Abstract
Neural stem cells (NSCs) generate new granule cells throughout life in the mammalian hippocampus. Canonical Wnt signaling regulates the differentiation of NSCs towards the neuronal lineage. Here we identified the prospero-related homeodomain transcription factor Prox1 as a target of β-catenin–TCF/LEF signaling in vitro and in vivo. Prox1 overexpression enhanced neuronal differentiation whereas shRNA-mediated knockdown of Prox1 impaired the generation of neurons in vitro and within the hippocampal niche. In contrast, Prox1 was not required for survival of adult-generated granule cells after they had matured, suggesting a role for Prox1 in initial granule cell differentiation but not in the maintenance of mature granule cells. The data presented here characterize a molecular pathway from Wnt signaling to a transcriptional target leading to granule cell differentiation within the adult brain and identify a stage-specific function for Prox1 in the process of adult neurogenesis.
New neurons are born throughout adulthood in two distinct areas of the adult brain, the subventricular zone (SVZ) of the lateral ventricles and the hippocampal dentate gyrus (DG) (1). Adult hippocampal neurogenesis is important for hippocampus-dependent learning and memory and has been implicated in a number of neuropsychiatric diseases such as major depression, epilepsy, and age-related cognitive decline (2, 3). Although neural stem cells (NSCs) that show neurogenic potential in vitro can be isolated from various adult brain regions, only the SVZ and the DG produce substantial numbers of new neurons throughout life (4). Previous reports suggested that the niche provides important cues to exploit the neurogenic potential of adult NSCs (5). Within the hippocampal niche, several signaling pathways have been identified that are critical for stem cell maintenance and neuronal differentiation, such as sonic hedgehog (Shh) and Wnt signaling (6, 7). Wnt3 is produced by local hippocampal astrocytes, regulating the generation of newborn granule cells (7, 8). Upon binding of Wnt to its receptors of the Frizzled and Lrp families, β-catenin becomes stabilized during canonical Wnt signaling, leading to its nuclear accumulation. There, β-catenin acts as a transcriptional coactivator of TCF/LEF transcription factors, resulting in the expression of Wnt target genes. Recently, it has been shown that NeuroD1, a basic helix-loop-helix transcription factor required for granule cell genesis in the embryonic and adult DG, is regulated by β-catenin–dependent signaling (9–12). However, NeuroD1 is not only important for hippocampal neurogenesis but is also involved in the generation of SVZ-derived olfactory neurons (11, 13, 14). Thus, the network of genes regulated by the Wnt pathway to selectively generate DG granule cells in the adult hippocampus remains unknown. Here we show that Prox1 is regulated with β-catenin–TCF/LEF signaling and controls the generation of newborn granule cells in the adult hippocampus.
Results
Prox1 Promoter Contains Functional TCF/LEF-Binding Sites.
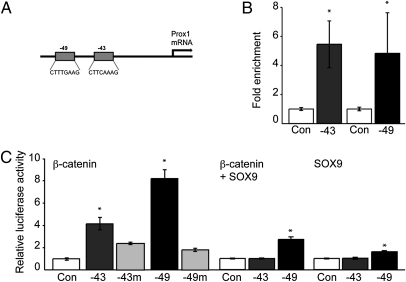
To identify potential targets of Wnt signaling within the hippocampal niche, we focused on genes expressed in the adult DG. Prox1 is a prospero-related homeodomain transcription factor, which is highly expressed in DG granule cells (10, 15, 16). Prox1 has previously been shown to be important for lens development, lymphangiogenesis, differentiation of certain spinal cord interneurons, and, very recently, in hippocampal granule cell genesis (17–20). We analyzed the murine Prox1 promoter/enhancer regions for TCF/LEF sites and found two TCF/LEF consensus sites 49 kb and 43 kb upstream of the Prox1 start codon (Fig. 1A and Fig. S1), in accordance with a previous report showing TCF/LEF sites at conserved positions in the human Prox1 enhancer sequence that appear to mediate Prox1 regulation in intestinal cancers (−43 kb, CTTCAAAG; −49 kb, CTTTGAAG) (21). First, we performed chromatin immunoprecipitations (ChIP) to analyze a potential association of β-catenin