Abstract
During early postnatal development in the rat hippocampus, synaptogenesis occurs in parallel with a developmental switch in the subunit composition of NMDA receptors from NR2B to NR2A. It is unclear how this switch affects the process of synaptogenesis, synapse maturation, and synapse stabilization. We investigated the role of NR2 subunits in synaptogenesis during the period in which expression and synaptic incorporation of the NR2A protein begins through the time when it reaches adult levels. We found that early expression of NR2A in organotypic hippocampal slices reduces the number of synapses and the volume and dynamics of spines. In contrast, overexpression of NR2B does not affect the normal number and growth of synapses; however, it does increase spine motility, adding and retracting spines at a higher rate. The C terminus of NR2B, and specifically its ability to bind CaMKII, is sufficient to allow proper synapse formation and maturation. Conversely, the C terminus of NR2A was sufficient to stop the development of synapse number and spine growth. Our results indicate that the ratio of synaptic NR2B over NR2A controls spine motility and synaptogenesis, and suggest a structural role for the intracellular C terminus of NR2 in recruiting the signaling and scaffolding molecules necessary for proper synaptogenesis.
Synaptic connectivity in the brain is the result of a delicate balance between synaptogenesis and synaptic pruning. Changes in synaptic connectivity drive the refinement of neuronal circuits during development (1) and are thought to underlie the formation of memories and acquisition of behaviors (2). Disruption of this balance has been linked to abnormal brain development and neuropsychiatric disorders such as Alzheimer's disease and schizophrenia (3).
In rat hippocampus, during the 2 wk following birth a large number of synaptic connections are assembled; some subsets of these are stabilized, and others are lost (4). During the same period, NMDA-type glutamate receptors (NMDARs) undergo a developmental switch from containing the NR2B subunit to containing the NR2A subunit (5). This switch accelerates the kinetics of NMDAR-mediated excitatory postsynaptic currents (EPSCs) (6, 7) and decreases the ability of the synapse to undergo potentiation (8, 9). Recent evidence also indicates that NMDARs play a structural role in the long-term stabilization of synapses and spines (10); however, it is not known whether NR2 subunit composition influences the process of synaptogenesis, synaptic pruning, and synapse stabilization.
NR2 subunits bind glutamate and determine the functional properties of NMDAR channels (11). NR2A and NR2B subunits are closely related in amino acid sequence; however, the large intracellular C terminus exhibits only 54% identical or similar amino acids (12). Differential interactions between intracellular signaling complexes and NMDAR C termini have been proposed to play important roles in synaptic plasticity (8, 9, 13, 14). Importantly, the interaction of active CaMKII and NR2B C terminus is necessary for long-term potentiation (LTP) (8, 9, 15), a cellular model of memory formation (2). Conversely, NR2A does not interact with CaMKII (16). Given the length of their C termini and the potential for differential interactions with scaffolding and signaling molecules, the NR2 subunits are good candidates to participate in controlling the process of synaptogenesis, synaptic pruning, and synapse stabilization. We investigated whether the subunit composition of synaptic NMDARs controls the number and structural plasticity of Schaffer collateral/commissural-CA1 synapses in the hippocampus. Cultured slices allowed us to manipulate the subunit composition of synaptic NMDARs while maintaining synaptic organization critical to understanding synapse development (17).
Our findings show that the ratio of NR2A to NR2B present at synapses controls the rate of synapse formation and maturation. We conclude that the developmentally regulated expression of NR2 subunits is a key component to controlling normal development of synapses through interactions mediated by the C terminus, in particular with CaMKII. We propose a model in which NR2A acts as a stabilizing force in the synapse, making both functional and structural change more difficult, whereas NR2B is required for structural changes, such as new spine formation and spine retraction, to occur.
Results
Development of Synapses in Cultured Hippocampal Slices.
We characterized the development of synapses in CA1 pyramidal cells from organotypic hippocampal slices prepared at postnatal day 6 (p6) and cultured for 4–5, 7–8, or 11–12 d. This allowed us to establish the normal rate of synaptogenesis with which to compare experiments where expression of NR2 subunits was manipulated. These age groups were chosen to bracket the developmental period in which expression and synaptic incorporation of NR2A begins through the time that it reaches adult levels (Fig. S1A) (18, 19).
