Abstract
The mechanotransducer channels of auditory hair cells are gated by tip-links, oblique filaments that interconnect the stereocilia of the hair bundle. Tip-links stretch from the tips of stereocilia in the short and middle rows to the sides of neighboring, taller stereocilia. They are made of cadherin-23 and protocadherin-15, products of the Usher syndrome type 1 genes USH1D and USH1F, respectively. In this study we address the role of sans, a putative scaffold protein and product of the USH1G gene. In Ush1g−/− mice, the cohesion of stereocilia is disrupted, and both the amplitude and the sensitivity of the transduction currents are reduced. In Ush1gfl/flMyo15-cre+/− mice, the loss of sans occurs postnatally and the stereocilia remain cohesive. In these mice, there is a decrease in the amplitude of the total transducer current with no loss in sensitivity, and the tips of the stereocilia in the short and middle rows lose their prolate shape, features that can be attributed to the loss of tip-links. Furthermore, stereocilia from these rows undergo a dramatic reduction in length, suggesting that the mechanotransduction machinery has a positive effect on F-actin polymerization. Sans interacts with the cytoplasmic domains of cadherin-23 and protocadherin-15 in vitro and is absent from the hair bundle in mice defective for either of the two cadherins. Because sans localizes mainly to the tips of short- and middle-row stereocilia in vivo, we conclude that it belongs to a molecular complex at the lower end of the tip-link and plays a critical role in the maintenance of this link.
Keywords: auditory mechanoelectrical transduction, Usher syndrome type 1, deafness, conditional knockout mice, organ of Corti
In the sensory hair cells of the auditory system, the hair bundle converts acoustic energy into an electrical receptor potential. The hair bundle is a morphologically and functionally polarized ensemble of stiff microvilli (stereocilia) that are arranged into three height-ranked rows forming a V- or U-shaped staircase pattern. Developing hair bundles also contain a genuine cilium, the kinocilium. Identification of the genes defective in Usher syndrome type 1 (USH1)—a disease characterized by congenital profound deafness, vestibular dysfunction, and delayed-onset retinopathy leading to blindness—has revealed the cohesive role of the transient fibrous lateral links that interconnect the stereocilia and connect the kinocilium to the stereocilia early in development (1). These links are made of two Ca2+-dependent adhesion molecules, the transmembrane proteins cadherin-23 and protocadherin-15 (2, 3), which are defective in the USH1D and USH1F (4) genetic forms, respectively. The tip-link (5, 6), a single oblique link running from the tip of one stereocilium to the side of its taller neighbor, also consists of cadherin-23 and protocadherin-15, which make up its upper and lower parts, respectively (7–9). According to the prevailing model for mechanoelectrical transduction, tip-link tension controls the open probability of one or two mechanoelectrical transduction (MET) channels in each stereocilium (10). These channels are located at the lower insertion point of the tip-link (11), i.e., at the very tips of stereocilia in the short and medium rows.
The other USH1 genes encode myosin VIIa (USH1B), the PDZ-domain–containing submembrane protein harmonin (USH1C), and another putative scaffold protein called sans (USH1G) (1). The direct in vitro interactions between the five USH1 proteins (2, 12–16) and the colocalization of cadherin-23, protocadherin-15, myosin VIIa, and harmonin in the growing hair bundle, along with the common early morphological defect in cochlear hair bundles of mouse mutants defective for any USH1 gene (17), suggest that USH1 proteins cooperate in hair bundle development. It has been proposed that harmonin-b anchors transient fibrous lateral links to the stereocilia actin filaments and that myosin VIIa creates tension on these links (2, 16), but the role played by sans is still unknown. Sans directly interacts in vitro with both the myosin VIIa tail (13) and harmonin (13, 18), but has so far not been detected within the hair bundle (13), thus questioning its participation in the USH1 molecular network that underlies cohesion of the growing hair bundle. To elucidate the role of sans, we studied the morphofunctional characteristics of the hair bundle in early ubiquitous and delayed conditional Ush1g knockout mice that lack sans from the early stages of hair cell development and in the later stages of hair bundle differentiation, respectively.
Results and Discussion
Mechanoelectrical Transduction Currents Are Defective in Cochlear and Vestibular Hair Cells of Ush1g−/− Mice.
The 460-amino-acid putative scaffolding protein sans contains a series of three ankyrin repeats and a sterile α-motif (SAM) domain, with an intervening a 95-aa central region (CENT) and a C-terminal PDZ domain-binding consensus motif. The previously described Ush1g mutant mice (js/js mice), which display profound deafness and a balance defect, have nucleotide insertions (19) that may result in a protein truncated just upstream or at the beginning of the SAM domain. These mutants may therefore not be appropriate to explore the consequences of the absence of sans in the inner ear. To produce bona fide sans-null (Ush1g−/−) mice, we first engineered Ush1gfl/fl mice by targeting exon 2 (Fig. S1A). These mice were then crossed with PGK-cre mice (20).
