Abstract
The restriction factor Fv1 confers resistance to murine leukemia virus (MLV), blocking progression of the viral life cycle after reverse transcription, but before integration into the host chromosome. It is known that the specificity of restriction is determined by both the restriction factor and the viral capsid (CA), but a direct interaction between Fv1 and MLV CA has not yet been demonstrated. With the development of a previously unexplored method for in vitro polymerization of MLV CA, it has now been possible to display a binding interaction between Fv1 and MLV CA. C-terminally His-tagged CA molecules were assembled on Ni-chelating lipid nanotubes, and analysis by electron microscopy revealed the formation of a regular lattice. Comparison of binding data with existing restriction data confirmed the specificity of the binding interaction, with multiple positions of both Fv1 and CA shown to influence binding specificity.
The gene Fv1 is one of a series of mouse genes that determine susceptibility to murine leukemia virus (MLV) (1). The precise mechanism by which Fv1 restricts MLV infection is unclear. It has been shown that viral progression is blocked at a stage after reverse transcription of the viral genome, but before integration of newly synthesized DNA into the host chromosome (2, 3).
There are two major alleles of Fv1, Fv1n and Fv1b, characterized by their ability to block infection by different strains of MLV. Fv1n, present in NIH-Swiss mice, blocks infection by B-tropic MLV, but permits infection by N-tropic MLV. Fv1b, present in BALB/c mice, has the reverse phenotype (4). Viruses that grow equally well in either cell type were termed NB-tropic (5). More recent studies have shown that when expressed at higher than natural levels, Fv1b exerts a moderate restriction activity against B- and NB-MLV (6, 7). Fv1n does not show such secondary effects (6, 7). A less common third allele, Fv1nr, restricts B-tropic viruses and some—but not all—N-tropic viruses (8). Those N-tropic viruses not restricted by Fv1nr are referred to as NR-tropic MLV (9, 10).
Genetic studies have shown that the viral target of Fv1 is the MLV capsid protein (CA) (11, 12), and subsequent work identified position 110 to be the major determinant of N/B-tropism (13). B-MLV has a glutamate at this position, and N-MLV has an arginine. More recently, many other residues in CA have been implicated in NB- and NR-tropism. When mapped onto the MLV CA structure, these residues suggest a potential Fv1 binding pocket (10). Among those viruses classified as NR-tropic are N-MLVs carrying mutations N82D, H114N, or L117H (9, 10).
The Fv1 gene was cloned in 1996 (14), and since then a number of features have been identified within the molecule it encodes. Fv1 contains a major homology region (MHR), a feature that is highly conserved among retroviral CAs (15). A predicted coiled-coil region containing a dimerization domain was identified toward the N terminus, and there is also evidence to suggest that a second multimerization domain exists in the C-terminal half of the molecule (16, 17). It is likely that multimerization is important for Fv1 function (18). A localization domain was also identified, as deletion of residues 109 to 118 were sufficient to cause redirection of Fv1 to the endoplasmic reticulum rather than the trans-golgi network, a property associated with loss of restriction activity (16). Residues involved in the specificity of restriction are found at the C terminus (6, 7), and it is probable that this part of the molecule directly interacts with CA. Fv1n and Fv1b differ at three sites: position 358, position 399, and the C terminus itself, all of which have been implicated in the specificity of restriction (6, 7). Fv1nr is identical to Fv1n, except for a single-point mutation at position 352 (10).
To date, a direct interaction between Fv1 and MLV CA has not been observed. However, such biochemical studies are complicated by the fact that an interaction does not occur between monomeric proteins but apparently requires assembly of CA into the hexameric lattice organization observed in a viral core (19). A restriction factor binding assay has been developed for HIV-1 (20), making use of the fact that purified HIV CA and CA-nucleocapsid (NC) molecules readily assembles into tubes and core-like structures that form a hexameric lattice arrangement (21, 22). MLV CA is less easy to polymerize in vitro, and in our hands the reported binding of TRIM5α to detergent-treated MLV (23) has not proved reproducible, presumably because of the relative instability of MLV cores. We therefore set out to develop a reliable method for the ordered assembly of MLV CA. Here, we have been able to achieve this by anchoring His-tagged CA molecules to Ni2+-chelating lipid nanotubes. Using these CA-coated tubes, it has been possible to investigate the interaction between Fv1 and MLV CA and demonstrate a pattern of binding consistent with restriction.
Results
Ordered Assembly of MLV CA on Lipid Nanotubes.