with the Prox1 enhancer region in adult NSCs. Chromatin from NSCs was precipitated with antibodies against β-catenin followed by quantitative RT-PCR (qPCR) using site-specific primers directed against the putative TCF/LEF sites within the Prox1 enhancer region. We found an enrichment of β-catenin on both the −49 kb and −43 kb sites compared with control ChIP using IgG antibody, suggesting that β-catenin is associated with these genomic regions of the Prox1 enhancer (Fig. 1B). To test the functionality of the two predicted TCF/LEF sites within the Prox1 enhancer, we cloned the −49 kb and −43 kb sites with approximately 1 kb surrounding genomic DNA into a luciferase reporter and analyzed the effect of β-catenin expression on luciferase activity. β-Catenin enhanced luciferase activity in adult hippocampal NSCs (Fig. 1C), demonstrating a functional relevance of β-catenin–mediated activity on the Prox1 enhancer in hippocampal NSCs. This finding was confirmed by using a heterologous cell system (293T cells; Fig. S1). The specificity of β-catenin–mediated induction of luciferase activity on Prox1 enhancer constructs was confirmed using site-directed mutagenesis against the consensus TCF/LEF sites: mutation of the TCF/LEF sites reduced the β-catenin–induced increase of luciferase activity (Fig. 1C). Furthermore, we found that lentiviral expression of Wnt3 led to an increase in Prox1 mRNA in NSCs (Fig. S1) using qPCR. We next analyzed if neuronal differentiation of adult NSCs upon exposure to retinoic acid/forskolin, which is associated with an increase in TOPFLASH reporter activity (Fig. S1), enhances Prox1 expression in hippocampal NSCs. Again supporting the association of TCF/LEF signaling and Prox1, we found a significant time-dependent increase of Prox1 mRNA in hippocampal NSCs upon neuronal differentiation (Fig. S1).
Fig. 1.
Prox1 enhancer contains functional TCF/LEF sites. (A) Two TCF/LEF sites are present in the Prox1 enhancer region −49 kb and −43 kb upstream of the Prox1 start codon. (B) ChIP analyses indicate that β-catenin is associated with the two TCF/LEF sites in the Prox1 enhancer region as shown by qPCR using primers surrounding the TCF/LEF sites, showing an enrichment of these sites with β-catenin precipitation compared with IgG precipitated chromatin. (C) β-Catenin expression enhanced luciferase activity of the two TCF/LEF sites containing regions cloned from the Prox1 promoter in reporter assays (dark gray bars, Left). This effect of β-catenin was blocked by site-directed mutagenesis deleting base pairs in the respective TCF/LEF sites (light gray bars, Left). SOX9 coexpression represses the effect of β-catenin at the −43 kb and, to a lesser extent, at the −49 kb site (middle bars), whereas SOX9 expression alone had no or only a small effect on luciferase activity (right bars); *P < 0.05.
Taken together, we identified two functional TCF/LEF sites in the murine Prox1 enhancer that become activated upon β-catenin expression in hippocampal NSCs. Interestingly, it has been recently shown that TCF/LEF sites and binding sites for the Sry-related HMG box (SOX) transcription factors often overlap, forming a SOX/LEF binding site (12), with the −43 kb site representing one of them. Thus, we analyzed if SOX proteins may repress the effects of β-catenin on the Prox1 enhancer region as it has been shown at comparable sites in the NeuroD1 promoter (12). We found a strong repression of β-catenin–induced luciferase activity on the −43 kb enhancer region when we coexpressed SOX9 (Fig. 1C), a SOX family member that is highly expressed in adult NSCs and that may function to repress neuronal differentiation (22, 23). Similarly, SOX9 repressed luciferase activity at the −49 kb site, albeit to a lesser extent compared with the −43 kb site (Fig. 1C). Given this finding, it appears that a switch on the Prox1 enhancer region from a suppressive state mediated by SOX proteins to an activated state mediated by β-catenin may be critical for Prox1 expression.
Hippocampal Prox1 Expression Is Associated with TCF/LEF Signaling in Vivo.