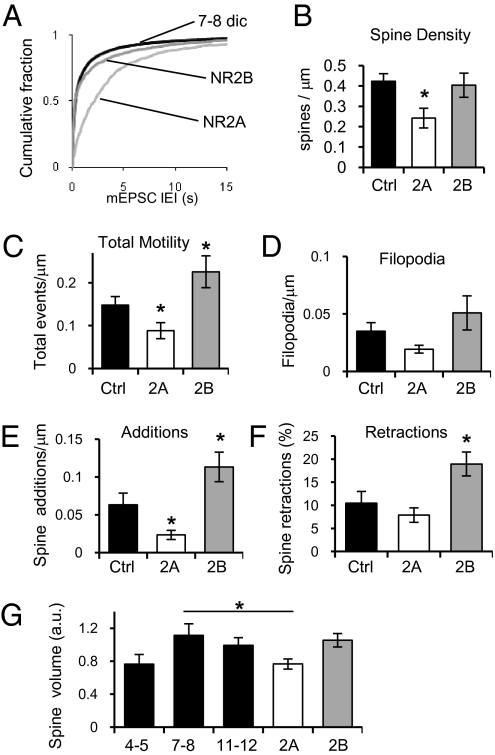
We determined the frequency of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate glutamatergic receptor (AMPAR)-mediated spontaneous miniature excitatory postsynaptic currents (mEPSCs) as a measure of functional synapses present at different stages of development. Importantly, paired pulse facilitation, an indicator of presynaptic release probability, did not change among the ages studied (Fig. S1B). A large increase in the frequency of mEPSCs is observed between 4–5 days in culture (dic) and 7–8 dic, indicating a period of strong synaptogenesis in hippocampal slices during the first week in culture. Between 7–8 dic and 11–12 dic, a smaller but still significant increase in mEPSC frequency is observed (Fig. 1 A and B). The average interevent interval (IEI) per cell was also calculated and pooled with age-matched cells, and a group average taken (Fig. 1B, Inset). Cumulative distribution plots were preferred in further figures to better display the total range of IEIs. Consistent with previous reports (17), the amplitude of mEPSCs did not change during the period studied. Only cells in the 7–8 dic group were observed to have slightly larger mEPSC amplitudes (∼5 pA), significantly different from the 11–12 dic group but not the 4–5 dic group, with an increase in the skew of the distribution (Fig. S1 E and F).
Fig. 1.
Development of synapses and spine motility in organotypic hippocampal slices. (A Left) Example traces from slices prepared at p6 and cultured as indicated showing mEPSCs recorded in the presence of 1 μM TTX and 100 μM PTX at −60 mV. (Scale bar, 20 pA and 1.3 s.) (Right) Example of apical dendritic spines from neurons transfected with GFP in slices as indicated. (Scale bar, 5 μm.) (B) Cumulative fraction of all IEIs of mEPSCs from slices cultured for 4–5 (n = 12), 7–8 (n = 27), or 11–12 (n = 9) days. Frequency of mEPSC increases significantly from 4–5 dic to 7–8 dic (KS test D = 0.3053, P < 0.01). A smaller but still significant increase occurs at 11–12 dic (KS test D = 0.0536, P < 0.01). (Inset) IEI cell average. Error bars represent SEM. *P < 0.05, Student's t test. (C) Quantification of dendritic spine density from cells from slices 4–5 dic (n = 9), 7–8 dic (n = 8), and 11–12 dic (n = 14). (D) Total spine motility decreases with age. Quantification of all spine motility events (filopodia extension + new spine emergences + spine retraction) per micrometer from cells transfected with GFP from slices 4–5 dic (n = 8), 7–8 (n = 8), and 11–12 dic (n = 6). Confocal live images were taken at intervals of 10 min for 2 h. (E–G) Quantification of filopodia extension (E) and new spines appearing per micrometer of dendrite (F), and percentage of spines retracting (G) from cells, as in D.
