Abstract
The 6-kDa early secretory antigenic target of Mycobacterium tuberculosis (ESAT-6) and the 10-kDa culture filtrate antigen (CFP-10), encoded in region of difference 1 (RD1) and secreted by the ESAT-6 system 1 (Esx-1) secretion system, are the most immunodominant and highly M. tuberculosis (MTB)-specific antigens. These attributes are responsible for their primary importance in tuberculosis (TB) immunodiagnosis and vaccine development. Rv3615c [Esx-1 substrate protein C (EspC)], encoded outside RD1, is similar in size and sequence homology to CFP-10 and ESAT-6, suggesting it might be a target of cellular immunity in TB. Using ex vivo enzyme-linked immunospot- and flow cytometry-based cytokine-secretion assay, we comprehensively assessed cellular immune responses to EspC in patients with active TB, latently infected persons, and uninfected bacillus Calmette–Guérin (BCG)-vaccinated controls. EspC was at least as immunodominant as ESAT-6 and CFP-10 in both active and latent TB infection. EspC contained broadly recognized CD4+ and CD8+ epitopes, inducing a predominantly CD4+ T-cell response that comprised functional T-cell subsets secreting both IFN-γ and IL-2 as well as functional T-cell subsets secreting only IFN-γ. Surprisingly, T-cell responses to EspC were as highly specific (93%) for MTB infection as responses to ESAT-6 and CFP-10, with only 2 of 27 BCG-vaccinated controls responding to each antigen. Using quantitative proteomics and metabolically labeled mutant and genetically complemented MTB strains, we identified the mechanism of the specificity of anti-EspC immunity as the Esx-1 dependence of EspC secretion. The high immunodominance of EspC, equivalent to that of ESAT-6 and CFP-10, makes it a TB vaccine candidate, and its high specificity confers strong potential for T-cell–based immunodiagnosis.
The inadequate protection afforded by bacillus Calmette–Guérin (BCG) vaccination provides continued impetus for the discovery of immunodominant Mycobacterium tuberculosis (MTB) antigens for development of improved vaccines. Although the immune mechanisms of protection against TB are incompletely understood, T-helper cell type 1 (Th1)-mediated immunity, in particular IFN-γ, is essential although not sufficient. All tuberculosis (TB) subunit vaccine candidates currently in clinical trials therefore incorporate MTB antigens that elicit strong Th1-type immune responses during natural infection and are recognized in latent TB infection (LTBI), where the bacillus has been contained successfully, as well as in active TB. A major challenge in vaccine development is to identify immunodominant antigens that elicit a strong IFN-γ and IFN-γ/IL-2 polyfunctional response from effector and memory T cells of both CD4+ and CD8+ T-cell subsets. Candidate vaccine antigens also need to be widely recognized in infected individuals to be immunogenic and effective in genetically heterogeneous outbred populations.
Antigens that elicit strong T-cell immunity but also are specific for MTB infection also are important for immunodiagnosis. For example, the 6-kDa early secretory antigenic target of MTB (ESAT-6), which is a leading vaccine candidate in the form of the ESAT-6-Ag85 fusion protein Hybrid 1 (1, 2) is also, together with 10-kDa culture filtrate antigen (CFP-10), the basis of T-cell–based diagnostic blood tests for MTB infection or IFN-γ release assays (IGRAs) (3, 4). ESAT-6 and CFP-10 are the most immunodominant MTB antigens hitherto identified, and their diagnostic specificity stems from their genomic location within region of difference 1 (RD1), a part of the MTB genome absent from all Mycobacterium bovis BCG vaccine strains (5–7). The limited diagnostic sensitivity of IGRAs is predicated on the availability of only two T-cell antigens that are simultaneously highly immunodominant and highly specific; the remaining seven RD1-encoded gene products, although potentially highly specific, do not encode proteins known to be strongly antigenic (8). RD1 is part of the larger ESAT-6 system 1 (Esx-1) type VII secretion system which is associated with virulence and necessary for secretion of ESAT-6, CFP-10, and other Esx-1 substrates (9–14).
As part of our program to identify new immunodominant MTB T-cell antigens, we identified the ESAT-6–like protein, Esx-1 substrate protein C (EspC; Rv3615c), as an interesting candidate. EspC is a small protein (103 amino acids), similar in length to ESAT-6, CFP-10, and other members of the ESAT-6 family, and has a low molecular mass (10.1 kDa) assigning it to the narrow fraction of proteins with a molecular mass of 6–12 kDa that is strongly recognized by T cells isolated from patients with TB (15). Motif analysis revealed that the N-terminal 20 amino acids of EspC are conserved in several ESAT-6 family members, suggesting that EspC may be distantly related to the ESAT-6 family of proteins (16), and BLAST searches reveal relatively high sequence similarity with CFP-10 and ESAT-6. Transcriptional response studies indicate that EspC is up-regulated at low pH (17) and in low-iron conditions (18) in vitro, conditions which pertain in the macrophage phagosome, suggesting that EspC is actively expressed and therefore is accessible to antigen-processing pathways during intracellular infection in vivo. We therefore hypothesized that EspC may be recognized by T cells from persons infected with MTB.