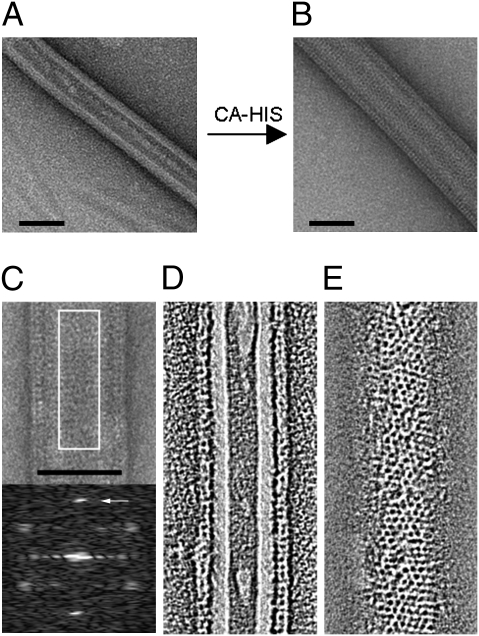
To become a target for Fv1 binding, CA must be assembled into the arrangement observed in virions (19). We therefore set out to develop a method for the ordered assembly of purified CA molecules. Compared with HIV CA (21, 22), MLV CA is less amenable to in vitro polymerization; however, assembly of His-tagged Moloney (Mo) MLV CA on Ni2+-chelating flat lipid sheets has been used to investigate CA structural organization (24–26). Based on these reports, we used the tube-forming lipid galactosylceramide (GalCer), which, with the inclusion of the Ni2+-chelating lipid 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl) iminodiacetic acid)succinyl] (DGS-NTA), produced a surface to which CA-His could be anchored (27). The CA protein was tagged at the C terminus to ensure a free N terminus, an apparent requirement both for MLV maturation and Fv1 binding (19). Fig. 1 shows Ni2+-chelating lipid tubes, before (Fig. 1A) and after (Fig. 1B) incubation with C-terminally His-tagged B-MLV CA, observed by electron microscopy following negative staining. In Fig. 1B, a protein layer increases the radius of the tube, and the surface is entirely covered with striations suggesting a regular organization of CA.
Fig. 1.
Ordered assembly of MLV CA on lipid nanotubes. Ni2+-chelating lipid nanotubes were generated from GalCer and DGS-NTA (30%). Tubes were observed by negative staining and electron microscopy, before (A) and after (B) incubation with C-terminally His-tagged B-MLV CA. (C) Image and computed diffraction pattern. (D) Tomogram cross-section of tube. (E) Tomogram section showing surface lattice. (Scale bars, 50 nm.) The arrow in the diffraction pattern points to the (1, 1) hexagonal reflection at 40- Å resolution.
Fig. 1C shows a negative-stain image of a tube and a computed diffraction pattern from its center, suggesting hexagonal ordering. The prominent spots at 40- Å resolution correspond to the (1, 1) reflection from a hexagonal lattice with a unit cell spacing of ∼70 Å. The lattice parameters are therefore similar to 2D crystals of MLV (25, 28) and smaller than those reported for MLV in ice (29) or for MLV by X-ray crystallography (30). We performed electron tomography (Movie S1) to further understand the tube structure. Fig. 1D shows the cross section of the CA-coated tube, and Fig. 1E shows a section through the surface layer showing the lattice. The images reveal a regular honeycomb-like organization of the CA surface layer decorating a hollow lipid nanotube.
Fv1n Specifically Binds B-MLV CA Assembled on Lipid Nanotubes.
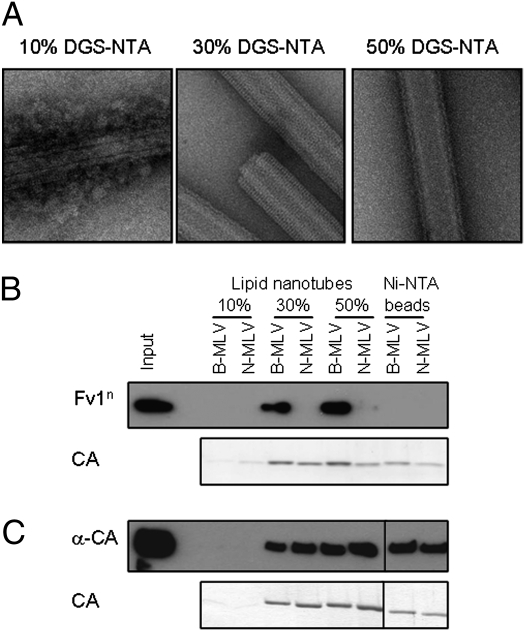
We next investigated whether it was possible to detect binding of Fv1 to CA-coated lipid tubes. Lipid tubes were generated comprising 10%, 30%, or 50% (mol/mol) DGS-NTA, and coated with either B- or N-MLV CA. Fig. 2A shows electron micrographs of B-MLV CA-coated tubes, and similar data were obtained for N-MLV CA. At 10% DGS-NTA, the tubes are incompletely coated with CA, evidenced by the irregular appearance of tubes and the presence of unbound protein (Fig. 2A). At 50%, tubes were completely covered by a protein layer but the best lattices were consistently obtained with 30% DGS-NTA. CA-coated tubes were then incubated with lysates from cells expressing Fv1n, and bound material separated from unbound by ultracentrifugation of the CA-tubes through a 40% sucrose cushion. A sample of lysate was saved for assessment of Fv1 expression, denoted “Input.” The pellet was analyzed for the presence of Fv1 by Western blotting with an anti-Fv1 antibody, and CA detected by SDS/PAGE and Coomassie staining. Consistent with expectations from restriction data (6, 7), Fv1n was found to cosediment with B- but not N-MLV CA (Fig. 2B), implying specific binding. As a negative control, Ni-NTA agarose beads were also coated with B- or N-MLV CA and used to detect binding of Fv1n. Although still proficient in pelleting CA protein, CA-coated Ni-NTA beads were not bound by Fv1n (Fig. 2B), indicating that the assembly of CA molecules seen on lipid tubes was a necessary requirement for Fv1n binding, as randomly orientated, high-density binding sites present on beads do not appear sufficient. In contrast to the specificity displayed by Fv1n, an anti-CA antibody was able to bind to and cosediment with both B- and N-MLV CA, whether bound to lipid nanotubes or Ni-NTA agarose beads (Fig. 2C). At 10% DGS-NTA, the limiting presence of Ni2+ binding sites resulted in tubes that bind less CA. Following centrifugation of these tubes, very little CA was detected in the pellet (Fig. 2 B and C), perhaps because the resulting tubes are too low in density to pass rapidly through the sucrose gradient, and the lack of Fv1n or anti-CA in the pellet fraction of these samples confirmed that neither binding partner was able to sediment through 40% sucrose in the absence of CA. Thus, the presence of Fv1n in the pellet confirmed a real and specific interaction with assembled CA.