Sound processing in the cochlea involves two types of sensory cells: specifically, the inner hair cells (IHCs), which are the genuine sensory cells that release neurotransmitters and induce action potentials in afferent neurons, and the outer hair cells (OHCs), which locally amplify the sound-induced motion of the sensory epithelium. Exploration of the auditory functions of Ush1g−/− mice in vivo showed a lack of identifiable auditory brainstem response (ABR) waves for all sound frequencies even at the highest intensity (105 dB SPL) tested. In addition, distortion-product otoacoustic emissions (DPOAEs) and cochlear microphonics (CM) were not detected in these mice (Fig. S2C), thus establishing the loss of OHC activity. IHC and OHC hair bundles of postnatal day 5 (P5) Ush1g−/− mice analyzed using scanning EM showed a disorganization of the stereocilia reminiscent of that observed in the js/js mutant mice (17). Notably, OHC hair bundles were often fragmented in two or more clumps of stereocilia, and their kinocilia were mispositioned (Fig. S3).
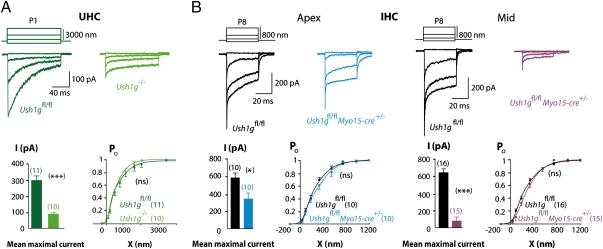
We analyzed the MET currents as a function of hair bundle displacement in cochlear and vestibular hair cells from Ush1gfl/fl and Ush1g−/− mice at P5 and P1, respectively, i.e., several days after the onset of MET in the two sensory organs (21–23). The maximal amplitudes of MET currents were markedly reduced in IHCs and OHCs from P5 Ush1g−/− mice (51 ± 9 pA in IHCs, 91 ± 24 pA in OHCs) compared with Ush1gfl/fl control mice (471 ± 40 pA in IHCs, 696 ± 50 pA in OHCs; unpaired t test, P < 10−4 for both comparisons), and the average sensitivity of the MET currents to hair bundle displacement was decreased by ∼50% in both IHCs and OHCs as well (unpaired t test, P < 10−4) (Fig. S3). Both MET current abnormalities could result from the morphologically abnormal hair bundles in these mice (Fig. S3). In contrast, residual MET current could not be detected in IHCs and OHCs from P5 cadherin-23-null (Cdh23−/−) mice (24) that lack tip-links (Fig. S4A). We then analyzed the MET currents in utricular hair cells (UHCs) of the vestibule from P1 Ush1g−/− mice. Notably, not all of the UHC hair bundles were fragmented, and MET current recordings were performed on selected cells that displayed morphologically intact hair bundles. The maximal amplitudes of the MET current in Ush1g−/− UHCs (88 ± 12 pA) were 30% those of Ush1gfl/fl UHCs (295 ± 30 pA) (unpaired t test, P < 10−4) (Fig. 1A). The normalized Po(X) curves, however, superimposed, indicating the lack of change in the sensitivity of the MET machinery in the UHCs from Ush1g−/− mice. Accordingly, the hair bundle displacement eliciting half of the maximal MET current (X0.5) was the same in the mutant and control UHCs (unpaired t test, P = 0.08 and P = 0.06, respectively). The results obtained in morphologically intact Ush1g−/− UHCs thus suggest that sans plays a role in the MET machinery. The fact that we found residual MET currents in the vestibular and cochlear Ush1g−/− hair cells at P1 and P5, respectively, however, indicates that sans is dispensable for the initial assembly of the MET machinery.
Fig. 1.