Results
Study Population.
Demographic and clinical characteristics of the study population are shown in Table S1.
T-Cell Recognition of EspC in Active TB.
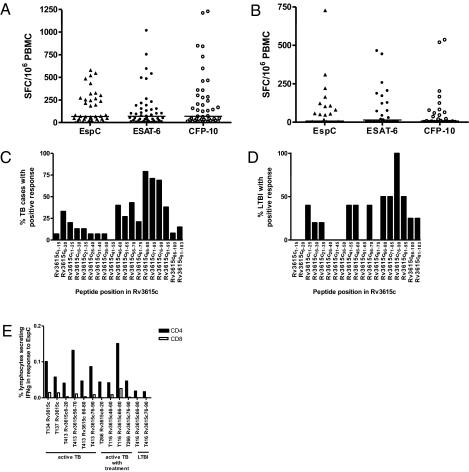
To test whether EspC is a target of T cells in MTB-infected humans, we tested peripheral blood mononuclear cells (PBMC) from 45 patients with TB against our comprehensive panel of EspC peptides in IFN-γ–ELISpot assays. T cells recognizing EspC were detected in 34 patients (76%), a rate of response very similar to that seen for ESAT-6 and CFP-10 (76% and 78%, respectively; Table 1) in the same patients, identifying EspC as a major MTB T-cell antigen. Moreover, the frequency of EspC peptide-specific IFN-γ responses was high (Fig. 1A), similar to the frequency of T cells recognizing ESAT-6 and CFP-10 (SI Results).
Table 1.
Response to ESAT-6, CFP-10, and EspC alone and in combination in an IFN-γ ELISpot assay
| TB cases (n = 45) |
LTBI suspects* (n = 27) |
Controls (n = 27) |
||||
| n | % | n | % | n | % | |
| ESAT-6 | 34/45 | 76 | 12/27 | 45 | 2/27 | 7 |
| CFP-10 | 35/45 | 78 | 12/27 | 45 | 2/27 | 7 |
| EspC | 34/45 | 76 | 13/27 | 48 | 2/27 | 7 |
| ESAT-6/CFP-10 | 42/45 | 93 | 15/27 | 56 | 2/27 | 7 |
| ESAT-6/CFP-10/EspC | 44/45 | 98 | 17/27 | 63 | 2/27 | 7 |
| ESAT-6/EspC | 41/45 | 91 | 15/27 | 56 | 2/27 | 7 |
| CFP-10/EspC | 44/45 | 98 | 15/27 | 56 | 2/27 | 7 |
*Of the 27 LTBI suspects, 15 were deemed to have LTBI on the basis of a positive TST and positive IFN-γ ELISpot response to ESAT-6 and/or CFP-10.
Fig. 1.
(A and B) Frequency of peptide-specific IFN-γ SFC for EspC, ESAT-6, and CFP-10 in 47 TB cases (A) and 27 subjects with risk factors for LTBI (B), enumerated by ex vivo ELISpot. Horizontal lines indicate median responses. (C and D) T-cell epitope maps of EspC for 15 TB cases (C) and five persons with LTBI (D). IFN-γ production in response to EspC epitopes was primarily by CD4+ T cells as shown by cytokine-secretion assay and flow cytometry (E). Results are expressed as percentages of lymphocyte populations staining CD4+IFN-γ+ or CD8+IFN-γ+. Subjects T134, T137, T413, T266, and T116 had active TB (T266 and T116 with 2-mo treatment); subject T416 had LTBI.
Forty-two of 45 TB cases (93%) responded to ESAT-6 and/or CFP-10, whereas 44 of 45 (98%) responded to ESAT-6 and/or CFP-10 and/or EspC (Table 1). Hence, two of the three cases that did not respond to either of the immunodominant antigens ESAT-6 and/or CFP-10 did respond to EspC. Combining responses to CFP-10 and/or EspC gave the highest sensitivity with the minimal number of antigens (Table 1).
T-Cell Recognition of EspC in LTBI.
To determine whether EspC is a target of T cells during long-term immune containment of MTB infection as well as in active TB, we studied 27 persons with risk factors for LTBI; 25 underwent a tuberculin skin test (TST), of whom 22 (88%) were TST-positive (≥10-mm induration), and the 15 who also were positive to ESAT-6 and/or CFP-10 were deemed to have LTBI (12 were positive to ESAT-6 and 12 to CFP-10). We included individuals with LTBI risk factors who were negative by TST and/or ESAT-6/CFP-10 ELISpot assays because, given the lack of a gold-standard test for LTBI, we wished to assess whether responses to EspC also can detect individuals with LTBI in the absence of responses to ESAT-6/CFP-10.