Fig. 2.
Specific binding of Fv1 to MLV CA-coated lipid nanotubes. (A) Ni2+-chelating lipid nanotubes were generated using 10, 30, or 50% DGS-NTA, incubated with C-terminally His-tagged B-MLV CA, and observed by negative staining and electron microscopy. (B) Lipid nanotubes (10, 30, or 50% DGS-NTA) were incubated with C-terminally His-tagged B- or N-MLV CA, before incubation with lysate from cells overexpressing Fv1n. Ni-NTA agarose beads were also coated with B- or N-MLV CA, and similarly used to detect binding of Fv1n. Before mixing, an aliquot of the lysate was removed, denoted “Input,” to assess the expression level of Fv1n. Following centrifugation of the sample through a sucrose cushion, the pellet was resuspended in SDS PAGE sample buffer. Bound Fv1n was detected in the pellet fraction by Western blotting with an anti-Fv1 antibody, and CA detected in the pellet by SDS/PAGE and Coomassie staining. (C) As in B, the ability of anti-CA antibody to bind CA-coated lipid tubes or Ni-NTA beads was assessed, detected by Western blotting with secondary antibody.
Specificity of Binding Correlates with Specificity of Restriction.
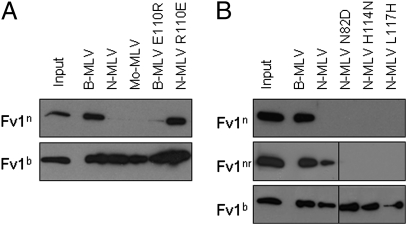
The ability of different Fv1 alleles to restrict various MLVs has been well studied, and a number of residues have been identified in CA that influence the specificity of restriction. To examine the correlation between binding and restriction, the binding behavior of a number of MLV CA variants was investigated. Cell lines were generated to stably express Fv1n, Fv1b, or Fv1nr, and CA-coated lipid tubes were prepared as described above. Fv1n was able to bind B-MLV CA, but not N- or Mo-MLV CA (Fig. 3A), consistent with existing restriction data (6, 7). In contrast, although Fv1b only restricts N-MLV when expressed at endogenous levels, significant activity against B- and Mo-MLV has been observed when Fv1b was overexpressed (6, 7). It was therefore unsurprising to discover that Fv1b was able to interact with all three CAs under the conditions of our assay (Fig. 3A). Among the many CA residues implicated in restriction specificity, position 110 has been demonstrated to be a major determinant of N/B-tropism (13). Mutating the residue at position 110 of B-MLV CA to that found in N-MLV CA was sufficient to ablate Fv1n binding, and the reverse was true when the same residue in N-MLV CA was mutated to that found in B-MLV (Fig. 3A). It seems that the presence of arginine (or the absence of glutamate) at this position was sufficient to ablate binding by Fv1n. However, Fv1nr, characterized by a single-point mutation in Fv1n, was able to overcome this block, binding both B- and N-MLV CA (Fig. 3B). Also consistent with restriction data, the NR-MLV CAs N82D, H114N, and L117H (9, 10) were bound by Fv1b, but not Fv1nr or Fv1n (Fig. 3B). The data show a correlation between the specificity of binding and the specificity of restriction.
Fig. 3.
The effect of CA mutations on the specificity of Fv1 binding. (A) CA-coated lipid nanotubes were generated using B-MLV, N-MLV, Mo-MLV, B-MLV (E110R), or N-MLV (R110E) CA. Tubes were then incubated with lysate from cells expressing Fv1n or Fv1b, and binding was detected as previously described. (B) CA-coated lipid nanotubes were generated using B-MLV, N-MLV, N-MLV (N82D), N-MLV (H114N), or N-MLV (L117H) CA. CA-coated tubes were then incubated with lysate from cells expressing Fv1n, Fv1nr, or Fv1b.