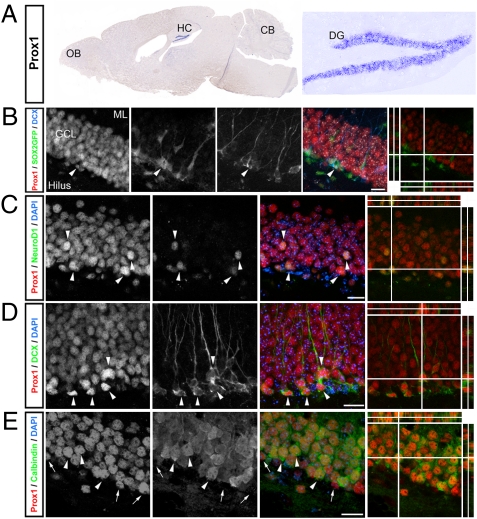
We next aimed to characterize the relationship between TCF/LEF signaling and Prox1 expression in vivo. First, we analyzed Prox1 mRNA and protein expression within the adult brain. By using in situ hybridization, we found that Prox1 mRNA expression was largely restricted to the DG granule cell layer, a finding confirmed by qPCR, wherein Prox1 mRNA levels among different brain regions were compared (Fig. 2A and Fig. S2). This is in contrast to earlier stages of neural development during which Prox1 is also expressed in the SVZ (16). Reflecting the mRNA expression pattern, we found Prox1 protein expression to be restricted to the DG in the adult brain (Fig. 2 B–E), confirming previous reports (16). By using single BrdU injections and previously characterized marker proteins for NSCs and their progeny, we found Prox1 to be expressed as early as in the nonradial stage of SOX2-expressing cells in the DG (24) (Fig. 2B and Fig. S2). Prox1 expression was also high in doublecortin (DCX)- and NeuroD1-expressing cells, two markers for newborn neuronal cells in the adult DG (25, 26). In contrast to other transcription factors associated with hippocampal neurogenesis, such as NeuroD1 (12), Prox1 remained to be expressed at high levels also in mature, calbindin-expressing hippocampal granule cells (Fig. 2 C–E) and was exclusively expressed in the DG but not in the SVZ, the second neurogenic region of the adult brain (Fig. S2).
Fig. 2.
Prox1 is expressed in the course of adult hippocampal neurogenesis. (A) In situ hybridization with a riboprobe against Prox1 shows the selective expression of Prox1 mRNA in the DG of adult mice. Shown are an overview of a sagittal brain section (Left) and a high-power view of the DG (Right). (B) Prox1 (red) is expressed in nonradial SOX2GFP-expressing cells (green) that are also weakly positive for DCX (blue, arrowheads). Grayscale images show single channels. Right: Collapsed z-stack overlay of single channels (nuclei were counterstained with DAPI, gray) and a 3D reconstruction of a triple-positive cell. (C–E) Prox1 (red) is expressed in early neuronal progeny expressing NeuroD1 (blue, C) and DCX (blue, D) but is also expressed at high levels in mature, calbindin-expressing granule cells (blue, E). Right: Collapsed z-stack overlay of single channels (nuclei were counterstained with DAPI, gray) and a 3D reconstruction of a triple-positive cell. OB, olfactory bulb; CB, cerebellum; HC, hippocampus; ML, molecular layer; GCL, granule cell layer. (Scale bars: B–E, 30 μm.)
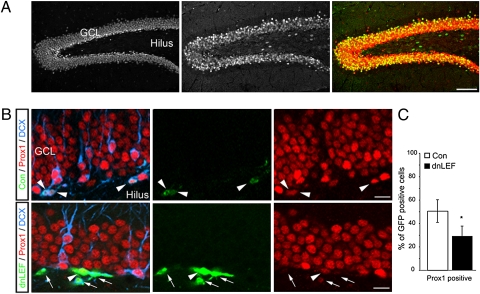
We then analyzed Prox1 expression in a transgenic mouse line (BAT-gal) expressing the lacZ gene coding for β-gal under the control of multiple TCF/LEF consensus sites (27). We observed a strong overlap of Prox1 and β-gal activity in the dentate area of BAT-gal mice (Fig. 3A), suggesting an association between TCF/LEF signaling and Prox1 expression in the adult hippocampus. To analyze a functional relationship between TCF/LEF signaling and Prox1 expression in the adult DG, we used a retrovirus, selectively targeting dividing cells and their progeny, to overexpress a dominant-negative LEF (dnLEF), thus inhibiting TCF/LEF-dependent transcription in vivo (Fig. S3). Given the importance of canonical Wnt signaling for dentate neurogenesis, inhibition of TCF/LEF-dependent transcription may lead to a block of hippocampal neurogenesis, making the interpretation of such loss-of-function experiments difficult. Nevertheless, we found 4 d after retroviral injection that the number of dnLEF-expressing cells colabeling with Prox1 was reduced compared with newborn cells labeled with a control virus (Fig. 3 B and C). These findings support the data showing the in vitro activity of β-catenin on the Prox1 promoter.
Fig. 3.