Eleven of the 15 persons (73%) deemed to have LTBI based on TST and ELISpot responses to ESAT-6 and/or CFP-10 had circulating EspC-specific IFN-γ−secreting T cells. Thirteen of the 27 persons (48%) with risk factors for LTBI responded to EspC, similar to the proportion responding to ESAT-6 and CFP-10 [12 of 27 (45%) for each antigen; Table 1]. EspC therefore is a major target of Th1-type T-cell immunity in LTBI as well as in active TB. There was no significant difference in the frequency of EspC- specific IFN-γ−secreting T cells in subjects responding to EspC, responders to ESAT-6, and responders to CFP-10 (Fig. 1B and SI Results).
Fifteen of 27 LTBI suspects (56%) had a response to ESAT-6 and/or CFP-10, but 17 of the 27 LTBI suspects (63%) responded to ESAT-6 and/or CFP-10 and/or EspC, identifying two additional persons as possibly infected with MTB (Table 1).
EspC Contains Multiple, Broadly Recognized T-Cell Epitopes.
To determine the location and immunodominance of T-cell epitopes within EspC, we quantified IFN-γ−secreting T cells specific for each of the 19 15mer peptides from EspC by ex vivo IFN-γ−ELISpot in 15 patients with TB and five LTBI donors (Fig. 1 C and D). In active TB, peptides located between amino acid positions 46 and 95 were commonly recognized, with peptides EspC66–80, EspC71–85, and EspC76–90 most frequently recognized (by 79%, 71%, and 69% of cases, respectively) (Fig. 1C). In LTBI, the spectrum of peptides recognized was similar (Fig. 1D).
The majority of patients with TB and persons with LTBI responded to more than one EspC peptide; of the 15 patients with active TB in Fig. 1C, three responded to one peptide only; one responded to two peptides; and 11 responded to four or more peptides. Of the five persons with LTBI in Fig. 1D, two responded to two peptides; one responded to three peptides; one responded to eight peptides; and one responded to 11 peptides.
Most EspC Peptide-Specific, IFN-γ–Secreting T Cells Are CD4+.
To determine the phenotype of EspC-specific T cells, we performed flow cytometric analysis on EspC peptide-stimulated PBMC after immunomagnetic enrichment for IFN-γ–secreting cells. T cells responding to the EspC peptide pool and to individual immunodominant peptides (as identified in Fig. 1 C and D) in active TB and LTBI were predominantly CD4+ (Fig. 1E), but six peptides also were recognized by CD8+ T cells. The frequency of EspC-specific IFN-γ–secreting CD4+ T cells [median (interquartile range; IQR) 0.05% (0.04, 0.09)] was substantially higher than the frequency of CD8+ T cells [median (IQR) 0.006% (0.001, 0.01)] (P < 0.001).
EspC Is a Target of Polyfunctional T Cells Secreting both IFN-γ and IL-2.
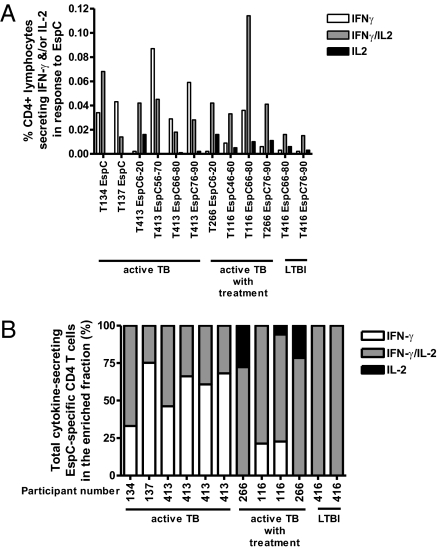
Given the role of T cells secreting both IFN-γ and IL-2 and T cells secreting only IL-2 in conferring protective immunity to intracellular pathogens, we quantified the frequency of EspC-specific CD4+ and CD8+ T cells secreting IFN-γ only, IFN-γ/IL-2, and IL-2 only in 10 patients with TB (four on treatment) and two persons with LTBI. In active TB, CD4+ T cells predominantly secreted IFN-γ only and IFN-γ/IL-2 in response to EspC (Fig. 2). In patients on treatment and in persons with LTBI the profile was distinct, with a dominance of dual IFN-γ/IL-2–secreting CD4+ T cells and a lower proportion of T cells secreting IFN-γ only and the presence of EspC-specific T cells secreting IL-2 only (Fig. 2 and Fig. S1). Although the frequency of EspC-specific IFN-γ– and/or IL-2–secreting CD8+ T cells was very low and often was below the detection limit of this assay in TB (0.007% of T cells) (19), the CD8+ T-cell response in active TB was IFN-γ only (frequency, 0.008–0.013%) (n = 4) compared with a codominance of CD8+ T cells secreting both IFN-γ and IL-2 (frequency, 0.014%) or secreting only IFN-γ (frequency, 0.012%) in the one patient with TB on treatment in whom CD8+ T cells were detectable.