Fv1 Binding Requires Assembly of CA Molecules into an Ordered Lattice.
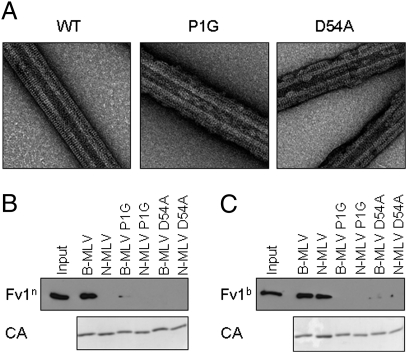
It has been proposed that assembly of CA is a necessary requirement for Fv1 binding, as monomeric CA does not compete for restriction factor binding (19). Whether CA molecules must be assembled into the hexameric lattice arrangement of a viral core or simply presented in close proximity has not been shown. To investigate this further, we generated CA proteins that would be impaired for lattice formation, and used these to investigate whether Fv1 could still bind. The N-terminal β-hairpin structure that forms on viral maturation is well conserved among retroviruses. Prevention of β-hairpin formation disrupts CA assembly, both in vivo and in vitro (31–33). In MLV, the β-hairpin is stabilized by a salt bridge between residues P1 and D54. We generated CA proteins mutated at position 1 (P1G) or position 54 (D54A), and used these for production of CA-coated lipid nanotubes. Fig. 4A shows electron micrograph images of wild-type and mutant N-MLV CA proteins assembled on lipid tubes, and similar data were obtained for B-MLV CA. Lipid tubes were still coated with CA, but rarely showed the complete covering of the tubes and were less well ordered than wild-type CA. Wild-type and mutant CA-coated lipid tubes were then used in a binding assay, to detect binding of either Fv1n (Fig. 4B) or Fv1b (Fig. 4C). Binding to assembly-impaired CAs was ablated or significantly reduced for both Fv1n and Fv1b, despite the presence of CA in the pellet fraction. It seemed these lattices, which lacked the integrity of the wild-type lattice, were not sufficient for Fv1 binding.
Fig. 4.
Disruption of CA β-hairpin impaired lattice formation and Fv1 binding. (A) Lipid nanotubes were incubated with N-MLV CA (WT), N-MLV P1G CA, or N-MLV D54A CA, and analyzed by negative staining and electron microscopy. (B) CA-coated lipid nanotubes were generated using wild-type or mutant B- or N-MLV CA before incubation with lysate from cells expressing Fv1n. Binding of Fv1 was detected as previously described. (C) As in B, using lysate from cells expressing Fv1b.
Multimerization Domains of Fv1 Are Required for CA Binding.
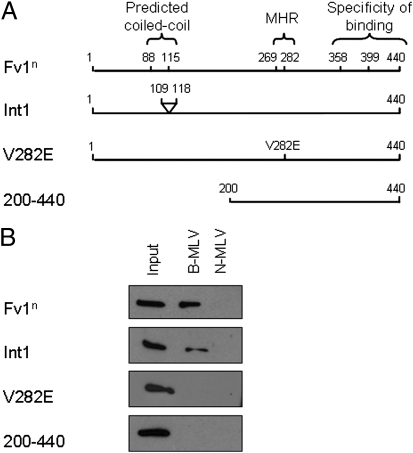
Mutation, deletion, and expression studies have identified a number of features in the Fv1 molecule that are important for restrictive function, as summarized in Fig. 5A (6, 7, 16, 17). Mutations were made in Fv1n to examine the importance of some of these features for CA binding (Fig. 5A). Cell lines were generated to stably express these proteins, and the ability to bind B- or N-MLV CA was investigated. Wild-type Fv1n was included for comparison. Despite being nonrestrictive (6), the deletion mutant Int1 (D109–118) was still able to bind to B-MLV CA. In contrast, the MHR mutant V282E and Fv1 200 to 440 were unable to bind (Fig. 5B). Fluorescence studies have shown that Int1 is mislocalized within the cell (16), and it is thought that this protein could be restrictive, but does not encounter the incoming virus. Fv1 V282E, in which the MHR is disrupted, is impaired for both self-association and restriction (6, 16). Fv1 200 to 440 retains the proposed binding domain as the MHR, but lacks the N-terminal dimer domain. As neither of these multimerization-impaired proteins were able to bind MLV CA (Fig. 5B) it seems that multimerization of Fv1 is required for CA binding.
Fig. 5.
Regions of Fv1 required for CA binding. (A) Schematic showing key features of Fv1n (Upper) and of Fv1n mutants (Lower panels). Numbers refer to amino acid positions. (B) CA-coated lipid nanotubes were generated using B- or N-MLV CA, before incubation with lysate from cells expressing Fv1n, or expressing the Fv1n mutant proteins, Int1 (D109-118), V282E or 200 to 440. Binding was detected as previously described.
Discussion
The ability to study the specific interaction between Fv1 and the MLV CA protein is essential for the detailed understanding of the mechanism of restriction. To facilitate this study, we have developed a method for the ordered assembly of MLV CA protein on the surface of lipid nanotubes. Using these CA-coated tubes, it has been uniquely possible to demonstrate qualitatively a binding interaction between Fv1 and MLV CA.