Because the vast majority of excitatory synapses are located on dendritic spines (20, 21), we used spine density as a second robust method to estimate the number of synapses during the developmental period of interest. Confocal fluorescence microscopy was used to calculate spine density on apical dendrites of CA1 pyramidal cells transfected with GFP. A large increase in spine density was observed between 4–5 dic and 7–8 dic. No change in spine density was observed between 7–8 and 11–12 dic (Fig. 1 A and C). These changes in spine density parallel changes in mEPSC frequency. Both methods underestimate the number of synapses because of our inability to detect events below the noise of our recordings (5–7 pA) and synapses made directly onto the dendritic shaft or spines that are normal to the imaging plane; however, they provide good and independent estimates of the relative number of synapses. Together, these experiments indicate that the number of synapses in CA1 pyramidal cells in cultured hippocampal slices increases rapidly during the first week in culture and stabilizes thereafter.
Normal Spine Motility in Cultured Hippocampal Slices.
To characterize the mechanism responsible for the observed increase in number of synapses, time-lapse fluorescence confocal microscopy was used to establish the normal dynamics of dendritic spines in CA1 pyramidal cells. Cultured hippocampal slices were transfected with GFP, and CA1 pyramidal cells imaged live 3 d later. Images were obtained every 10 min for a period of 2 h, and spine motility quantified. Motility events were defined as new spines appearing, spines retracting, and extension of filopodia (Materials and Methods). The total number of motility events observed in neurons from organotypic slices 4–5 dic and 7–8 dic was similar; however, at 11–12 dic, total motility events decrease significantly (Fig. 1D), consistent with reports indicating that spine motility decreases with age (22). In neurons from slices 7–8 dic, it was common to observe filopodia growth or extension; however, in cells 11–12 dic, the occurrence of filopodia movements or appearance decreased significantly (Fig. 1E), consistent with previous reports (23). The number of new spines added during the period of observation per unit of length decreased slightly between 4–5 and 7–8 dic. A large reduction in the number of new spines added is observed in cells 11–12 dic (Fig. 1F). Because the probability of observing spines retracting during imaging sessions is a function of the number of spines present, we normalized the number of spines retracted to the number of spines at the beginning of the imaging session and expressed it as a percentage. Similarly to new additions, spine retractions decrease slightly between 4–5 and 7–8 dic, and a large reduction in the number of spines retracted is observed in cells 11–12 dic (Fig. 1G and Fig. S1C). These data indicate that spine motility in general decreases drastically after the first week in culture. However, in cells from slices 4–5 and 7–8 dic, the number of new spines added is larger than the number of spines being retracted per unit of length (net gain; Fig. S1D), explaining the increase in the number of synapses and spine density observed during the first week in culture. In slices 11–12 dic, where spine motility is significantly lower, new spines can still be observed; however, the number of additions and retractions is equivalent, producing a net gain close to zero, and therefore maintaining a stable number of synapses (Fig. S1D).
This period of synaptogenesis and synapse stabilization in cultured organotypic hippocampal slices coincides with the beginning of expression and synaptic incorporation of NR2A subunits. We next manipulated the level of synaptic NR2A-containing NMDARs to test the hypothesis that NR2A controls the ratio of spine additions to retractions.
Early Expression of NR2A Decreases Number of Synapses.
Organotypic hippocampal slices 4–5 dic were transfected with GFP-tagged NR2A and NR1 and cultured for an additional 3 d. Only a few cells in the slice were transfected; therefore, we could not determine whether the total level of NMDARs was affected, but electrophysiological assays allowed us to determine whether the subunit composition of synaptic NMDARs was altered. Transfected CA1 pyramidal cells were compared with age-matched cells, i.e., 7–8 dic. At this age, evoked NMDAR EPSCs in CA1 pyramidal cells are dominated by NR2B-containing receptors. Ro25-6981, an NR2B-specific blocker (24), decreases the amplitude of NMDAR currents by ∼63% (Fig. S1G) and accelerates the decay kinetics (Fig. S1H), consistent with a larger proportion of NR2B-containing receptors and some synaptic incorporation of endogenous NR2A at this age. In cells transfected with NR2A, Ro25-6981 does not block NMDAR currents significantly (Fig. S1G) and has no effect on the decay kinetics of NMDAR currents (Fig. S1H). These experiments confirm that expression of recombinant NR2A significantly increases the proportion of NR2A-containing receptors at synapses (25).