Fig. 2.
The IFN-γ and IL-2 cytokine profiles of CD4+ T cells specific for EspC in active TB, during anti-TB therapy, and in LTBI. (A) Percentages plotted indicate the frequency of CD4+ T cells producing IFN-γ and/or IL-2 in response to EspC peptides. Background levels of nonspecific cytokine production have been subtracted. (B) Relative proportions of CD4+ T cells producing IFN-γ and/or IL-2 in the enriched fraction.
EspC-Specific T Cells Are Absent in BCG-Vaccinated Controls.
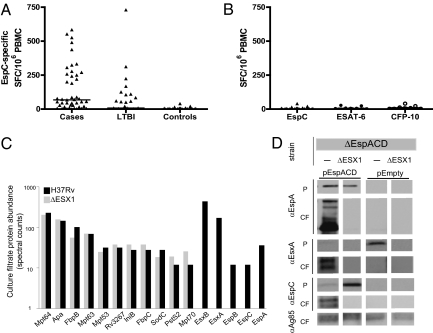
We next compared the T-cell responses to EspC in BCG-vaccinated controls who had no risk factors for MTB infection with the responses in our MTB-infected study population. Of the 27 BCG-vaccinated controls, only two (7%) responded to EspC (Fig. 3A and Table 1). All subjects also were screened for responses to ESAT-6 and CFP-10, and the same two subjects responded to both antigens (Fig. 3B and Table 1). IFN-γ responses to EspC, ESAT-6, and CFP-10 were low in frequency and borderline positive [spot-forming cells (SFC)/106 PBMC for the two subjects for each of the following antigens: EspC 20, 40, ESAT-6 24, 28, and CFP-10 22, 40] (Fig. 3 A and B), suggesting these responses may have been weak false-positive responses in uninfected persons, as occasionally observed with ESAT-6/CFP-10 (4, 8). However, in the absence of a gold standard for LTBI, it is possible that these two subjects might have had unrecognized LTBI that was identified by the responses to EspC, ESAT-6, and CFP-10.
Fig. 3.
(A and B) Comparison of frequency of EspC-specific IFN-γ–secreting T cells in TB cases, LTBI, and controls (A) and IFN-γ–secreting T cells specific for EspC, ESAT-6, and CFP-10 in 27 BCG-vaccinated controls (B). Horizontal lines indicate median responses. EspC, identified by mass spectrometry, is present in H37Rv culture filtrates but not in the culture filtrate of the ΔRD1 (denoted as ΔEsx1) deletion mutant (C). EspC secretion is dependent on RD1; localization of EspC, EspA, and EsxA (ESAT-6) in MTB H37Rv strains carrying deletions of EspA, EspC, and EspD (denoted ΔEspACD) with or without additional deletion of RD1 (denoted ΔEsx1). Blots are shown for transposon of empty vector (pEmpty) and for transposon insertion of EspACD (pEspACD). Cell pellet (P) and culture filtrate (CF) proteins were resolved through SDS/PAGE, and the proteins were identified by Western blotting with specific antibodies (D).
Mechanism Underlying the Specificity of EspC-Specific T Cells for TB Infection is Esx-1–Dependent Secretion.
BCG-vaccinated control subjects failed to generate a detectable T-cell response to EspC, even though the EspC gene is not encompassed by RD1. Because the absence of RD1 disrupts the Esx-1 secretion system in BCG, we hypothesized that EspC might not be recognized by PBMC from BCG-vaccinated individuals because it is secreted by the ESX-1 system and thus not secreted by BCG. To determine whether EspC secretion is dependent on the Esx-1 secretion system and hence on the RD1 locus, the identities and relative abundance of proteins secreted into the culture filtrate by wild-type MTB strain H37Rv and the mutated strain H37RvΔRD1 were determined by quantitative proteomics. Although the abundance of most proteins predicted to be secreted by the general secretory system was similar in the two strains, EspC was present in wild-type culture filtrates but absent from H37RvΔRD1 culture filtrates (Fig. 3C), as were ESAT-6 (EsxA), CFP-10 (EsxB), EspA, and EspB.