Examination of a number of Fv1 and CA variants allows us to show a strong correlation between the specificity of binding and the specificity of restriction. Consistent with previous data, CA residue 110 was of prime importance for Fv1n binding. As previous studies have shown that a neutral residue at this position resulted in a virus that could still be restricted by Fv1n (10), it seems that the presence of arginine (rather than the absence of glutamate) is required to disrupt the binding interaction. As well as adding to the array of residues involved in binding, results with Fv1nr and NR-MLV CA point to the coevolution of restriction factor and virus, illustrating how a single-point mutation in either molecule is sufficient to allow or escape binding, respectively.
The binding behavior of Fv1b in our assay was markedly different from Fv1n and Fv1nr. Binding was not affected by the identity of the amino acids found at positions 82, 110, 114, or 117 of N-MLV CA, all of which span the proposed Fv1 binding site and all of which affect the functional interaction with Fv1b, as measured by restriction (10). Our previous observations that overexpression of Fv1b results in inhibition of B-MLV replication, whereas overexpressed Fv1n does not restrict N-MLV, suggest that the difference in binding affinity with restricted and nonrestricted targets might be much lower for Fv1b than Fv1n. In any event, mutations in CA like P1G and D54A that prevent Fv1n binding of B-MLV, possibly by interfering with the ordered assembly of CA (see below), also prevent Fv1b binding of N- or B-MLV, suggesting similarity in mode of binding.
At present, it is unclear whether Fv1 binding of CA is alone sufficient to induce restriction or whether secondary mechanisms exist. The development of a binding assay will allow a detailed examination of this question. However, artificial restriction factors comprising only a multimerization domain and a CA-binding domain are able to restrict viral infection (18), and as yet no data has been obtained to suggest a more complex mechanism for Fv1 analogous to the proteasomal degradation associated with TRIM5α. Throughout the course of this work, only one example was identified where binding and restriction did not correlate. Int1, despite being nonrestrictive (6), was still able to interact with B-MLV CA. However, as this mutated protein, Fv1 with amino acids 109–118 deleted, has been observed in a cellular location where it is not expected to encounter incoming virions (16), these data do not have implications for the mechanism of binding. Rather, they underline the importance of correct localization for Fv1 restriction function.
This work has also been informative about the multimerization requirements for Fv1-CA binding. It has previously been hypothesized that the affinity of CA binding is low, requiring multimerization of both binding partners to increase avidity sufficiently to allow a stable association to form (18, 30, 34). It has now been possible to confirm that both the assembly of CA and multimerization of Fv1 are indeed required for a measurable binding interaction to occur.
Fv1 possesses two multimerization domains, a dimerization domain associated with a predicted coiled-coil region located toward the N terminus, and a second, less well-defined multimerization domain in the C-terminal half of the molecule (17). Despite the fact that Fv1n mutants V282E and 200 to 440 would each retain one of these multimerization domains, neither was able to bind B-MLV CA. The related restriction factor TRIM5α is better understood, both in terms of domain structure and binding behavior, and so it is of interest to draw comparisons between this protein and Fv1. It has recently been shown that binding of TRIM5α to HIV CA requires not only the dimerization associated with the coiled-coil domain, but also higher order multimerization mediated by the B-box (34, 35). It seems likely that higher order multimerization is also important for Fv1 binding, and it will be interesting to pursue this idea further by investigating the binding and association behavior of a more extensive panel of Fv1 mutants.
Our binding data indicate that CA multimerization is required for binding by Fv1. We have previously suggested that binding sites either form during MLV maturation or assume a suitable spacing to allow stable binding (19, 30). Although we do not yet know the precise arrangement of CA molecules in the lipid nanotubes, it appears that they form a polymer sufficiently closely related to that found in virions to allow Fv1 binding. Again, the parallels to TRIM5 appear close, binding of HIV-1 CA being dependent on CA forming a regular array (20, 36). Very recently, it has been shown that TRIM5 can form a hexagonal lattice allowing the correct alignment of multiple B30.2 domains over CA (37). It will be of considerable interest to determine whether the same holds true for Fv1. If so, the failure to form an ordered long-range structure might provide an explanation for the absence of Fv1 binding by P1G and D54A.
In addition to Fv1 binding, the methodological advances described here will also be of use for the study of MLV CA assembly and structure. In vitro assembled HIV CA has proved a valuable tool for examining CA structural organization, in some cases revealing information that was not obtained from X-ray crystallography (21, 22, 38). It will now be possible to obtain similar information about the structural organization of CA of MLV and other retroviruses. This being the case, these unique methodologies will open up new avenues for the comparative study of both the structural organization and also the binding characteristics of a wide range of retroviral CA proteins.
Materials and Methods
Cells and Viruses.
TE671 and 293T cells were cultivated in DMEM containing 10% FCS and antibiotics. Fv1-expressing cell lines were generated by transduction with retroviral delivery vectors. The viruses were generated by simultaneous transfection of 293T cells with three plasmids containing the vector, gag-pol, and env functions (7, 39). Virus-containing supernatant was harvested, filtered, and either used to transduce cells directly or stored at −70 °C.