(A) Mechanoelectrical transduction current recordings in UHCs from Ush1g−/− P1 mice. In the graduated shading of green, examples of transduction current recordings in UHCs from Ush1gfl/fl and Ush1g−/− P1 mice are shown. Mean maximum amplitudes are 295 ± 30 pA and 88 ± 23 pA in Ush1gfl/fl and Ush1g−/− UHCs, respectively (unpaired t test, P < 10−4). Po(X) curves plotted for Ush1gfl/fl and Ush1g−/− UHCs can be superimposed, with average sensitivity values of 0.88 ± 0.11 μm−1 and 1.18 ± 0.21 μm−1 for Ush1gfl/fl and Ush1g−/− UHCs, respectively (unpaired t test, P = 0.08). No change in X0.5 can be detected in the mutant UHCs (X0.5 = 686 ± 138 nm and 620 ± 75 nm for Ush1gfl/fl and Ush1g−/− UHCs, respectively; unpaired t test, P = 0.06). (B) Mechanoelectrical transduction currents were recorded in IHCs from Ush1gfl/flMyo15-cre+/− P8 mice. We analyzed mechanoelectrical transduction currents in cochlear inner hair cells from the apical region (∼35% of the total length of the cochlea from the apex) and the middle region (∼55% of the total length) of the cochlea in Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− P8 mice. (Left) Examples of transduction currents in apical IHCs from Ush1gfl/fl (black) and Ush1gfl/flMyo15-cre+/− (blue) mice. Mean maximum current amplitude is 584 ± 52 pA and 346 ± 71 pA for Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− IHCs, respectively (unpaired t test, P = 0.015). The Po(X) curves, however, can be superimposed with average sensitivity values of 1.85 ± 0.16 μm−1 and 1.72 ± 0.15 μm−1 for Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− IHCs, respectively (unpaired t test, P = 0.58). In addition, no change in X0.5 can be detected in the mutant IHCs (X0.5 = 218 ± 13 nm and 421 ± 109 nm for Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− IHCs, respectively; unpaired t test, P = 0.08). (Right) Examples of transduction currents in Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− IHCs from the middle of the cochlea at P8. Mean maximum current amplitude is 652 ± 44 pA and 84 ± 44 pA for Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− IHCs, respectively (unpaired t test, P < 10−4). The Po(X) curves, however, can be superimposed with average sensitivity values of 1.95 ± 0.12 μm−1 and 2.00 ± 0.61 μm−1 for Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− IHCs, respectively (unpaired t test, P = 0.94). In addition, no change in X0.5 can be detected in the mutant IHCs (X0.5 =220 ± 19 nm and 489 ± 122 nm in Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− mice, respectively; unpaired t test, P = 0.07).
Ush1gfl/flMyo15-cre+/− Mice Are Profoundly Deaf and Develop a Balance Defect.
To circumvent the early morphogenetic defect present in the hair bundles of Ush1g−/− mice, we engineered Ush1gfl/flMyo15-cre+/− mutant mice, in which Ush1g was deleted postnatally. These mice were obtained by crossing Ush1gfl/fl mice with Myo15-cre+/− mice in which cre recombinase gene expression was driven by the Myo15a promoter (Fig. S1B). The presence of the Myo15-cre allele leads to the sequential inactivation of a lacZ reporter gene from the basal to the apical region of the cochlea, starting at P0 at the base (Fig. S1C). ABRs, DPOAEs, and CM were analyzed in Ush1gfl/flMyo15-cre+/− mice at P13, P15, and P21 (Fig. S2). At P13, these mice lacked identifiable ABR waves for all sound frequencies, even at the highest intensity tested (105 dB SPL). This indicates that IHCs are impaired because even a complete loss of OHC function cannot account for more than a 60-dB threshold elevation (25). In the P13 mutant mice, the round-window CM in response to sound stimulation could not be detected either, thus indicating that the high-frequency OHCs from the basal end of the cochlea were severely impaired. Moreover, the DPOAE amplitudes at 15 kHz did not significantly differ from background noise at all sound intensities tested, thus indicating that the OHC functional defect extends to cells tuned to this lower frequency. DPOAE amplitudes at 10 kHz were, however, still close to those obtained in Ush1gfl/fl P13 mice in response to 70 dB SPL stimuli, indicating partly preserved OHC function at this lower frequency (Fig. S2A). From P15 onward, neither CMs nor DPOAEs could be recorded in Ush1gfl/flMyo15-cre+/− mice at all sound intensities and frequencies tested, indicating that the activity of all OHCs was now severely impaired (Fig. S2B). Notably, these mice also displayed a progressive vestibular dysfunction starting at P21, as assessed by their circling behavior and abnormal vestibular tests (26).
Amplitude of MET Currents Is Reduced in IHCs from Ush1gfl/flMyo15-cre+/− Mice.
We studied the MET currents in hair cells from Ush1gfl/flMyo15-cre+/− mice. Recordings were performed in Ush1gfl/flMyo15-cre+/− and Ush1gfl/fl mice at positions located in the apical end (at ∼35% the total length of the cochlea from its apex) and in the middle (∼55% the total length) of the cochlea. At P7, at both cochlear sites, MET currents in IHCs and OHCs of Ush1gfl/flMyo15-cre+/− mice were indistinguishable from those of Ush1gfl/fl mice (Fig. S5). In contrast, P8 Ush1gfl/flMyo15-cre+/− IHCs displayed a decrease in the maximal amplitude of their MET current. At the apex and middle of the cochlea, the maximal amplitudes in Ush1gfl/flMyo15-cre+/− mice (346 ± 71 pA and 84 ± 44 pA, respectively) were on average 60% and 13% those of IHCs from Ush1gfl/fl mice (584 ± 52 pA and 652 ± 44 pA; unpaired t test, P = 0.015 and P < 10−4), respectively (Fig. 1B). The Po(X) curves of Ush1gfl/flMyo15-cre+/− and Ush1gfl/fl IHCs at both cochlear sites could, however, be superimposed, thus indicating that the MET sensitivity to hair bundle displacement is preserved. We then investigated the characteristics of MET current adaptation, the process that resets the sensitivity of the MET channel close to its normal resting value during sustained stimuli. No changes were observed in the extent of adaptation or the kinetics when analyzed in IHCs from apical cochlear regions (Fig. S6). Therefore, the lack of sans in IHCs affects only the amplitude of the MET current without affecting the sensitivity of the hair bundle to displacement or adaptation. This suggests that the MET machinery functions normally in some of the stereocilia, but ∼40% and 87% of the MET complexes are entirely nonfunctional at the apex and at the mid region of the cochlea, respectively. The role of sans in the MET process thus markedly differs from that of harmonin-b, which is involved in the extent of adaptation but does not play a significant role in the amplitude of the MET current (22). No difference in the OHC MET currents could be detected between Ush1gfl/flMyo15-cre+/− and Ush1gfl/fl P8 mice (Fig. S7).