To confirm that EspC is secreted in an ESX-1–dependent fashion, we epitope-tagged espC and ectopically expressed this tagged construct as part of a three-gene cassette, espACD, mimicking the native operon. We expressed this cassette in both wild-type MTB lacking the espACD genes and in MTB lacking both an intact RD1 locus and the espACD genes. We and others have shown previously that mutations in EspA, C, and D abolished export of ESAT-6 (EsxA) (16, 20). Genetic complementation of the espACD deletion with the cassette expressing epitope-tagged espC restored ESAT-6 secretion and allowed EspA secretion (Fig. 3D), demonstrating that epitope-tagged EspC is functional. As expected, we found that EspC is secreted into the culture filtrates in the wild-type background. Interestingly, EspC appears in multiple isoforms in the culture filtrates but not in the cell pellets, suggesting that it is modified upon secretion (Fig. 3D). However, in MTB lacking RD1 and hence Esx-1 function, EspC was not present in the culture filtrate and accumulated in the cell pellet, indicating that secretion of EspC is dependent on RD1 (Fig. 3D).
Discussion
We identify EspC as a major target of cellular immunity in human TB infection. Remarkably, EspC was as immunodominant as ESAT-6 and CFP-10 in patients with TB and in latently infected persons. The prevalence and magnitude of IFN-γ–ELISpot responses to EspC in both active and latent TB were at least as high as for responses to ESAT-6 and CFP-10 and also identified MTB-infected persons who did not respond to ESAT-6 or CFP-10.
Several EspC-derived peptides were widely recognized by T cells from an ethnically diverse range of patients with TB and persons with LTBI, suggesting that these peptides may be recognized in the context of a wide range of HLA class II haplotypes, similar to the multiple promiscuous epitopes previously found in ESAT-6 and CFP-10 (21–24). Peptide mapping revealed that EspC contains multiple T-cell epitopes throughout its length with particularly frequent recognition of peptides toward the C terminus in both active and latent TB infection, analogous to the concentration of frequently recognized epitopes near the C terminus of CFP-10 (25) and perhaps reflecting the 33% sequence identity (75% similarity) between EspC (amino acids 75–98) and CFP-10 (amino acids 71–94) in this region.
Both CD4+ and CD8+ T cells are required for protective immunity against TB and therefore are important in vaccine development (26), with candidate vaccine antigens typically inducing both T-cell populations during natural infection (1). We determined that EspC-derived peptides are recognized by both CD4+ and CD8+ IFN-γ–secreting T cells, induction of the latter being consistent with EspC secretion and access to the cytosolic MHC class I antigen-processing pathway during intracellular infection in vivo (27–29). The high density of multiple CD4+ and CD8+ epitopes in EspC, with a dominance of CD4+ responses, is reminiscent of ESAT-6 (21, 22, 30, 31) and CFP-10 (21, 22, 30, 31).
The quality or cytokine profile of antigen-specific T cells, and, in particular, antigen-specific T cells secreting both IFN-γ and IL-2, is increasingly considered to be important for vaccine design and evaluation (32). In mice, ESAT-6 elicits a strong IFN-γ and dual IFN-γ/IL-2 response and induces protection, whereas polyfunctional T cells in the lung are associated with protection induced by Ag85A recombinant adenovirus (33). In humans, dual IFN-γ–/IL-2–secreting T cells are preferentially associated with self-healed TB (34) and LTBI (35), consistent with a role in long-term immune control. Accordingly, candidate vaccine antigens should be recognized by T cells secreting both IFN-γ and IL-2 as well as by T cells secreting only IFN-γ during natural infection, as observed for ESAT-6, CFP-10 (19, 35), Ag85B, and TB10.4 (36), and new vaccines aim to induce such polyfunctional T cells (37–39). We found that EspC induces a strong IFN-γ and dual IFN-γ/IL-2 response in active TB. As previously observed for ESAT-6 and CFP-10, this functional signature of EspC-specific T cells shifts in successfully treated patients to a dominance of dual IFN-γ–/IL-2–secreting T cells, and this shift also occurs in LTBI.
Collectively, these findings identify EspC as an MTB vaccine candidate. Although immunodominance and induction of polyfunctional T-cell responses remain dominant criteria for selecting antigens as vaccine candidates, two recent studies suggest that protective immunity may be more complex, with implications for vaccine design. A cohort study of BCG-vaccinated infants in South Africa found that BCG-specific polyfunctional T cells measured from blood 10 wk postvaccination did not correlate with BCG-induced protection against TB (40). Furthermore, whole-genome sequencing of 21 MTB strains found that genes encoding known T-cell antigens were highly conserved, suggesting that purifying selection may act on T-cell epitopes and raising the intriguing possibility that MTB might benefit from recognition by human T cells (41).
Remarkably, T-cell immunity to EspC also was highly specific (93%) for MTB infection, as evidenced by the very low proportion (7%) of BCG-vaccinated unexposed controls that had EspC-specific IFN-γ–secreting T cells. In our study population, T-cell responses to EspC were as specific for MTB infection as responses to ESAT-6 and CFP-10. Given the limited number of gene products encoded in RD1, proteins that are specific for MTB are very rare, and only two, ESAT-6 and CFP-10, are strong T-cell antigens. Our findings define EspC as being as immunodominant and as highly specific for MTB infection as ESAT-6 and CFP-10, indicating potential for immunodiagnosis as well as vaccine development. In this regard, it is notable that two of the three patients with TB who did not respond to ESAT-6 or CFP-10 did respond to EspC. Given the limited diagnostic sensitivity of ESAT-6–/CFP-10–based IGRAs, large prospective studies to quantify the incremental diagnostic sensitivity conferred by EspC when used in combination with ESAT-6 and CFP-10 are now a priority.
