed consent, and completed an intake questionnaire to ensure that inclusion and exclusion criteria were met. Next, 30 min after arrival, a first saliva sample was taken, followed by another sample 15 min later, to measure a reliable baseline level. Participants were asked to complete a first Profile of Mood States (POMS) questionnaire (20–22), after which they were briefly trained in the WM task to ensure proper understanding during scanning. Immediately after the second saliva sample (at t = −240 min) participants received the first capsule. During the entire period (∼3.5 h) before scanning, participants waited in a quiet room where they were free to conduct any activities except for anything potentially arousing (e.g., video games). At 30 min before scanning, participants were asked to complete another POMS questionnaire, and received the second capsule. Both drug capsules, containing either 10 mg CORT or placebo (cellulose), were administered orally. This dose is known to elevate salivary cortisol levels to moderate to high stress levels (19, 54, 55), and has been shown to be successful in the induction of corticosteroid effects on declarative memory (54, 55). Depending on the group to which the participant was (randomly) assigned he received either the first capsule containing placebo, the second containing placebo (placebo group); the first capsule hydrocortisone, the second capsule placebo (slow CORT group); or the first capsule placebo, the second capsule hydrocortisone (rapid CORT group).
n-Back task.
At about 4.5 h after arrival participants were taken to the scanner room were they were asked to conduct an n-back task. Using a blocked design, participants completed eight cycles of alternating 0-, 1-, 2-, and 3-back conditions, interleaved by a short fixation period (2.4 s) (Fig. S2). Within each block, a pseudorandom digit sequence (no more than two repetitions) consisting of 12 single digits was shown to participants. Each digit was presented for 400 ms, followed by an interstimulus interval of 1400 ms. Each block started with a 2-s cue presentation indicating the 0-, 1-, 2-, or 3-back condition, resulting in an interblock interval of 26 s. Blocks were presented in a mirrored design avoiding covariation with linear drift. During the 0-back condition, participants were asked to decide whether or not the current item on the screen was a “1.” During the 1-back condition, participants were asked to detect whether the current item had appeared one position back in the sequence. Similarly, in the 2- and 3-back condition, participants were instructed to detect whether the current item had appeared two or three positions back, respectively. Each sequence contained either two or three targets, and participants were asked to make a button press with their right index finger as fast as possible when detecting a target. To ensure proper understanding and sufficient performance, participants practiced each condition twice earlier that day outside the MRI scanner (at t = −240 min) and twice inside immediately before the actual task (t = 0 min).
Physiological and Psychological Measures.
Salivary cortisol measure.
Cortisol levels were measured from saliva at 10 time points (Fig. 1): baseline measurements twice at the beginning of the experiment (t = −255 and −240 min), and eight samples (t = −210, −180, −150, −30, 0, 30, 60, and 90 min) to assess cortisol changes throughout the experiment. Saliva was collected using a commercially available collection device (Salivette, Sarstedt).
Heart rate.
Cardiac rhythm of the participants was measured during scanning using a pulse oximeter placed on their left index finger. Participants were instructed to keep their hands as still as possible during the measurement. Heart rate frequency was calculated using in-house software.
Mood state.
Mood state was assessed using the POMS questionnaire (20–22) at three time points: at the beginning of the experimental day (t = −255 min), just before entering the fMRI scanner (t = −30 min), and just before departure (t = 90 min).
Physiological and Psychological Statistical Analysis.
Behavioral and physiological data were analyzed in SPSS 15.0 (SPSS, Inc) using mixed-model ANOVAs with WM load (3- vs. 2- vs. 1- vs. 0-back) as within-subject factor and CORT manipulation (placebo vs. slow CORT vs. rapid CORT) as between-subjects factor. Participant age was included as covariate. Due to the high levels of skewness and kurtosis of the POMS questionnaire (20–22), mood data were analyzed using nonparametric tests (Friedman test). The two measures of working memory performance, accuracy and reaction times, were analyzed both separately and combined as one overall WM performance measure using Stouffer's z-score method (65). This method first applies a z-transformation to both independent variables and subsequently combines them (here by subtraction) into one overall z-score. Alpha was set at 0.05 throughout.
MRI Acquisition.
Participants underwent scanning with a Siemens MAGNETOM Avanto 1.5 Tesla MRI scanner equipped with an eight-channel head coil. A series of blood oxygenation level dependent (BOLD) T2*-weighted gradient echo EPI images was acquired with the following parameters: TR = 2340 ms, TE = 35 ms, FA = 90 °, 32 axial slices approximately aligned with AC–PC plane, slice matrix size = 64 × 64, slice thickness = 3.5 mm, slice gap = 0.35 mm, field of view (FOV) = 212 × 212 mm2. Owing to its relatively short TE, this sequence yields optimal contrast-to-noise ratio in the medial temporal lobes. High-resolution anatomical images were acquired for individuals by a T1-weighted 3D Magnetization-Prepared RApid Gradient Echo (MP-RAGE) sequence, which used the following parameters: TR = 2250 ms, TE = 2.95 ms, FA = 15 °, orientation: sagittal, FOV = 256 × 256 mm2, voxel size = 1.0 mm isotropic.
fMRI Data Analysis.