Prox1 is associated with TCF/LEF-dependent signaling in vivo. (A) Prox1 (Left, red in overlay) protein expression shows substantial overlap with β-gal immunoreactivity (Middle, green in overlay) in BAT-gal reporter mice, indicating active TCF/LEF-dependent transcription in Prox1-expressing cells. (B and C) Inhibition of TCF/LEF-dependent transcription by retrovirus-based overexpression of dnLEF reduces the number of newborn Prox1-expressing cells. Shown are examples of 4-d-old control cells expressing GFP (green, Upper) that colabel with Prox1 (red, arrowheads). In contrast to newborn cells labeled with a control virus, the majority of GFP-labeled cells expressing dnLEF (green, Lower) were negative for Prox1 (red, arrows), even though a fraction of dnLEF-expressing newborn cells remained Prox1-positive (arrowhead). GCL, granule cell layer. *P < 0.05. (Scale bars: A, 200 μm; B, 20 μm.)
Overexpression of Active β-Catenin Induces Ectopic Prox1 Expression.
We next aimed to analyze if β-catenin is sufficient to induce Prox1 expression. As the vast majority of newborn cells in the adult hippocampus, under physiological conditions, express Prox1 (Fig. S2), we switched to the developing forebrain. We decided to address whether elevated levels of active β-catenin induce Prox1 expression. To enhance active β-catenin levels, we used a mouse line in which exon 3 of the β-catenin gene is flanked by loxP sites (28). Exon 3 encodes all amino acids essential for phosphorylation of β-catenin and its subsequent degradation. Deletion of exon 3 results in the generation of a truncated but stabilized form of β-catenin, which is not targeted for degradation and retains its transcriptional activity (28). We used Emx1IRESCre mice to express Cre recombinase under the Emx1 promoter, which is active in cortical progenitors and excitatory neurons in the embryonic forebrain (29), and crossed heterozygous mice to produce animals with the genotype Emx1IRESCre/ß-cateninexo3.
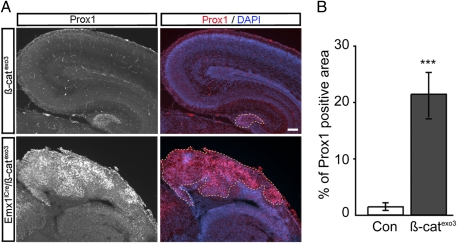
In mice overexpressing the dominant active form of β-catenin, the Prox1 expression domain was dramatically increased in the Emx1IRESCre/β-cateninexo3 mutants compared with WT littermates. At embryonic day 18 to postnatal day 0, we found robust ectopic expression of Prox1 in dorsal cortical areas of Emx1IRESCre/β-cateninexo3 mutants whereas Prox1 expression in controls was largely restricted to the developing DG (Fig. 4). Taken together, these experiments show that β-catenin is sufficient to induce ectopic Prox1 expression in the developing forebrain, supporting a regulation of Prox1 by β-catenin–TCF/LEF signaling.
Fig. 4.
Expression of dominant-active β-catenin in dorsal telencephalon induces ectopic Prox1 expression. (A) Coronal sections of embryonic day 18 β-cateninflox/flox (Con) and Emx1IRESCre/β-cateninexo3 (mutant) brains were stained using antibodies against Prox1 (red). Upon stabilization of β-catenin in Emx1IRESCre/β-cateninexo3 mice, Prox1 is dramatically up-regulated and ectopically expressed in cortical tissue. Note that overexpression of β-catenin in the telencephalon caused by deletion of exon 3 of β-catenin after Emx1-driven Cre recombination disrupts hippocampal development. Nuclei were counterstained with DAPI (blue). Prox1 expression is delineated by yellow dashed line in the DG (Upper) and in large areas of the dorsal cortex in mutant mice (Lower). (B) Quantification of the cortical areas expressing Prox1 in β-cateninflox/flox controls and Emx1IRESCre/β-cateninexo3 mutant mice (***P < 0.001). (Scale bars: 100 μm).
Prox1 Has a Stage-Specific Role in the Course of Adult Hippocampal Neurogenesis.
It has been recently shown that Prox1 is required for granule cell genesis during embryonic and adult neurogenesis in the hippocampus (17). Given the expression pattern of Prox1 in newborn but also fully mature granule cells, we aimed to analyze the functional role of Prox1 in early and late steps of adult neurogenesis. Thus, we used shRNA-expressing lentiviruses—transducing dividing but also postmitotic cells in the DG to target Prox1 expression levels in the adult hippocampus (Fig. S3)—in combination with endogenous markers and thymidine analogue labeling of cells in S-phase and their progeny.