The potential for the M. bovis counterpart of EspC (Mb3645c) to increase incrementally the sensitivity of antigen mixtures for immunodiagnosis of M. bovis infection in cattle was noted following a screen of 14 M. bovis proteins highly expressed at the mRNA level during in vitro culture (42). Four of these proteins were recognized by T cells in bovine-TST–positive cattle, with Mb3645c eliciting responses in 11 of 30 cattle (37%).
The EspC gene is encoded outside the RD1 locus and thus is present in BCG (7). A BCG strain complemented with RD1 and flanking genes from Esx-1 expresses and exports ESAT-6 and induces ESAT-6–specific T-cell responses in infected mice, conferring better protection against TB than provided by BCG (14), and ESAT-6 export correlates with the generation of ESAT-6–specific T-cell responses (9). We therefore hypothesized that EspC may be recognized specifically in MTB infection but not in BCG-vaccinated persons because the protein might not be secreted in BCG if secretion were via the Esx-1 secretion system and therefore were RD1 dependent. Indeed, we found that EspC was detected in MTB H37Rv culture filtrate but was absent from culture filtrate of MTB H37RvΔRD1, as also observed for ESAT-6, CFP-10, EspB, and EspA. This offers a previously unrecognized example of an MTB antigen where high diagnostic specificity is conferred by RD1-dependent secretion. An alternative explanation might be that EspC is transcriptionally down-regulated or is unstable in the absence of an intact Esx-1 locus; however, these explanations are less likely, because the protein was detectable and stable in cell pellets of both wild-type MTB and the ΔRD1 deletion mutant. Given the high concentration of frequently recognized T-cell epitopes and the sequence similarity in the C termini of EspC and CFP-10, it is notable that secretion of the homologs of EspC and CFP-10 in Mycobacterium marinum is dependent on C-terminal amino acid motifs that direct them to the Esx-1 system for secretion through interaction with distinct Esx-1–associated cytosolic ATPases (43). Thus, it is tempting to speculate that the C termini of these proteins may be recognized frequently by T cells and yet be invariant because they are constrained by their biological function.
ESAT-6 (EsxA) and CFP-10 (EsxB) are the founding members of the WXG100 family of proteins (characterized by a central WXG motif and an approximate size of 100 amino acids) which are part of the EsxAB dimer-like protein clan. Taken together, our immunologic, genetic, and proteomic data indicate that EspC, EsxA, and EsxB are the three most immunodominant MTB antigens and also are the only members of the EsxAB dimer-like protein clan known to be secreted by the virulence-associated secretion system Esx-1. The underlying reason for the high immunodominance of these three remarkable proteins currently is unclear but presumably is related to one or more of the properties they share. Because of the prominent role of Esx-1 in the virulence of MTB, it now will be important to delineate the precise contributions of EspC and the other Esx-1 substrates to pathogenesis.
The observation that only live but not dead BCG elicits substantial protection is understood to indicate that active export of antigenic proteins is essential for maximizing protective immune responses, and many of the most promising vaccine candidates are secreted antigens. A corollary of our data is that the enhanced protective efficacy of BCG complemented with RD1 and several surrounding genes, as compared with that of BCG alone, may in fact be attributable to EspC secretion as well as secretion of ESAT-6 and CFP-10 (14). The notable immunodominance of EspC could be exploited for vaccination through delivery of EspC alone (as an adjuvanted protein or in recombinant viral vectors) or as part of a recombinant BCG strain. New TB vaccines will be deployed, at least initially, in BCG-vaccinated persons and, unlike vaccines based on antigens expressed by BCG, EspC-based vaccination would broaden the repertoire of potentially protective antigen-specific immune responses.
The global effort to develop improved tools for diagnosis, vaccination, and treatment of TB has made substantial advances in recent years, and deployment of the outputs of this research will require strategic coordination. There are 12 TB vaccine candidates in clinical trials, but, until this study, only two immunodominant and specific immunodiagnostic T-cell antigens were known. Hybrid 1, a leading vaccine, is an ESAT-6–based fusion protein (1, 2); its widespread deployment would diminish the specificity of IGRA, thereby undermining diagnosis and targeted treatment of LTBI, a fundamental pillar of TB control. Our data indicate that EspC could readily replace ESAT-6 to maintain diagnostic sensitivity and specificity of the IGRA platform, thus providing a practical immunological solution to this emerging strategic conundrum in global TB control.