Early expression of the NR2A subunit resulted in a significant decrease in mEPSC frequency compared with age-matched nontransfected cells (Fig. 2A). No change in the average amplitude of mEPSCs was observed (Fig. S1 E and F). To study the effect of early NR2A expression in spine density, mCherry-NR2A and NR1 were cotransfected with GFP. Cells expressing NR2A earlier than normal exhibit a significantly lower number of dendritic spines compared with cells expressing only GFP (Fig. 2B). Thus, increasing the level of NR2A during a period of synaptogenesis reduces the number of synapses formed. In contrast, coexpression of NR2B and NR1 did not affect the frequency of mEPSCs (Fig. 2A) nor the number of spines observed per unit length (Fig. 2B; for examples, see Fig. S2).
Fig. 2.
Early expression of NR2A in hippocampal slices decreases synapse number. (A) Cumulative fraction of all IEIs of mEPSCs from neurons cotransfected with NR1 and NR2A (n = 9), NR1 and NR2B (n = 8), or nontransfected age-matched neurons (black; n = 27). NR2A significantly decreases mEPSC frequency (KS test P < 0.01, D = 0.4989 vs. Ctrl and D = 0.51226 vs. NR2B). (B) Spine density from neurons cotransfected with GFP, NR1, and NR2A (n = 13), or NR2B (n = 12) and control age-matched neurons transfected with GFP (n = 8). (C) Quantification of all spine motility per micrometer from control age-matched neurons transfected with GFP (n = 8) or cells cotransfected with GFP, NR1, and NR2A (n = 13) or NR2B (n = 9). (D–F) Quantification of filopodia extension (D) and new spines per micrometer of dendrite (E), and percentage of spines retracting (F) from cells, as in C. (G) Quantification of spine volume from control neurons transfected with GFP from slices cultured for 4–5 (n = 4), 7–8 (n = 5), or 11–12 (n = 7) dic, and neurons cotransfected with GFP, NR1, and NR2A (n = 7) or NR2B (n = 4). Error bars represent SEM.
Early Expression of NR2A Blocks Addition of New Spines.
Cells expressing NR2A exhibit a significant decrease in the total number of motility events observed (Fig. 2C). A small nonsignificant decrease in the normal extension of filopodia is observed (Fig. 2D) as well as a significant decrease in the number of new spines added (Fig. 2E). Importantly, the number of spine retractions does not differ significantly from age-matched control cells (Fig. 2F; P = 0.4035). In contrast, expression of NR2B increases the total number of motility events observed (Fig. 2C). This increase in motility is due to a small increase in the number of filopodia appearing or extending and to significant increases in addition of new spines and spine retractions (Fig. 2 D–F). Interestingly, expression of NR2B does not alter the total number of synapses (Fig. 2 A and B), indicating that additions and retractions are occurring with similar frequency. In cells transfected with GFP, the average spine volume increases between 4–5 dic and 7–8 dic, but stabilizes between 7–8 dic and 11–12 dic (Fig. 2G). Early expression of NR2A decreases the volume of spines compared with age-matched (7–8 dic) control cells (Fig. 2G). The resulting decreased spine volumes are similar to those seen in 4–5 dic cells. Expression of NR2B does not affect the spine volume (Fig. 2G).
Thus, the decreased number of synapses seen with early synaptic incorporation of NR2A is not the result of a change in the rate of spine retractions, but rather due to a decrease in the appearance of new spines. Though NR2B does not change the total number of synapses, it stimulates motility of spines and filopodia. This suggests that NR2B may be critical for the initial setup of the synapse, because it allows spines to move and find appropriate presynaptic partners. To test whether NR2B is necessary for synapse formation, we next decreased the level of expression of endogenous NR2B.
NR2B Is Required for Synaptogenesis.