Recombinant DNA.
Plasmids expressing Fv1n (pLFv1nIEG), Fv1b (pLFv1bIEG), Fv1nr (pLFv1nrIEG), Int1 (pLFv1nD109-118IEG), and Fv1nV282E (pLFv1nV282EIEG) have been previously described (6, 7, 10). To generate the plasmid pLFv1n200–440IRESYFP, residues 200 to 440 of Fv1n were amplified by PCR mutagenesis, from the template pLFv1nIEG, using the primers 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTATGGCCTCTCCATCC-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTCAGAGTTTTGTAGC-3′. The PCR product was subcloned into pENTR/d-TOPO then recombined into the retroviral vector pLgatewayIRESYFP using the Invitrogen Gateway cloning system.
The expression plasmids for C-terminally His-tagged N-tropic MLV CA (pET22-N-MLV-CA) and B-tropic MLV CA (pET22-B-MLV-CA) are as previously described (30), and a similar plasmid for Mo- MLV CA (pET22-Mo-MLV-CA) was prepared using pHIT60 (39) as a template. Briefly, restriction endonuclease sites were introduced to the ends of the Mo-MLV-CA coding sequence by PCR using primers 5′-CATATGCCCCTCCGCGCAGGA-3′ (Nde1 site added, underlined) and 5′-GCGGCCGCCAATAGCTTGCTCAT-3′ (Not1 site added, underlined). The PCR product was digested with Nde1/Not1 and ligated into the large fragment of Nde1/Not1 digested pET22 vector.
Expression plasmids with point mutations were generated by QuikChange site-directed mutagenesis (Stratagene) using the following primers; P1G forward 5′-TATACATATGGGGCTCCGTTTGG-3′, P1G reverse 5′-CCAAACGGAGCCCCATATGTATA-3′, D54A forward 5′-CAGCCCACCTGGGATGCCTGCCAGCAATTATTAG-3′, D54A reverse 5′-CTAATAATTGCTGGCAGGCATCCCAGGTGGGCTG-3′, N82D forward 5′-CTGTCCGGGGCGATGATGGGCGC-3′, N82D reverse 5′-GCGCCCATCATCGCCCCGGACAG-3′, R110E forward 5′-CACCACCCAAGAAGGTAGGAACC-3′, R110E reverse 5′-GGTTCCTACCTTCTTGGGTGGTG-3′, E110R forward 5′-CACCACTACAAGAGGTAGGAACC-3′, E110R reverse 5′-GGTTCCTACCTATTGTAGTGGTG-3′, H114N forward 5′-GGTAGGAACAACCTAGTCCTC-3′, H114N reverse 5′-GAGGACTAGGTTGTTCCTACC-3′, L117H forward 5′-CCACCTAGTCCACTATCGCCAG-3′, L117H reverse 5′-CTGGCGATAGTGGACTAGGTGG-3′.
CA Expression and Purification.
C-terminally His-tagged MLV CA protein was expressed in Rosetta 2 DE3 (Novagen) Escherichia coli cells grown at 37 °C, induced at an OD of 0.6 with 0.5 mM IPTG, and grown for 3 h. Cells were harvested by centrifugation, and protein was purified by Ni2+-affinity chromatography, followed by size-exclusion gel filtration on a HiLoad 16/60 200 column (GE Healthcare). Fractions containing protein were then pooled, concentrated to 3 to 6 mg/mL, aliquoted, and stored at −70 °C.
Generation of CA-Coated Lipid Nanotubes.
Lipid nanotubes were generated as described by Wilson-Kubalek et al. (27, 40). Tube forming lipids, d-galactosyl-β-1,1' N-Nervonoyl-d-erythro-sphingosine (GalCer), and Ni2+-chelating lipids, DGS-NTA were purchased from Avanti polar lipids and stored in chloroform at −20 °C. Ni2+-chelating lipid nanotubes were prepared by combining GalCer with DGS-NTA at a ratio of 7:3, to generate a nanotube comprising 30% Ni-chelating lipid (unless stated otherwise). After mixing, the chloroform was removed under a gentle stream of nitrogen, the lipids were rehydrated with 20 mM Tris-HCl pH 8, 100 mM NaCl to a concentration of 500 μg/mL and sonicated for 5 min. To generate CA-coated nanotubes, 2 mg/mL CA protein was combined with lipid nanotubes at a ratio of 1:3 with 10 mM imidazole. The mixture was incubated at room temperature for 1 h before use.
MLV Binding Assay.