Materials and Methods
Participants.
Forty-five consenting adult patients with culture-confirmed (n = 27) or highly probable (n = 18) active TB, as previously defined (44), and 27 persons with risk factors for LTBI were prospectively recruited. Control participants (n = 27) were healthy, BCG-vaccinated persons from regions of low TB incidence who had no known MTB exposure. Further details are given in SI Materials and Methods and Table S1.
Ex Vivo IFN-γ ELISpot Assays.
Ex vivo IFN-γ ELISpot assays were performed as previously described using 15mer peptides (overlapping their adjacent peptides by 10 amino acids) and 2.5 × 105 PBMC per well (19). Pairs of duplicate ELISpot (Mabtech AB) wells contained positive (mitogen) and negative (no stimulus) controls and peptide pools spanning EspC (n = 19 peptides), ESAT-6 (n = 17 peptides), and CFP-10 (n = 18 peptides) at 10 μg/mL Further details are given in SI Materials and Methods.
CD4 and CD8 Phenotyping of IFN-γ and IL-2 Single- and Dual-Secreting Antigen-Specific T Cells.
Cytokine secretion assays, flow-cytometry (Cyan 7; Dako), and analysis (FlowJo version 6.1; Tree Star) were performed as previously described (19) (SI Materials and Methods).
Bacteria and Culture for Mass Spectrometry.
To compare the abundance of proteins secreted into culture filtrate by wild-type H37Rv and H37RvΔRD1 (12), we used quantitative proteomics in which the number of peptide spectra from a given protein detected by tandem mass spectrometry provides a measure of protein abundance (45). Strain-specific metabolic labeling and peptide elution are described in SI Materials and Methods.
Mass Spectrometric Analysis.
Purification, fractionation, and mass spectrometric analysis of culture filtrates from labeled wild-type bacteria and the unlabeled ΔRD1 deletion mutant are described in SI Materials and Methods.
Data Analysis for Quantitative Mass Spectrometry.
Peptide identifications were made using the database search algorithm, SEQUEST (Thermo Scientific) as described in SI Materials and Methods.
Mutation and Genetic Complementation of MTB Strains.
To assess secretion of EspC in the presence and absence of the RD1 locus, we replaced the native copy of the EspACD locus with an ectopic copy of this locus in which EspC was epitope-tagged with an HA tag, as recently described (46). The same vector and two-step deletion strategy was used to delete the EspACD locus from H37RvΔRD1 (12). H37RvΔEspACD and H37RvΔRD1ΔEspACD then were complemented with pEspACD, an ectopic vector expressing EspACD under the control of a tetracycline-inducible promoter (47), or an empty vector, pEmpty, as a control as previously described (46). Secretion was assessed as previously described (20, 46). EspC was detected using antibody to the HA-epitope (Novus Biologicals).
Statistical Analysis.
Methods used in statistical analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank all participants and the staff of St Mary's Tuberculosis Service. Financial support was provided by the Wellcome Trust and by National Institute of Allergy and Infectious Diseases Grant 5R21AI70871. A.L. is a Wellcome Senior Clinical Research Fellow and National Institute of Health Research Senior Investigator.
Footnotes
Conflict of interest statement: A.L. and K.A.M. are inventors in the field of T-cell–based diagnosis. The ESAT-6/CFP-10 IFN-γ ELISpot was commercialized by an Oxford University spin-out company (Oxford Immunotec Ltd.) in which Oxford University and A.L. have a minority share of equity and entitlement to royalties.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015153108/-/DCSupplemental.