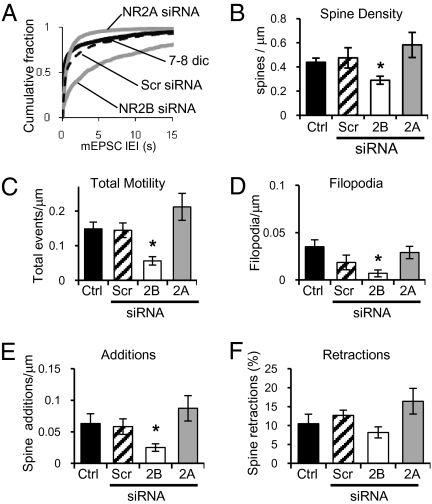
To selectively knock down NR2 subunits, we used a plasmid that coexpresses GFP and a siRNA directed against the sequence of either NR2A or NR2B, as previously described (13). Electrophysiological measurements were made to confirm knockdown of the corresponding NR2 subunit. Knockdown of NR2B reduces the peak amplitude of NMDAR-mediated EPSCs by ∼61%, compared with nearby nontransfected cells stimulated under the same conditions (Fig. 3A). As expected, the kinetics of decay of remaining NMDAR-mediated responses are faster, indicating that at 7–8 dic, a small amount of endogenous NR2A-containing receptors have been incorporated into synapses (Fig. 3B). Knockdown of endogenous NR2B reduces the number of functional synapses, as indicated by a significant reduction in the frequency of mEPSCs (Fig. 4A), and significantly lowers spine density (Fig. 4B). In contrast, knockdown of NR2A has no major effect, because the level of expression of endogenous NR2A and synaptic incorporation at this age, 7–8 dic, is low. Knockdown of NR2A produces only a small, nonsignificant decrease in the peak amplitude (∼16%) compared with nearby control cells (Fig. 3A). However, the decay kinetics of evoked NMDAR-mediated EPSCs are significantly slower (Fig. 3B), indicating removal of what NR2A subunits were present from synaptic NMDARs. Knocking down expression of endogenous NR2A slightly increases the frequency of mEPSCs (Fig. 4A) and spine density (Fig. 4B), although not significantly. Scrambled control siRNA had no effect on mEPSCs frequency or spine density.
Fig. 3.
Expression of NR2B-siRNA reduces synaptic NMDAR currents. (A) Peak amplitude of NMDAR-mediated EPSCs at +40 mV from neurons transfected with NR2B-siRNA (white; n = 14 pairs) or NR2A-siRNA (gray; n = 11) and adjacent nontransfected neurons (black bars). Values are normalized to the peak amplitude of the nontransfected cell in the pair. (B) Time to half-decay of NMDAR-mediated responses from neurons, as in A. Error bars represent SEM.
Fig. 4.
Knockdown of NR2B in hippocampal slices decreases synapse number and reduces spine motility. (A) Cumulative fraction of all IEIs of AMPAR-mediated mEPSCs from neurons transfected with 2B-siRNA (n = 8; KS D = 0.4876 vs. Ctrl), 2A-siRNA (n = 11; KS D = 0.1176 vs. Ctrl), or scrambled siRNA (dashed line; n = 11) and age-matched control neurons (black; n = 4 cells). (B) Quantification of dendritic spine density in control neurons (n = 8), scrambled siRNA (n = 6), and neurons cotransfected with GFP and NR2B-siRNA (n = 10) or NR2A-siRNA (n = 6). (C) Quantification of all spine motility events per micrometer from control neurons transfected with GFP (n = 8), scrambled siRNA (n = 6), 2B-siRNA (n = 8), or 2A-siRNA (n = 6). (D–F) Quantification of filopodia extension (D) and new spines per micrometer of dendrite (E), and percentage of spines retracting (F) from cells, as in C. Error bars represent SEM.