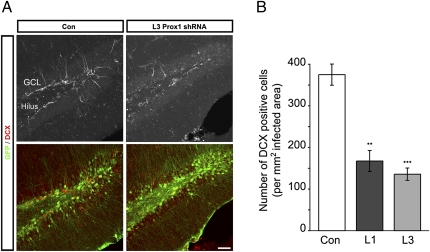
By using DCX to analyze the levels of neurogenesis (26), we found a substantial reduction of newborn neurons after shRNA-mediated Prox1 knockdown compared with animals injected with nontargeting shRNA-expressing control virus (Fig. 5). As a complementary strategy, we injected the thymidine analogue chlorodeoxyuridine (CldU) 2 wk after stereotactic injections of Prox1-targeting or nontargeting shRNA-expressing lentiviruses. Animals were killed 3 wk after CldU injection. We found that Prox1 knockdown led to impaired differentiation and reduced numbers of CldU-DCX–labeled cells (Fig. S4).
Fig. 5.
Prox1 has a stage-specific role for neurogenesis in the adult hippocampus. (A) Intrahippocampal injections of lentiviruses expressing control, nontargeting shRNA and GFP (green, Left), or shRNA against Prox1 mRNA (green, Right) show a decrease in newborn DCX-expressing neurons (red) after Prox1 knockdown. Upper: DCX. Lower: Overlay of DCX and GFP. (B) The density of DCX-expressing cells is reduced after Prox1 knockdown by using two independent shRNAs. GCL, granule cell layer. **P < 0.01 and ***P < 0.001. (Scale bar: 100 μm.)
We next aimed to analyze the effects of Prox1 knockdown on the NSC compartment. Notably, the number of hippocampal, radial glia-like NSCs expressing SOX2 and GFAP was not affected by Prox1 knockdown (Fig. S4), suggesting that Prox1 knockdown affects the level of newborn neurons but not the proliferative activity and/or maintenance of hippocampal NSCs at this time point after Prox1 knockdown. However, this may change in a situation in which Prox1 is chronically depleted: Lavado and colleagues found defective maintenance of radial glia-like NSCs 2 mo after Prox1 KO (17).
We then asked if Prox1 overexpression selectively in newborn cells of the adult DG would cell-autonomously enhance neuronal differentiation. Animals were injected with retroviruses expressing Prox1 or control retroviruses and killed 1 wk later. We found that Prox1 overexpression enhanced neuronal differentiation as the number of GFP-labeled cells coexpressing DCX was enhanced compared with cells transduced with a control retrovirus (Fig. S5).
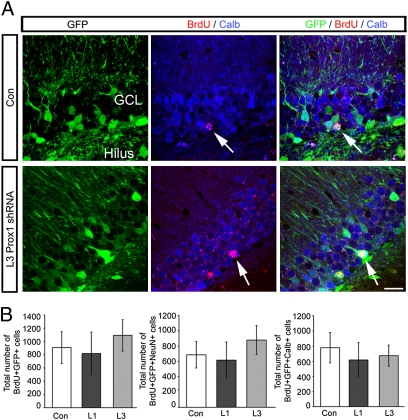
To analyze if Prox1 is required for granule cell maintenance after cells have fully matured, we again used a lentivirus-based strategy. Adult-born granule cells were birthdated with BrdU labeling and injected 16 wk later with shRNA-expressing virus targeting Prox1 mRNA or control lentiviruses, at a time point when newborn granule cells are stably integrated into the hippocampal circuitry and many of them express calbindin (15, 30, 31). In contrast to the obvious loss of differentiating granule cells, we found no differences in lentivirus-infected BrdU-labeled cells expressing shRNAs against Prox1 compared with control cells (Fig. 6). There were also no differences in the total number of BrdU-labeled cells expressing NeuN or calbindin, suggesting that Prox1 is not required for the survival of granule cells after they have matured (Fig. 6).
Fig. 6.
Prox1 knockdown does not affect survival of mature granule cells. (A) Representative images of BrdU (red)-labeled, calbindin-positive (blue, arrows) cells in areas expressing control lentivirus (green, Upper) and after Prox1 knockdown (green, Lower). (B) The numbers of cells labeled with BrdU 16 wk before lentivirus injection were not different between control and Prox1 knockdown conditions. In analogy, the number of BrdU/NeuN colabeled cells was not different between groups. ML, molecular layer; GCL, granule cell layer. (Scale bar: 50 μm.)