Disappearance of the Tip-Links and Reduction of Stereocilia Height from the Small and Middle Rows in Ush1gfl/flMyo15-cre+/− Mice.
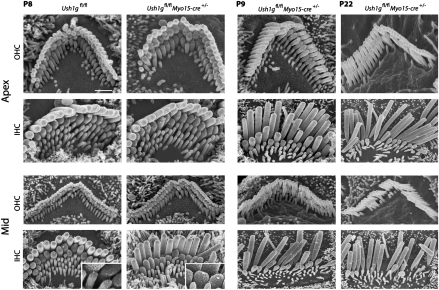
We analyzed the structure of the hair bundles in IHCs and OHCs from the apical and middle regions of the cochlea in Ush1gfl/flMyo15-cre+/− mice at P8, P9, and P22 by scanning EM. At P8, hair bundles of IHCs from the apical region were cohesive with a normal U shape, and the tip-links could be detected and had prolate-shaped tips, a form thought to result from the tension exerted on the stereocilia tips by tip-links (27). In the mid region, however, only a few prolate-shaped stereocilia tips (<10%) and tip-links were observed within the medium row of stereocilia (Figs. 2 and 3). In contrast, the Ush1gfl/flMyo15-cre+/− OHC hair bundles could not be distinguished from the Ush1gfl/fl ones on this day (Fig. 2). At P9, additional hair bundle anomalies appeared in the mutant IHCs from the middle to the apex of the cochlea; specifically, some stereocilia within the small and medium rows had reduced heights (Figs. 2 and 3). These features were also present in mutant OHCs from the cochlear base. At P22, the reduction of the stereocilia length was much more pronounced, and some of the stereocilia from the small row had even disappeared in both the IHCs and the OHCs throughout the cochlea. Notably, the size of the stereocilia that compose the tallest row was unchanged in the mutants.
Fig. 2.
Hair bundle morphology in OHCs and IHCs from Ush1gfl/flMyo15-cre+/− P8 mice. Scanning electron microscopy analysis of OHCs and IHCs from the apex (Upper) and from the middle region of the cochlea (Lower) in Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− P8 mice. In the apical region, the hair bundles of Ush1gfl/flMyo15-cre+/− IHCs and OHCs are cohesive. In the apical region, the hair bundles of Ush1gfl/flMyo15-cre+/− IHCs and OHCs are cohesive, the tip-links are present, and prolate-shaped stereocilia tips are systematically observed. In the middle region, no differences were detected between Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− OHCs. In contrast, the presence of nonprolate-shaped stereocilia tips within the middle row of stereocilia was frequently detected in Ush1gfl/flMyo15-cre+/− IHCs, and the number of tip-links that could be detected in these cells was reduced. At P9, additional hair bundle anomalies appeared in the Ush1gfl/flMyo15-cre+/− IHCs from the mid to the apex of the cochlea; specifically, some stereocilia within the small and middle rows had reduced heights. No defects were found in OHCs from the cochlear apex at this stage, but in the middle region of the cochlea, we found the same anomalies as in IHCs. At P22, the reduction of the stereocilia length dramatically worsened, and some of the stereocilia from the small row had even disappeared in both IHCs and OHCs. Notably, the size of the stereocilia that compose the tall row was unchanged in Ush1gfl/flMyo15-cre+/− mutants. (Scale bar: 1 μm.)
Fig. 3.
Analysis of stereocilia length in IHCs and OHCs from P8 and P9 Ush1gfl/flMyo15-cre+/− mice. Data corresponding to Ush1gfl/fl and Ush1gfl/flMyo15-cre+/− mice are indicated in blue and in red, respectively. Five cells were analyzed in each group. The length of every measurable stereocilium from the middle and small rows was normalized to the mean length of stereocilia in the tall row (L2/L1 and L3/L1, respectively; mean ± SEM). The numbers (mean ± SEM) of tip-links detected in the apical (apex) and middle (mid) regions of the cochlea are indicated by histograms (Right panels). In Ush1gfl/flMyo15-cre+/− IHCs (Upper panels), there is a progressive reduction of the stereocilia length in the middle and small rows and a parallel decrease of the number of tip-links detected, compared with Ush1gfl/fl IHCs (two-way ANOVA, P < 10−4 for both comparisons). In Ush1gfl/flMyo15-cre+/− OHCs (Lower panels), a decrease of the stereocilia length and number of tip-links is also observed (two-way ANOVA, P < 10−4 for both comparisons). In Ush1gfl/flMyo15-cre+/− P9 mice, note that some stereocilia have completely disappeared in both IHCs and OHCs (red dots on the x axis), specifically, 4% of stereocilia from the small row in IHCs of the mid cochlear region, 3% of stereocilia from the small row in OHCs of the apical region, and 15% and 32% of the stereocilia from the middle and small rows in OHCs of the mid region, respectively.