Our findings suggest that NR2B is necessary for spinogenesis/synaptogenesis. Our model predicts that this decrease in the number of synapses formed is due to a lack of structural plasticity in the absence of NR2B subunits. Therefore, we used time-lapse fluorescence imaging to estimate spine motility. As predicted, a decrease in the total number of motility events in cells expressing siRNA-NR2B is observed (Fig. 4C). Knockdown of NR2B decreased the number of filopodia extensions and the number of spine additions (Fig. 4 D and E) but did not alter the number of retractions (Fig. 4F; P = 0.4428). In contrast, knockdown of NR2A caused a small, nonsignificant increase in the total number of motility events observed (Fig. 4C) with a trend to increase both additions and retractions (Fig. 4 E and F). These experiments indicate that the ratio of NR2B to NR2A controls spine motility, with NR2B subunits increasing motility and allowing synaptogenesis, and NR2A subunits inducing stability.
NR2B and NR2A exhibit significant dissimilarity in their long, intracellular C termini, resulting in different binding affinities for structural and signaling proteins—in particular CaMKII, a key enzyme responsible for functional plasticity (26). We next tested the hypothesis that the C termini of NR2 subunits are responsible for the differences observed in synapse formation.
NR2 Subunits C Termini Control Synaptogenesis.
We created chimeric NR2 subunits with swapped intracellular C termini to test whether the C terminus of NR2A was responsible for preventing the normal increase in number of synapses when expressed prematurely, and whether the C terminus of NR2B was sufficient to allow normal development of synapses.
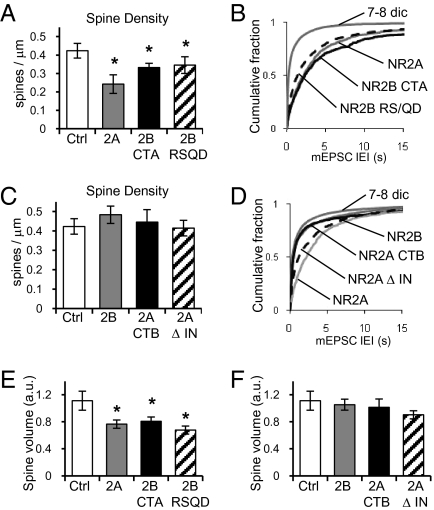
As mentioned, early expression of NR2A decreases the number of synapses formed at 7–8 dic, measured by decreases in spine density and frequency of AMPAR-mediated mEPSCs (Fig. 2; shown again in Fig. 5 for easy comparison). Expression of chimeric NR2B with the C terminus of NR2A (2B-CTA) reduced the number of spines formed at 7–8 dic (Fig. 5A, black bar) and the frequency of AMPAR-mediated mEPSCs (Fig. 5B, black line), similar to the effect of expressing WT NR2A. Importantly, 2B-CTA also decreases the volume of spines (Fig. 5E, black bar). Confirming the importance of the C terminus, cells expressing chimeric NR2A with the C terminus of NR2B (2A-CTB) exhibited normal spine density (Fig. 5C, black bar) and normal frequency of AMPAR-mediated mEPSCs (Fig. 5D, black line). In addition, the presence of the NR2B C terminus allowed spines to grow properly, as the spine volume was not different from cells expressing GFP or WT NR2B (Fig. 5F, black bar). These findings indicate that the C terminus of NR2B is sufficient to allow proper synaptogenesis.
Fig. 5.
C termini of NR2 subunits control synaptogenesis and spine growth. (A) Quantification of dendritic spine density in control neurons (n = 8), neurons cotransfected with GFP, NR1, and NR2A (gray; n = 13), chimeric NR2B with the C terminus of NR2A (2B-CTA; black; n = 13), or NR2B RS/QD mutant (hatched; n = 15). (B) Cumulative fraction of IEIs of AMPAR-mediated mEPSCs from nontransfected neurons (n = 27) or neurons cotransfected with NR1 and NR2A (n = 7), NR1 and 2B-CTA (black; n = 8), or NR1 and NR2B RS/QD mutant (dashed; n = 8). NR2B-CTA reduces frequency compared with control cells (KS test D = 0.3794) and shows no difference with NR2A (KS test D = 0.0785). NR2B RS/QD decreases frequency compared with control (KS test D = 0.4286). (C) Quantification of dendritic spine density in control neurons (n = 8), neurons cotransfected with GFP, NR1, and NR2B (gray; n = 12), chimeric NR2A with the C terminus of NR2B (2A-CTB; black; n = 6), or NR2AΔIN mutant (hatched; n = 14). (D) Cumulative fraction of IEIs of AMPAR-mediated mEPSCs from nontransfected neurons (n = 27) or cotransfected with NR1 and NR2B (n = 8), NR1 and NR2A (n = 9), NR1 and 2A-CTB (black; n = 10), or NR1 and NR2AΔIN mutant (dashed; n = 8). NR2A-CTB recovers frequency and is not different from control cells (D = 0.0002) but different from WT NR2A (D = 0.36). NR2AΔIN partially recovers frequency (D = 0.1543 vs. NR2A). (E) Quantification of spine volume from control neurons (n = 5), or cotransfected with NR1 and NR2A (n = 7), NR1 and 2B-CTA (n = 7), or NR1 and NR2B RS/QD (n = 6). (F) Quantification of spine volume from control neurons (n = 5), or cotransfected with NR1 and NR2B (n = 4), NR1 and 2A-CTB (n = 6), or NR1 and NR2AΔIN (n = 7). Error bars represent SEM.