Supporting these in vivo findings, we found that Prox1 overexpression enhanced neuronal differentiation of cultured NSCs, whereas shRNA-mediated knockdown of Prox1 impaired neuronal differentiation (Figs. S6 and S7). Taken together, our data suggest that Prox1 is (i) required for proper neuronal differentiation of granule cells, (ii) sufficient to enhance neuronal differentiation of newborn cells in the adult DG, and (iii) not required for granule cell survival after they have fully matured. Thus, our findings confirm and extend the results recently obtained by using a transgenesis-based approach (17).
Discussion
Here we show that the homeodomain transcription factor Prox1 is regulated with β-catenin–TCF/LEF activation downstream of Wnt signaling and that Prox1 has a stage-specific function in the course of adult hippocampal neurogenesis. Thus, we have identified a molecular mechanism of how niche-derived signals are translated into the production of new granule cells within the adult hippocampus.
The hippocampus is one of the two regions within the brain that generates new neurons throughout adulthood (1). Despite recent advances in understanding the molecular mechanisms underlying lifelong neurogenesis (2), it remains largely unknown how local signals become translated into the production of new neurons. One of the key molecules expressed by local astrocytes within the neurogenic niche of the adult hippocampus is Wnt3, which is required for the generation of new granule cells (7, 32). Supporting the critical role of Wnt signaling in adult neurogenesis, it has been recently shown that NeuroD1, a basic helix-loop-helix transcription factor required for granule cell and olfactory neuron generation in the embryonic and adult brain, is regulated by β-catenin (11, 12). We now show that Prox1, expressed in newborn but also mature granule cells selectively in the DG, is a downstream target of β-catenin–TCF/LEF signaling in the context of neurogenesis and is required for the proper differentiation and survival of newborn granule cells but not for the maintenance of granule cells after they have fully matured. Interestingly, Wnt signaling has previously been shown to be a tissue-specific regulator of Prox1 in the context of intestinal cancer biology, during which Prox1 promotes dysplasia and tumor growth (21). In contrast, Wnt signaling in the adult hippocampus appears to mainly regulate the differentiation, but not primarily the growth, of NSCs (7, 12), suggesting that unknown context-dependent effects of Wnt signaling are critically involved in the regulation and distinct functions of target genes such as Prox1.
The transcriptional code regulating the progression from presumably uncommitted NSCs to a differentiating progeny in the adult hippocampus is only poorly understood. A hypothetical picture emerging is that the nuclear orphan receptor TLX (33) and Sry-related HMG box transcription factors such as SOX2 and SOX9 maintain hippocampal NSCs in an undifferentiated state (24, 33, 34). Upon activation of the Wnt signaling pathway, a switch in transcription factor occupation on the NeuroD1 promoter may then convert the promoter from a repressed to an activated state, resulting in NeuroD1 expression and allowing for differentiation and survival of newborn granule cells (11, 12). Our data suggest that similar events take place on the Prox1 enhancer region, given the repression of SOX9 on β-catenin–induced activation in luciferase reporter constructs containing parts of the Prox1 enhancer region. Notably, NeuroD1 is also required for the generation of certain olfactory neurons (11, 13, 14). Thus, the data presented here add an important piece to the transcriptional mechanisms regulating adult hippocampal neurogenesis by showing that β-catenin–TCF/LEF–dependent transcription selectively up-regulates Prox1 expression in the adult hippocampus. Future studies will have to characterize if Wnt-regulated Prox1 and NeuroD1 may function together or independently in the formation of new granule cells in the adult hippocampus.
What could be the downstream targets of Prox1 that are important for neuronal differentiation in vitro and in vivo? Several transcriptional targets of Prox1 have been identified, among them a number of genes that could be also important for hippocampal neurogenesis, such as VEGF receptor 3, FGF receptor 3 (35), and α9-integrin (36). Furthermore, a recent study suggested that Prox1 deletion in NSCs and their progeny results in impaired Notch signaling in NSCs, leading to the depletion of the stem cell pool over time (17). However, significant targets of Prox1 in the context of adult neurogenesis are still unknown.