Data were analyzed using Statistical Parametric Mapping software (SPM5; University College London). Following standard preprocessing procedures (SI Materials and Methods), data were analyzed using a general linear model, in which individual events were modeled based on drug condition and working memory load [1-, 2- or 3-back contrasted vs. 0-back (baseline)]. Regressors were temporally convolved with the canonical hemodynamic response function of SPM5. The six covariates corresponding to the movement parameters obtained from the realignment procedure were also included in the model. To reduce nonspecific differences between scan sessions, global normalization using proportional scaling was applied. The single-subject parameter estimates from each session and condition obtained from the first-level analysis were included in subsequent random effects analyses. For the second-level analysis, a factorial ANOVA was used, with working memory load (1-, 2-, 3-back) as within-subject factor, drug condition (placebo vs. slow CORT vs. rapid CORT) as between-subjects factor, and participant age as covariate.
Given strong neurophysiological evidence for the locus of corticosteroid receptors (5) and its involvement in WM processing (18), the DLPFC was a region of interest. Data concerning this region was corrected for reduced search volume through an anatomical mask as defined by the WFU PickAtlas Tool, version 2.4 (bilateral middle frontal gyrus). A threshold of P < 0.05, familywise error whole-brain corrected, was applied to all other regions.
Supplementary Material
Acknowledgments
This work was supported by Grant 021.002.053 from The Netherlands Organization for Scientific Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019128108/-/DCSupplemental.
References
- 1.McEwen BS. Influences of adrenocortical hormones on pituitary and brain function. Monogr Endocrinol. 1979;12:467–492. doi: 10.1007/978-3-642-81265-1_25. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 3.Sapolsky RM, McEwen BS, Rainbow TC. Quantitative autoradiography of [3H]corticosterone receptors in rat brain. Brain Res. 1983;271:331–334. doi: 10.1016/0006-8993(83)90295-0. [DOI] [PubMed] [Google Scholar]
- 4.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 5.de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol. 1991;12:95–164. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Karst H, et al. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlides C, Kimura A, Magariños AM, McEwen BS. Hippocampal homosynaptic long-term depression/depotentiation induced by adrenal steroids. Neuroscience. 1995;68:379–385. doi: 10.1016/0306-4522(95)94332-s. [DOI] [PubMed] [Google Scholar]
- 8.Wiegert O, Pu Z, Shor S, Joëls M, Krugers H. Glucocorticoid receptor activation selectively hampers N-methyl-D-aspartate receptor dependent hippocampal synaptic plasticity in vitro. Neuroscience. 2005;135:403–411. doi: 10.1016/j.neuroscience.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Joëls M. Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 11.Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1213–1223. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 13.Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007 doi: 10.1155/2007/60803. 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerqueira JJ, et al. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17:1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- 16.Dedovic K, D'Aguiar C, Pruessner JC. What stress does to your brain: A review of neuroimaging studies. Can J Psychiatry. 2009;54:6–15. doi: 10.1177/070674370905400104. [DOI] [PubMed] [Google Scholar]
- 17.van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joëls M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem. 2010;93:56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Baddeley A. Working memory: Looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 19.Morgan CA, 3rd, et al. Hormone profiles in humans experiencing military survival training. Biol Psychiatry. 2000;47:891–901. doi: 10.1016/s0006-3223(99)00307-8. [DOI] [PubMed] [Google Scholar]
- 20.Wald FD, Mellenbergh GJ. De verkorte versie van de Nederlandse vertaling van de Profile of Mood States (POMS) Ned Tijdschr Psychol. 1990;45:86–90. [Google Scholar]
- 21.de Groot MH. Psychometrische aspecten van een stemmingsschaal (Verkorte POMS) Gedrag Gezond. 1992;20:46–51. [Google Scholar]
- 22.Reddon JR, Marceau R, Holden RR. A confirmatory evaluation of the Profile of Mood States: Convergent ans discriminant item validity. J Psychopathol Behav Assess. 1985;7:243–259. [Google Scholar]
- 23.Prinzmetal W, McCool C, Park S. Attention: Reaction time and accuracy reveal different mechanisms. J Exp Psychol Gen. 2005;134:73–92. doi: 10.1037/0096-3445.134.1.73. [DOI] [PubMed] [Google Scholar]
- 24.Pachella RG. In: Human Information Processing: Tutorials in Performance and Cognition. Kantowitz B, editor. Hillsdale, NJ: Erlbaum; 1974. pp. 41–82. [Google Scholar]
- 25.Neubauer AC, Bauer C, Holler G. Intellegence, attention, motivation and speed-accuracy trade-off in the Hick-paradigm. Pers Individ Dif. 1992;13:1325–1332. [Google Scholar]