These observations suggested that the tip-link and/or the tip-link molecular complex control the polymerization of F-actin in the stereocilia. Alternatively, sans might exert a direct role in F-actin polymerization. To address the first possibility, we produced a postnatal conditional knockout of the cadherin-23 gene by crossing Cdh23fl/fl mice (24) with Myo15-cre+/− mice. In the Cdh23fl/flMyo15-cre+/− mice, ABR recordings first showed a hearing impairment at P16. At P22, ABR recordings lacked identifiable waves even at the highest sound intensity (105 dB SPL) for all frequencies tested, thus indicating IHC impairment (Fig. S4B). At this stage, we did not detect CM and DPOAEs either, which indicated that the function of OHCs was also severely impaired (Fig. S4 C and D). Scanning EM performed at P16 and P22 established that IHC and OHC hair bundles had normal shapes and were cohesive. However, both IHC and OHC hair bundles displayed morphological anomalies that were similar to those observed in Ush1gfl/flMyo15-cre+/− mice (Fig. S4E), specifically, the loss of the prolate shape of stereocilia tips within the medium row and decrease of the length of stereocilia from the small and medium rows, followed by progressive loss of the stereocilia in these rows (Fig. S4F). This strongly suggests that the tip-link, which is lost in the absence of cadherin-23, has a positive effect on the size of the stereocilia from these two rows. We conclude that the decrease of stereocilia length that we observed in the Ush1g conditional knockout mice is likely to be the consequence of the tip-link loss. We suggest that the size anomalies of the small and medium rows of stereocilia (occurring between P1 and P5), which failed in Ush1g−/− mice (Fig. S3) and also in Cdh23−/− mice and protocadherin-15–deficient mutant mice (Pcdh15av3J/av3J) (17), in fact results from the same mechanism. As soon as the tip-link can operate (at P1 in the mouse cochlea), it may play a role in F-actin polymerization in the two smallest rows of stereocilia, a role that, we suggest, is maintained during the continuous steady-state renewal of the stereocilia actin filaments in the mature hair bundle. The presence of the MET machinery may provide the conditions that allow F-actin polymerization to occur in the stereocilia of the two smallest stereocilia rows. Alternatively, the activity of the MET machinery may be directly coupled to the F-actin polymerization. These two hypotheses are not mutually exclusive. The tension exerted by the tip-link on the plasma membrane may be coupled to the kinetics of actin polymerization that takes place at the stereociliary distal end. As a result of these forces, the activity of proteins that promote F-actin elongation and that are sensitive to forces in the piconewton range, such as the formins, may be modulated (28). Tip-link tension may also enhance actin polymerization by increasing MET channel opening probability, and hence the influx of Ca2+ ions at the stereocilia tip may modulate actin polymerization (27). The rapid [in about 2 d, the time period for the renewal of stereociliary actin (29)] and total disappearance/collapse of the small and medium stereocilia rows contrasts with the maintenance of stereocilia of reduced size in mice defective for myosin XV, whirlin, or espin (30, 31) and suggests that there is a complete failure of actin polymerization in the absence of the tip-link. The present findings provide experimental results in support of the role proposed for tip-link tension in the control of the stereocilia length (30).
Sans Belongs to the Tip-Link Molecular Complex and Interacts with Protocadherin-15 and Cadherin-23.
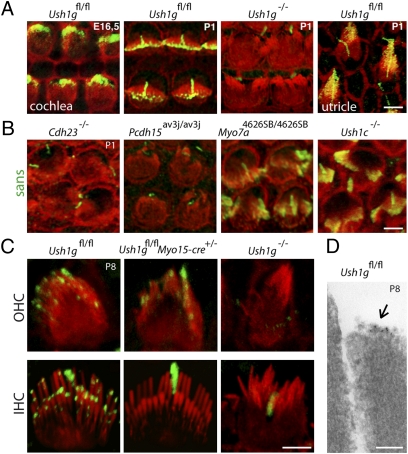
The anti-sans antibodies described previously (13) were tested on Ush1g−/− mice. The labeling observed in the cochlear hair cells from wild-type mice persisted unchanged in the mutant mice, indicating the nonspecificity of these antibodies. We produced another polyclonal antibody directed against the SAM domain of sans (Materials and Methods). This antibody labeled the stereocilia in Ush1gfl/fl, but not in Ush1g−/− mice, thereby proving the specificity of the stereocilia labeling (Fig. 4A). In the cochlea, sans was detected in the apical region of the hair bundles as early as embryonic day 16.5 (E16.5), and at P1 it was present at the hair bundle tips, as were the other four USH1 proteins (17). We conclude that sans belongs to the USH1 protein network that is required for the cohesion of hair bundles in cochlear hair cells during the early stages of their development. Thereafter, at P8, spots of sans labeling were observed in the apical region of all three stereocilia rows (Fig. 4C). Immunogold labeling confirmed the presence of sans at the tip of the small and middle stereocilia rows (Fig. 4D). Notably, the labeling of the small and medium rows was always located at the tips of stereocilia. In Ush1gfl/flMyo15-cre+/− mice at P8, sans labeling was no longer detected in IHCs, but it was still present in OHCs, in accordance with the preservation of their MET currents at this stage (Fig. 4C).