The levels of Prox1 remain high in fully mature granule cells, which is also reflected by high TCF/LEF-dependent transcriptional activity in mature granule cells as seen in the BAT-gal reporter mouse (7). Thus, two models could explain the functions of Prox1: (i) Prox1 regulates the expression of differentiation/survival factors that are required for early and late steps of hippocampal neurogenesis or (ii) downstream targets of Prox1 are distinct between early and late steps of neurogenesis (e.g., in immature vs. mature granule cells). At this time, neither of the two possibilities can be formally ruled out. However, the finding that the survival of fully mature granule cells was not affected by Prox1 knockdown suggests that the function of Prox1 in mature granule cell is distinct to its role during initial neuronal differentiation of adult-born granule cells.
The data presented here identify Prox1 as a critical stage-specific regulator of hippocampal neurogenesis in the adult brain. Understanding the transcriptional code governing the neuronal differentiation of newborn granule cells is a prerequisite for the development of new therapeutic strategies to target adult neurogenesis in the context of neuropsychiatric diseases.
Materials and Methods
Plasmids and Viruses.
For Prox1 overexpression, full-length mouse Prox1 cDNA (ImaGenes) was subcloned into a retroviral backbone containing a chicken β-actin promoter followed by an internal ribosome entry site GFP as described previously (37). To block β-catenin signaling, we cloned a dnLEF (7) into the same retroviral backbone. As control, a chicken β-actin–driven GFP vector was used. For knockdown experiments, two different shRNAs directed against Prox1 rat and mouse mRNA were used (target sequence L1, GGGCAATGAAAACGAAAGA; L3, CGGCAAACCAAGAGGAGAA). As control shRNA we used a target sequence not matching to any rodent mRNA sequence (CCTAAGGTTAAGTCGCCCT). For retroviral experiments, shRNAs were cloned into a vector backbone that contains an U6-driven cloning site and a chicken β-actin–driven GFP as described previously (37). For lentiviral experiments, the same shRNA oligos were cloned into the LentiLox 3.7 vector (Addgene) that contains a U6-driven cloning site and a CMV-driven GFP. For overexpression of Wnt3 we used lentiviruses described previously (7). Viruses were produced as described previously (38).
Luciferase Assays and ChIP.
For ChIP, we used murine NSCs as described previously (39). Chromatin was isolated 5 d after growth factor withdrawal to induce neuronal differentiation. Samples were prepared according to the manufacturers recommendations using the EZ ChIP Kit (Millipore). For immunoprecipitation of the crosslinked protein-DNA complexes, 5 μg of rabbit anti–β-catenin antibody (C2206; Sigma) or 1 μg of rabbit IgG antibody (Sigma) were used. For qPCR detection, Power SYBR Green PCR Master Mix (Applied Biosystems) was used. Primer sequences are provided in SI Materials and Methods. The amount of DNA pulled down from each immunoprecipitation was calculated using Ct values and expressed as fold enrichment compared with the DNA amount pulled down by the IgG immunoprecipitation.
For luciferase assays, we cloned the genomic regions of the Prox1 enhancer containing a consensus TCF/LEF site into the reporter plasmid pGL4.25 (Promega) using PCR. Primer sequences are provided in SI Materials and Methods. Plasmids used for β-catenin expression, TOPFLASH, and FOPFLASH reporters have been described previously (7). NSCs were electroporated with reporter plasmids and β-catenin plus/minus SOX9 by a Nucleofector device (Amaxa) according to the manufacturer's recommendations. phLR-TK (Promega) was coelectroporated as an internal control. Forty-eight hours after electroporation, luciferase activity in the lysis supernatant was measured with a Dual-Luciferase Reporter Assay System (Promega) using a MicroLumat Plus (Berthold).
Animals and Stereotactic Injections.
Animal experiments were approved by the veterinary office of the Canton of Zurich, Switzerland, or by the Bezirksregierung, Braunschweig, Germany. For all experiments in the adult brain, the animals used were 6- to 8-wk-old female C57BL/6 mice (Janvier) or SOX2GFP transgenic mice (24). Mice were stereotactically injected with 1.5 μL of lentiviral suspension (or 1 μL retrovirus for overexpression experiments) into the DG as described previously (38). According to the experimental paradigm for lentiviral analysis, either BrdU (100 mg/kg) or CldU (85.5 mg/kg; Sigma) was injected to the animals intraperitoneally at the times indicated. Perfusion and preparation of brain sections were described previously (40). Group sizes for all experimental groups were greater than three. For qPCR of Prox1 mRNA isolated from cerebral subregions, brains from 8-wk-old C57BL/6 mice (n = 2) were microdissected and RNA was obtained, reverse-transcribed, and used for expression analysis. For experiments in the embryonic and postnatal day 0 forebrain, we used the previously described mouse lines Emx1IRESCre, β-cateninexo3 (28, 29).