- 26.Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci. 2001;14:675–689. doi: 10.1046/j.0953-816x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- 27.Henckens MJ, van Wingen GA, Joëls M, Fernández G. Time-dependent effects of corticosteroids on human amygdala processing. J Neurosci. 2010;30:12725–12732. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joëls M, Velzing E, Nair S, Verkuyl JM, Karst H. Acute stress increases calcium current amplitude in rat hippocampus: Temporal changes in physiology and gene expression. Eur J Neurosci. 2003;18:1315–1324. doi: 10.1046/j.1460-9568.2003.02845.x. [DOI] [PubMed] [Google Scholar]
- 29.Morsink MC, et al. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol. 2006;18:239–252. doi: 10.1111/j.1365-2826.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- 30.Joëls M, de Kloet ER. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15:25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- 31.Elzinga BM, Roelofs K. Cortisol-induced impairments of working memory require acute sympathetic activation. Behav Neurosci. 2005;119:98–103. doi: 10.1037/0735-7044.119.1.98. [DOI] [PubMed] [Google Scholar]
- 32.Oei NY, Everaerd WT, Elzinga BM, van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress. 2006;9:133–141. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- 33.Schoofs D, Preuss D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33:643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Birnbaum SG, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 35.Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: Effects of antidepressants. Cereb Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- 37.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henckens MJ, Hermans EJ, Pu Z, Joëls M, Fernández G. Stressed memories: How acute stress affects memory formation in humans. J Neurosci. 2009;29:10111–10119. doi: 10.1523/JNEUROSCI.1184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Marle HJ, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: How acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Qin S, Hermans EJ, van Marle HJ, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Kumsta R, et al. Working memory performance is associated with common glucocorticoid receptor gene polymorphisms. Neuropsychobiology. 2010;61:49–56. doi: 10.1159/000262180. [DOI] [PubMed] [Google Scholar]
- 42.Monk CS, Nelson CA. The effects of hydrocortisone on cognitive and neural function: A behavioral and event-related potential investigation. Neuropsychopharmacology. 2002;26:505–519. doi: 10.1016/S0893-133X(01)00384-0. [DOI] [PubMed] [Google Scholar]
- 43.Oei NY, Tollenaar MS, Spinhoven P, Elzinga BM. Hydrocortisone reduces emotional distracter interference in working memory. Psychoneuroendocrinology. 2009;34:1284–1293. doi: 10.1016/j.psyneuen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Yuen EY, et al. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf OT, et al. Cortisol differentially affects memory in young and elderly men. Behav Neurosci. 2001;115:1002–1011. doi: 10.1037//0735-7044.115.5.1002. [DOI] [PubMed] [Google Scholar]
- 46.Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans. Behav Neurosci. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- 47.Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 49.Yuen EY, et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pecci A, et al. New lead compounds in the search for pure antiglucocorticoids and the dissociation of antiglucocorticoid effects. J Steroid Biochem Mol Biol. 2009;113:155–162. doi: 10.1016/j.jsbmb.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Heikinheimo O, Kekkonen R. Dose-response relationships of RU 486. Ann Med. 1993;25:71–76. doi: 10.3109/07853899309147861. [DOI] [PubMed] [Google Scholar]
- 52.Heikinheimo O, et al. Plasma concentrations and receptor binding of RU 486 and its metabolites in humans. J Steroid Biochem. 1987;26:279–284. doi: 10.1016/0022-4731(87)90083-5. [DOI] [PubMed] [Google Scholar]
- 53.Wickelgren WA. Speed-accuracy tradeoff and information processing dynamics. Acta Psychol (Amst) 1977;41:67–85. [Google Scholar]
- 54.Tops M, et al. Acute cortisol effects on immediate free recall and recognition of nouns depend on stimulus valence. Psychophysiology. 2003;40:167–173. doi: 10.1111/1469-8986.00018. [DOI] [PubMed] [Google Scholar]
- 55.Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- 56.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- 57.Lupien SJ, et al. The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology. 2002;27:401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- 58.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Kloet ER, Karst H, Joëls M. Corticosteroid hormones in the central stress response: Quick-and-slow. Front Neuroendocrinol. 2008;29:268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 62.de Quervain DJ, et al. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci. 2003;17:1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- 63.Oei NY, et al. Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain Imaging Behav. 2007;1:31–41. doi: 10.1007/s11682-007-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buss C, Wolf OT, Witt J, Hellhammer DH. Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology. 2004;29:1093–1096. doi: 10.1016/j.psyneuen.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 65.Stouffer SA, et al. The American Soldier, Vol. 1: Adjustment during Army Life. Princeton: Princeton University Press; 1949. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.