Fig. 4.
(A) Distribution of sans in mouse cochlear and vestibular hair cells. Hair bundles from E16.5 and P1 mouse cochlear and utricular sensory cells stained using phalloidin to detect F-actin (red) and the antibody to sans (green). From E16.5, sans is detected in the actin-rich protrusions that grow on top of the newly differentiated cochlear hair cells, with a particular concentration at their actin-free distal end. In the P1 cochlea, sans immunoreactivity becomes restricted to stereocilia tips. The stereocilia immunoreactivity is specific to sans as it is absent in Ush1g−/− P1 mice. In P1 vestibular hair cells, the sans signal is also restricted to stereocilia tips. (B) Distribution of sans in different Ush1 mutant mice at P1. Hair bundles of sensory cells from the cochlear basal turn of Cdh23−/−, Pcdh15av3J/av3J, Myo7a4626SB/4626SB, and Ush1c−/− P1 mice are shown. F-actin (red) and sans (green) stainings as in A. Note the absence of immunoreactivity in the hair bundles of Cdh23−/− and Pcdh15av3J/av3J mice. (C) Distribution of sans in cochlear hair cells from P8 Ush1gfl/fl, Ush1gfl/flMyo15-cre+/−, and Ush1g−/− mice. F-actin (red) and sans (green) stainings as in A. In OHCs from Ush1gfl/fl mice, sans is detected in the apical region of the stereocilia. In IHCs from Ush1gfl/fl mice, sans is detected at the distal ends of the stereocilia from the short, medium, and tall rows. Occasional subapical labeling of some tall stereocilia is compatible with a presence of sans at the upper insertion point of the tip-link. In Ush1gfl/flMyo15-cre+/− P8 mice, sans is still detected in OHCs, but is no longer present in IHCs. The immunoreactivity of stereocilia is specific as it is absent in Ush1g−/− P8 mice. Note the nonspecific immunolabeling of the kinocilium. (D) Sans immunogold labeling of a stereocilium from the intermediate stereocilia row in an Ush1gfl/fl P8 OHC (shown in a transmission electron micrograph). The gold particles (5-nm gold particles, arrow.) are located at the tip of the stereocilium. (Scale bars: 2 μm in A–C and 100 nm in D.)
In the absence of harmonin (Ush1c−/−) or myosin VIIa (Myo7a4626SB/4626SB), sans was properly located at the apical end of the hair bundles in IHCs and OHCs at P1. In contrast, sans could not be detected in Cdh23−/− and Pcdh15av3J/av3J mutant mice that lack cadherin-23 and protocadherin-15, respectively (Fig. 4B). We thus asked whether sans interacts with the cytoplasmic regions of these cadherins. By coexpressing sans with a chimeric cadherin composed of the extracellular region of E-cadherin and the cytoplasmic region of either cadherin-23 or protocadherin-15 (CD1, CD2, or CD3 isoforms; Materials and Methods) in transfected COS-7 cells (2), we found that sans is indeed recruited to the plasma membrane when either cadherin-23 or protocadherin-15 (except the CD1 isoform) is present (Fig. S8). In the developing hair bundle, sans is thus likely to be present at both ends of the transient lateral links that are made of cadherin-23 and protocadherin-15. On the basis of the immunodetection of sans in the region of the tip-link lower insertion point in the stereocilia from the small and middle rows at later stages, as well as the interaction, in cotransfected cells, between sans and the CD3 isoform of protocadherin-15 that is believed to form the lower part of the tip-link (8, 32), we conclude that sans belongs to the molecular complex present at the lower insertion point of the tip-link. However, we cannot exclude the possibility that sans also belongs to the molecular complex associated with the upper insertion point of the tip-link because the protein was also detected on the lateral side of some stereocilia in the taller row (Fig. 4) and was recruited to the plasma membrane by cadherin-23 in cotransfected cells (Fig. S8). Notably, the disappearance of the tip-links that we observed in IHCs from Ush1gfl/flMyo15-cre+/− P8 mice fully accounts for the decrease of the MET current amplitude in these cells. The less-than-1-d delay between the time when sans becomes undetectable and the time when tip-links disappear and the MET current decreases suggests that sans is involved in either the maintenance of the tip-link or its renewal.