Immunostaining, Western Blotting, in Situ Hybridization, and qPCR.
Tissue and cells were prepared for immunostaining as described earlier (37). Briefly, mice were perfused transcardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer. Brains were removed and postfixed overnight. Brains were then transferred into 30% sucrose overnight and cut on a dry ice-cooled microtome into 40-μm-thick coronal sections. Cells were fixed for 15 min in 4% PFA followed by extensive washes in phosphate buffer. A list of antibodies is provided in SI Materials and Methods. For in vivo analyses, areas transduced by lentiviruses were identified by GFP coverage by using ImageJ. Virus-infected cells (50–100 cells per animal: n ≥ 3) in the granule cell layer of one-in-12 series of sections were analyzed by using high-resolution confocal microscopy in sequential scanning mode (SP2; 20× objective, 0.7 N.A.; Leica). The presented total number of cells per DG was adjusted accordingly. Imaris version 6.1.x (Bitplane) was used to quantify colocalization of neurogenesis markers and thymidine analogues in lentivirus-infected areas. Radioactive and nonradioactive in situ hybridizations were performed as described before (37) or according to standard protocols (41). qPCR was performed using standard procedures. Further details are provided in SI Materials and Methods.
Statistical Analysis.
All numerical analyses were performed using Excel (Microsoft) with appropriate add-ons or SPSS Statistics (IBM). For all comparisons, two-sample t tests or ANOVA if appropriate were used. Differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank the Swiss Federal Institute of Technology Zurich Light Microscopy Facility for valuable help with imaging; Drs. Aigner and Gage and members of the S.J. laboratory for comments on the manuscript; and Drs. Suh and Gage for SOX2GFP tissue. This study was supported by National Competence Center in Research Neural Plasticity and Repair Research Grant ETH-01 08-1, Swiss National Science Foundation Grant 3100A0-117744/1, the Theodore Ott foundation, and the Novartis Foundation (S.J.). M.K. was supported by the Janggen–Pöhn Foundation, and V.T. was supported by German Research Foundation Grant SFB665.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013456108/-/DCSupplemental.
References
- 1.Gage F. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Ann Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 6.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 7.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 8.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 9.Pleasure SJ, Collins AE, Lowenstein DH. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci. 2000;20:6095–6105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, et al. Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc Natl Acad Sci USA. 2000;97:865–870. doi: 10.1073/pnas.97.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Z, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwabara T, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutin C, et al. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc Natl Acad Sci USA. 2010;107:1201–1206. doi: 10.1073/pnas.0909015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roybon L, Deierborg T, Brundin P, Li JY. Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci. 2009;29:232–243. doi: 10.1111/j.1460-9568.2008.06595.x. [DOI] [PubMed] [Google Scholar]
- 15.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Lavado A, Oliver G. Prox1 expression patterns in the developing and adult murine brain. Dev Dyn. 2007;236:518–524. doi: 10.1002/dvdy.21024. [DOI] [PubMed] [Google Scholar]
- 17.Lavado A, Lagutin OV, Chow LML, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010;8:e1000460. doi: 10.1371/journal.pbio.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misra K, Gui H, Matise MP. Prox1 regulates a transitory state for interneuron neurogenesis in the spinal cord. Dev Dyn. 2008;237:393–402. doi: 10.1002/dvdy.21422. [DOI] [PubMed] [Google Scholar]
- 19.Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- 20.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 21.Petrova TV, et al. Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell. 2008;13:407–419. doi: 10.1016/j.ccr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Sottile V, Li M, Scotting PJ. Stem cell marker expression in the Bergmann glia population of the adult mouse brain. Brain Res. 2006;1099:8–17. doi: 10.1016/j.brainres.2006.04.127. [DOI] [PubMed] [Google Scholar]
- 23.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suh HK, et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steiner B, et al. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- 26.Couillard-Despres S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 27.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorski JA, et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 31.Brandt MD, et al. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 32.Jessberger S, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats Learning Memory. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 34.Favaro R, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- 35.Shin JW, et al. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: A role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17:576–584. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishima K, et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell. 2007;18:1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jessberger S, et al. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6:e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11:888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray J, Gage FH. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cell Neurosci. 2006;31:560–573. doi: 10.1016/j.mcn.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson DG, editor. In Situ Hybridization: A Practical Approach. 2nd Ed. New York: Oxford Univ Press; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.