Mammalian cells (TE671) stably expressing Fv1 protein were plated in 6-cm dishes 1 d before use. Cells from one confluent plate were resuspended in 5 mL PBS and collected by centrifugation (5 min, 1,000 × g), then lysed in 750 mL of cold hypotonic lysis buffer (10 mM Tris-HCl pH8, 10 mM KCl). Cells were left for 15 min on ice then homogenized using a 2-mL dounce homogenizer (20 strokes) and insoluble material removed by centrifugation (10 min, 20,000 × g). The protein concentration of the cleared cell lysate was adjusted to 300 mg/mL (measured using Bradford reagent). To account for the differences in expression levels, lysate from cells expressing Fv1n (or Fv1n-related mutants) was diluted fourfold with TE671 cell lysate. For the binding reaction, 200 μL of lysate was combined with 4 μL of CA-coated lipid nanotubes, and the NaCl concentration adjusted to 150 mM. The samples were incubated at room temperature for 1 h with gentle mixing, then layered on top of 2 mL 40% (wt/vol) sucrose in PBS. The samples were centrifuged at 110,000 × g for 1 h in a SW55 rotor, the supernatant aspirated, and the pellet resuspended in 50 μL of SDS sample buffer. CA in the pellet was detected by SDS PAGE and Coomassie staining, and Fv1 pull-down was measured by Western blotting with a rabbit anti-Fv1 antibody directed against the N terminus of the molecule (17). Fv1n 200 to 440 was detected with a rabbit anti-Fv1 antibody directed against the whole molecule (6).
Electron Microscopy and Image Analysis.
Two to three microliters of sample were applied to glow-discharged, continuous carbon-coated grids, stained with 1% uranyl acetate. Imaging was performed in an FEI Spirit TWIN microscope at 120 keV using an Eagle 2K camera (FEI) at 4.3 Å/pixel magnification or an FEI G2 Polara operating at liquid nitrogen temperature and 200 keV with a 224 HD detector (TVIPS) at 4.4 Å/pixel. Tilt series (0 to ±66° in 3° steps) for tomography were recorded on an FEI G2 Polara at 2.3 Å/pixel using Xplor3D (FEI) and reconstructed using IMOD (41). The final 2× binned tomogram was 4.6 Å/pixel. A movie showing tomogram sections can be found at Movie S1.
Supplementary Material
Acknowledgments
We thank John Berriman and Melvyn Yap for helpful discussions. This work was supported by the United Kingdom Medical Research Council, File References U117581334 (to P.B.R.), U117565647 (to I.A.T.), and U117512710 (to J.P.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100118108/-/DCSupplemental.
References
- 1.Lilly F. Fv-2: Identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970;45:163–169. [PubMed] [Google Scholar]
- 2.Jolicoeur P, Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci USA. 1976;73:2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sveda MM, Soeiro R. Host restriction of Friend leukemia virus: Synthesis and integration of the provirus. Proc Natl Acad Sci USA. 1976;73:2356–2360. doi: 10.1073/pnas.73.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lilly F, Steeves RA. B-tropic Friend virus: A host-range pseudotype of spleen focus-forming virus (SFFV) Virology. 1973;55:363–370. doi: 10.1016/0042-6822(73)90176-1. [DOI] [PubMed] [Google Scholar]
- 5.Hartley JW, Rowe WP, Huebner RJ. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970;5:221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop KN, Bock M, Towers G, Stoye JP. Identification of the regions of Fv1 necessary for murine leukemia virus restriction. J Virol. 2001;75:5182–5188. doi: 10.1128/JVI.75.11.5182-5188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bock M, Bishop KN, Towers G, Stoye JP. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J Virol. 2000;74:7422–7430. doi: 10.1128/jvi.74.16.7422-7430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steeves R, Lilly F. Interactions between host and viral genomes in mouse leukemia. Annu Rev Genet. 1977;11:277–296. doi: 10.1146/annurev.ge.11.120177.001425. [DOI] [PubMed] [Google Scholar]
- 9.Jung YT, Kozak CA. A single amino acid change in the murine leukemia virus capsid gene responsible for the Fv1(nr) phenotype. J Virol. 2000;74:5385–5387. doi: 10.1128/jvi.74.11.5385-5387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens A, et al. Retroviral capsid determinants of Fv1 NB and NR tropism. J Virol. 2004;78:9592–9598. doi: 10.1128/JVI.78.18.9592-9598.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkins N, Schindler J, Hynes R. Six-NB-tropic murine leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J Virol. 1977;21:309–318. doi: 10.1128/jvi.21.1.309-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rommelaere J, Donis-Keller H, Hopkins N. RNA sequencing provides evidence for allelism of determinants of the N-, B- or NB-tropism of murine leukemia viruses. Cell. 1979;16:43–50. doi: 10.1016/0092-8674(79)90186-7. [DOI] [PubMed] [Google Scholar]
- 13.Kozak CA, Chakraborti A. Single amino acid changes in the murine leukemia virus capsid protein gene define the target of Fv1 resistance. Virology. 1996;225:300–305. doi: 10.1006/viro.1996.0604. [DOI] [PubMed] [Google Scholar]
- 14.Best S, Le Tissier P, Towers G, Stoye JP. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature. 1996;382:826–829. doi: 10.1038/382826a0. [DOI] [PubMed] [Google Scholar]