Materials and Methods
Experiments on mice were carried out according to Institut National de la Santé et de la Recherche Médicale and Institut Pasteur welfare guidelines.
Whole-Mount Immunofluorescence.
Rabbit antisera were raised against recombinant proteins encompassing the SAM domain (aa 369–455) of sans (GenBank accession no. NM_176847), and intracellular fragments of the protocadherin-15 CD1 (aa 1612–1726; GenBank accession no. Q99PJ1), CD2 (aa 1652–1790; GenBank accession no. Q0ZM28), or CD3 (aa 1522–1682; GenBank accession no. Q0ZM20) isoforms. Antibodies were affinity-purified against the corresponding antigens coupled to an NHS column (GE Healthcare). We used the N1 polyclonal antibody to detect cadherin-23 (3).
Expression Vectors.
A recombinant pCMV vector encoding sans and recombinant pcDNA3 vectors encoding the extracellular and transmembrane domains of human E-cadherin fused to the cytoplasmic domain of either human cadherin-23 lacking the alternative exon 68-encoded fragment (GenBank accession no. NP_001165405) or mouse protocadherin-15 (CD1, CD2, and CD3 isoforms; GenBank accession nos. NM_001142740, HQ420254, and HQ404375, respectively) were engineered for expression in COS-7 cells (2).
Scanning Electron Microscopy.
Mouse inner ears were processed with osmium tetroxide/thiocarbohydrazide method as previously described (33). Samples were analyzed by field emission scanning electron microscopy operated at 5 kV (Jeol JSM6700F).
Immunogold Labeling.
For transmission electron microscopy, gold-labeled tissues were washed, refixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer pH 7.2 containing 1% tannic acid, washed in buffer, and postfixed in 1% osmium tetroxide as previously described (32).
In Vivo and in Vitro Electrophysiological Recordings and Data Analysis.
ABRs, CM, and DPOAEs were recorded in anesthetized mice and analyzed as described previously (34, 35).
Electrophysiological cell recordings were performed on cochlear and utricular explants from mice aged between P1 and P8 (cochlear hair cells, n = 286; vestibular hair cells, n = 54), as reported (22). The probe used for mechanical stimulation of the hair bundles was secured to a stack-type piezoelectric actuator (PA8/12; Piezosystem Jenas) driven by a low-voltage power supply (30V300, Piezosystem Jena). As measured offline with a displacement monitor containing photodiodes, the first two milliseconds of the time course of probe motion were well described by an exponential rise with a time constant of 100 μs (22). Data were analyzed in Matlab, version 7.0 (MathWorks). Po(X) curves were fitted with a three-state Boltzmann relation (22). For sensitivity measurements, the mean value of the three-state Boltzmann relation derivative was calculated for displacements corresponding to P0 values between 0.2 and 0.8.
Statistical Analysis.
Statistical significance was tested by using either two-way analysis of variance (two-way ANOVA) coupled to the Bonferroni posttest or two-tailed unpaired t test with Welch's correction using Prism software (GraphPad). Statistical significances are indicated on the figures (Figs. 1 and 3 and Figs. S2–S7); the designations (ns), (*), (**), and (***) correspond to nonsignificant (P > 0.05), P < 0.05, P < 0.01, and P < 0.001, respectively.
Supplementary Material
Acknowledgments
We thank Jacqueline Levilliers for her help in the preparation of the manuscript and Sylvie Nouaille for technical help. We are grateful to the Institut Clinique de la Souris (Illkirch, France) for engineering Ush1g and Cdh23 recombinant mice. This work was supported by FAUN Stiftung (Suchert Foundation), Fondation Raymonde and Guy Strittmatter, LHW-Stiftung, the Conny Maeva Charitable Foundation, Fondation Orange, and the Louis-Jeantet Foundation. R.J.G. and G.P.R. are supported by The Wellcome Trust.
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017114108/-/DCSupplemental.