References
- 1.Barker LF, Brennan MJ, Rosenstein PK, Sadoff JC. Tuberculosis vaccine research: The impact of immunology. Curr Opin Immunol. 2009;21:331–338. doi: 10.1016/j.coi.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 2.van Dissel JT, et al. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naïve human volunteers. Vaccine. 2010;28:3571–3581. doi: 10.1016/j.vaccine.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 3.Lalvani A, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. 2001;163:824–828. doi: 10.1164/ajrccm.163.4.2009100. [DOI] [PubMed] [Google Scholar]
- 4.Mori T, et al. Specific detection of tuberculosis infection: An interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med. 2004;170:59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 5.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole ST, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 7.Behr MA, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 8.Liu XQ, et al. Evaluation of T-cell responses to novel RD1- and RD2-encoded Mycobacterium tuberculosis gene products for specific detection of human tuberculosis infection. Infect Immun. 2004;72:2574–2581. doi: 10.1128/IAI.72.5.2574-2581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodin P, et al. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun. 2006;74:88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guinn KM, et al. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu T, et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA. 2003;100:12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis KN, et al. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J Infect Dis. 2003;187:117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majlessi L, et al. Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system. J Immunol. 2005;174:3570–3579. doi: 10.4049/jimmunol.174.6.3570. [DOI] [PubMed] [Google Scholar]
- 14.Pym AS, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9:533–539. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- 15.Boesen H, Jensen BN, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacGurn JA, Raghavan S, Stanley SA, Cox JS. A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol Microbiol. 2005;57:1653–1663. doi: 10.1111/j.1365-2958.2005.04800.x. [DOI] [PubMed] [Google Scholar]
- 17.Fisher MA, Plikaytis BB, Shinnick TM. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J Bacteriol. 2002;184:4025–4032. doi: 10.1128/JB.184.14.4025-4032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez GM, Voskuil MI, Gold B, Schoolnik GK, Smith I. ideR, An essential gene in Mycobacterium tuberculosis: Role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect Immun. 2002;70:3371–3381. doi: 10.1128/IAI.70.7.3371-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millington KA, et al. Dynamic relationship between IFN-gamma and IL-2 profile of specific T cells and antigen load. J Immunol. 2007;178:5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortune SM, et al. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci USA. 2005;102:10676–10681. doi: 10.1073/pnas.0504922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shams H, et al. Characterization of a Mycobacterium tuberculosis peptide that is recognized by human CD4+ and CD8+ T cells in the context of multiple HLA alleles. J Immunol. 2004;173:1966–1977. doi: 10.4049/jimmunol.173.3.1966. [DOI] [PubMed] [Google Scholar]
- 22.Pathan AA, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: Associations with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–5225. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 23.Mustafa AS, et al. Multiple epitopes from the Mycobacterium tuberculosis ESAT-6 antigen are recognized by antigen-specific human T cell lines. Clin Infect Dis. 2000;30(Suppl 3):S201–S205. doi: 10.1086/313862. [DOI] [PubMed] [Google Scholar]
- 24.Ravn P, et al. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 25.Chapman AL, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002;16:2285–2293. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 26.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grotzke JE, et al. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 2009;5:e1000374. doi: 10.1371/journal.ppat.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewinsohn DM, et al. Secreted proteins from Mycobacterium tuberculosis gain access to the cytosolic MHC class-I antigen-processing pathway. J Immunol. 2006;177:437–442. doi: 10.4049/jimmunol.177.1.437. [DOI] [PubMed] [Google Scholar]
- 29.Grotzke JE, Siler AC, Lewinsohn DA, Lewinsohn DM. Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway. J Immunol. 2010;185:4336–4343. doi: 10.4049/jimmunol.1000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathan AA, et al. High frequencies of circulating IFN-gamma-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur J Immunol. 2000;30:2713–2721. doi: 10.1002/1521-4141(200009)30:9<2713::AID-IMMU2713>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Lewinsohn DM, et al. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: Definition of antigenic specificity. J Immunol. 2001;166:439–446. doi: 10.4049/jimmunol.166.1.439. [DOI] [PubMed] [Google Scholar]
- 32.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: Implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 33.Forbes EK, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millington KA, et al. Mycobacterium tuberculosis-specific cellular immune profiles suggest bacillary persistence decades after spontaneous cure in untreated tuberculosis. J Infect Dis. 2010;202:1685–1689. doi: 10.1086/656772. [DOI] [PubMed] [Google Scholar]
- 35.Caccamo N, et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 36.Tena-Coki NG, et al. CD4 and CD8 T-cell responses to mycobacterial antigens in African children. Am J Respir Crit Care Med. 2010;182:120–129. doi: 10.1164/rccm.200912-1862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beveridge NE, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol. 2007;37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aagaard C, et al. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One. 2009;4:e5930. doi: 10.1371/journal.pone.0005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abel B, et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med. 2010;181:1407–1417. doi: 10.1164/rccm.200910-1484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagina BM, et al. other members of the South African Tuberculosis Vaccine Initiative Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guérin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comas I, et al. Human T cell epitopes of MTB are evolutionarily hyperconserved. Nature Genetics. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sidders B, et al. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the MTB complex. Infect Immun. 2008;76:3932–3939. doi: 10.1128/IAI.00150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiGiuseppe Champion PA, Champion MM, Manzanillo P, Cox JS. ESX-1 secreted virulence factors are recognized by multiple cytosolic AAA ATPases in pathogenic mycobacteria. Mol Microbiol. 2009;73:950–962. doi: 10.1111/j.1365-2958.2009.06821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dosanjh DP, et al. Improved diagnostic evaluation of suspected tuberculosis. Ann Intern Med. 2008;148:325–336. doi: 10.7326/0003-4819-148-5-200803040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 46.Garces A, et al. EspA acts as a critical mediator of ESX1-dependent virulence in Mycobacterium tuberculosis by affecting bacterial cell wall integrity. PLoS Pathog. 2010;6:e1000957. doi: 10.1371/journal.ppat.1000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehrt S, et al. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 2005;33:e21. doi: 10.1093/nar/gni013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.