- 15.Bénit L, et al. Cloning of a new murine endogenous retrovirus, MuERV-L, with strong similarity to the human HERV-L element and with a gag coding sequence closely related to the Fv1 restriction gene. J Virol. 1997;71:5652–5657. doi: 10.1128/jvi.71.7.5652-5657.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap MW, Stoye JP. Intracellular localisation of Fv1. Virology. 2003;307:76–89. doi: 10.1016/s0042-6822(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 17.Bishop KN, et al. Characterization of an amino-terminal dimerization domain from retroviral restriction factor Fv1. J Virol. 2006;80:8225–8235. doi: 10.1128/JVI.00395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yap MW, Mortuza GB, Taylor IA, Stoye JP. The design of artificial retroviral restriction factors. Virology. 2007;365:302–314. doi: 10.1016/j.virol.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Dodding MP, Bock M, Yap MW, Stoye JP. Capsid processing requirements for abrogation of Fv1 and Ref1 restriction. J Virol. 2005;79:10571–10577. doi: 10.1128/JVI.79.16.10571-10577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stremlau M, et al. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci USA. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganser BK, Li S, Klishko VY, Finch JT, Sundquist WI. Assembly and analysis of conical models for the HIV-1 core. Science. 1999;283:80–83. doi: 10.1126/science.283.5398.80. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Hill CP, Sundquist WI, Finch JT. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature. 2000;407:409–413. doi: 10.1038/35030177. [DOI] [PubMed] [Google Scholar]
- 23.Sebastian S, Luban J. TRIM5α selectively binds a restriction-sensitive retroviral capsid. Retrovirology. 2005;2:40. doi: 10.1186/1742-4690-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barklis E, et al. Structural analysis of membrane-bound retrovirus capsid proteins. EMBO J. 1997;16:1199–1213. doi: 10.1093/emboj/16.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayo K, McDermott J, Barklis E. Hexagonal organization of Moloney murine leukemia virus capsid proteins. Virology. 2002;298:30–38. doi: 10.1006/viro.2002.1452. [DOI] [PubMed] [Google Scholar]
- 26.Zuber G, et al. Assembly of retrovirus capsid-nucleocapsid proteins in the presence of membranes or RNA. J Virol. 2000;74:7431–7441. doi: 10.1128/jvi.74.16.7431-7441.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson-Kubalek EM. Preparation of functionalized lipid tubules for electron crystallography of macromolecules. Methods Enzymol. 2000;312:515–519. doi: 10.1016/s0076-6879(00)12936-2. [DOI] [PubMed] [Google Scholar]
- 28.McDermott J, Mayo K, Barklis E. Three-dimensional organization of retroviral capsid proteins on a lipid monolayer. J Mol Biol. 2000;302:121–133. doi: 10.1006/jmbi.2000.4030. [DOI] [PubMed] [Google Scholar]
- 29.Ganser BK, Cheng A, Sundquist WI, Yeager M. Three-dimensional structure of the M-MuLV CA protein on a lipid monolayer: A general model for retroviral capsid assembly. EMBO J. 2003;22:2886–2892. doi: 10.1093/emboj/cdg276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortuza GB, et al. Structure of B-MLV capsid amino-terminal domain reveals key features of viral tropism, gag assembly and core formation. J Mol Biol. 2008;376:1493–1508. doi: 10.1016/j.jmb.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 31.Oshima M, et al. Effects of blocking individual maturation cleavages in murine leukemia virus gag. J Virol. 2004;78:1411–1420. doi: 10.1128/JVI.78.3.1411-1420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Schwedler UK, et al. Proteolytic refolding of the HIV-1 capsid protein amino-terminus facilitates viral core assembly. EMBO J. 1998;17:1555–1568. doi: 10.1093/emboj/17.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiegers K, et al. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J Virol. 1998;72:2846–2854. doi: 10.1128/jvi.72.4.2846-2854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz-Griffero F, et al. A B-box 2 surface patch important for TRIM5alpha self-association, capsid binding avidity, and retrovirus restriction. J Virol. 2009;83:10737–10751. doi: 10.1128/JVI.01307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Sodroski J. The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J Virol. 2008;82:11495–11502. doi: 10.1128/JVI.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forshey BM, Shi J, Aiken C. Structural requirements for recognition of the human immunodeficiency virus type 1 core during host restriction in owl monkey cells. J Virol. 2005;79:869–875. doi: 10.1128/JVI.79.2.869-875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganser-Pornillos BK, et al. Hexagonal assembly of a restricting TRIM5α protein. Proc Natl Acad Sci USA. 2011;108:534–539. doi: 10.1073/pnas.1013426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byeon IJ, et al. Structural convergence between Cryo-EM and NMR reveals intersubunit interactions critical for HIV-1 capsid function. Cell. 2009;139:780–790. doi: 10.1016/j.cell.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soneoka Y, et al. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dang TX, Milligan RA, Tweten RK, Wilson-Kubalek EM. Helical crystallization on nickel-lipid nanotubes: Perfringolysin O as a model protein. J Struct Biol. 2005;152:129–139. doi: 10.1016/j.jsb.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Mastronarde DN. Dual-axis tomography: An approach with alignment methods that preserve resolution. J Struct Biol. 1997;120:343–352. doi: 10.1006/jsbi.1997.3919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.