References
- 1.Petit C, Richardson GP. Linking genes underlying deafness to hair-bundle development and function. Nat Neurosci. 2009;12:703–710. doi: 10.1038/nn.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boëda B, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michel V, et al. Cadherin 23 is a component of the transient lateral links in the developing hair bundles of cochlear sensory cells. Dev Biol. 2005;280:281–294. doi: 10.1016/j.ydbio.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 4.El-Amraoui A, Petit C. Usher I syndrome: Unravelling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. J Cell Sci. 2005;118:4593–4603. doi: 10.1242/jcs.02636. [DOI] [PubMed] [Google Scholar]
- 5.Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984;15:103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- 6.Furness DN, Hackney CM. Cross-links between stereocilia in the guinea pig cochlea. Hear Res. 1985;18:177–188. doi: 10.1016/0378-5955(85)90010-3. [DOI] [PubMed] [Google Scholar]
- 7.Siemens J, et al. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed ZM, et al. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–7034. doi: 10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazmierczak P, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- 10.Hudspeth AJ. How the ear's works work: Mechanoelectrical transduction and amplification by hair cells. C R Biol. 2005;328:155–162. doi: 10.1016/j.crvi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Beurg M, Fettiplace R, Nam JH, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci. 2009;12:553–558. doi: 10.1038/nn.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siemens J, et al. The Usher syndrome proteins cadherin 23 and harmonin form a complex by means of PDZ-domain interactions. Proc Natl Acad Sci USA. 2002;99:14946–14951. doi: 10.1073/pnas.232579599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adato A, et al. Interactions in the network of Usher syndrome type 1 proteins. Hum Mol Genet. 2005;14:347–356. doi: 10.1093/hmg/ddi031. [DOI] [PubMed] [Google Scholar]
- 14.Senften M, et al. Physical and functional interaction between protocadherin 15 and myosin VIIa in mechanosensory hair cells. J Neurosci. 2006;26:2060–2071. doi: 10.1523/JNEUROSCI.4251-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan L, Yan J, Wu L, Zhang M. Assembling stable hair cell tip link complex via multidentate interactions between harmonin and cadherin 23. Proc Natl Acad Sci USA. 2009;106:5575–5580. doi: 10.1073/pnas.0901819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahloul A, et al. Cadherin-23, myosin VIIa and harmonin, encoded by Usher syndrome type I genes, form a ternary complex and interact with membrane phospholipids. Hum Mol Genet. 2010;19:3557–3565. doi: 10.1093/hmg/ddq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefèvre G, et al. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135:1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- 18.Yan J, Pan L, Chen X, Wu L, Zhang M. The structure of the harmonin/sans complex reveals an unexpected interaction mode of the two Usher syndrome proteins. Proc Natl Acad Sci USA. 2010;107:4040–4045. doi: 10.1073/pnas.0911385107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikkawa Y, et al. Mutations in a new scaffold protein Sans cause deafness in Jackson shaker mice. Hum Mol Genet. 2003;12:453–461. doi: 10.1093/hmg/ddg042. [DOI] [PubMed] [Google Scholar]
- 20.Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- 21.Lelli A, Asai Y, Forge A, Holt JR, Géléoc GS. Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol. 2009;101:2961–2973. doi: 10.1152/jn.00136.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalski N, et al. Harmonin-b, an actin-binding scaffold protein, is involved in the adaptation of mechanoelectrical transduction by sensory hair cells. Pflugers Arch. 2009;459:115–130. doi: 10.1007/s00424-009-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waguespack J, Salles FT, Kachar B, Ricci AJ. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci. 2007;27:13890–13902. doi: 10.1523/JNEUROSCI.2159-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etournay R, et al. Cochlear outer hair cells undergo an apical circumference remodeling constrained by the hair bundle shape. Development. 2010;137:1373–1383. doi: 10.1242/dev.045138. [DOI] [PubMed] [Google Scholar]
- 25.Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978;41:365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- 26.Steel KP, Harvisty R. What's Wrong With My Mouse? New Interplays Between Mouse Genes and Behavior. Washington, DC: Society for Neuroscience; 1996. Assessing hearing, vision and balance in mice; pp. 26–38. [Google Scholar]
- 27.Tilney LG, Tilney MS, Cotanche DA. Actin filaments, stereocilia, and hair cells of the bird cochlea. V. How the staircase pattern of stereociliary lengths is generated. J Cell Biol. 1988;106:355–365. doi: 10.1083/jcb.106.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: A time to experiment, and a time to theorize. Curr Opin Cell Biol. 2006;18:472–481. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Schneider ME, Belyantseva IA, Azevedo RB, Kachar B. Rapid renewal of auditory hair bundles. Nature. 2002;418:837–838. doi: 10.1038/418837a. [DOI] [PubMed] [Google Scholar]
- 30.Manor U, Kachar B. Dynamic length regulation of sensory stereocilia. Semin Cell Dev Biol. 2008;19:502–510. doi: 10.1016/j.semcdb.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillespie PG, Müller U. Mechanotransduction by hair cells: Models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodyear RJ, Forge A, Legan PK, Richardson GP. Asymmetric distribution of cadherin 23 and protocadherin 15 in the kinocilial links of avian sensory hair cells. J Comp Neurol. 2010;518:4288–4297. doi: 10.1002/cne.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furness DN, Katori Y, Nirmal Kumar B, Hackney CM. The dimensions and structural attachments of tip links in mammalian cochlear hair cells and the effects of exposure to different levels of extracellular calcium. Neuroscience. 2008;154:10–21. doi: 10.1016/j.neuroscience.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Le Calvez S, Avan P, Gilain L, Romand R. CD1 hearing-impaired mice. I: Distortion product otoacoustic emission levels, cochlear function and morphology. Hear Res. 1998;120:37–50. doi: 10.1016/s0378-5955(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 35.Verpy E, et al. Stereocilin-deficient mice reveal the origin of cochlear waveform distortions. Nature. 2008;456:255–258. doi: 10.1038/nature07380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.