An important difference between the C termini of NR2A and NR2B is their ability to interact with CaMKII. NR2B interacts with CaMKII, and NR2A does not; therefore, NR2A decreases functional synaptic plasticity (8, 9). In light of this, we next tested whether the interaction of CaMKII with the NR2 subunits is necessary for normal synaptogenesis. Expression of a mutant NR2B that cannot bind CaMKII, NR2B RS/QD (8, 16), decreased spine density (Fig. 5A, hatched bar) and frequency of AMPAR-mediated mEPSCs (Fig. 5B, dotted line). Disruption of NR2B interaction with CaMKII also decreased spine volume (Fig. 5E, hatched bar). These results indicate that recruitment of CaMKII to synapses is essential for proper spine growth and synapse formation. To test whether the interaction of CaMKII with NMDARs was sufficient for normal synaptogenesis, we used a mutant of NR2A that is able to bind CaMKII, NR2AΔIN (8). Cells expressing NR2AΔIN exhibited normal spine density (Fig. 5C, hatched bars) compared with cells expressing GFP or WT NR2B. Expression of NR2AΔIN also results in a small increase in AMPAR-mediated mEPSC frequency, although this increase does not represent a full recovery to the frequency seen in age-matched control cells (Fig. 5D, dotted line). Increasing the ability of NR2A to bind CaMKII also increased spine volume to values not significantly different from age-matched control cells expressing GFP or WT NR2B (Fig. 5F, hatched bar). Thus, lack of interaction between CaMKII and NR2A C terminus seems to explain the diminished spinogenesis/synaptogenesis observed when NR2A is overexpressed, although other effects, such as the total charge transferred, may also play a role.
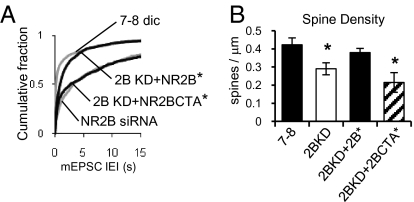
Finally, we performed a molecular replacement experiment to confirm that NR2B is necessary for formation of the proper number of spines and synapses and that this property resides in the C terminus. This experiment is also the best control to rule out possible off-target effects of the siRNA used. Endogenous NR2B was knocked down using siRNA and simultaneously recovered with WT NR2B carrying a silent mutation that renders it resistant to the siRNA (NR2B*). As shown in Fig. 6, the mEPSC frequency and spine density values are recovered with NR2B*. However, replacement of endogenous NR2B with a chimeric NR2B* with the C terminus of NR2A (2B-CTA*) was not able to recover the mEPSC frequency or spine density.
Fig. 6.
Molecular replacement of endogenous NR2B. (A) Cumulative fraction of all IEIs of AMPAR-mediated mEPSCs from control neurons (n = 23), transfected with 2B-siRNA (n = 8 cells), and neurons cotransfected with 2B-siRNA and WT-resistant NR2B* (n = 13) or chimeric-resistant NR2B-CTA* (n = 12). (B) Quantification of dendritic spine density in control neurons (n = 8), neurons transfected with 2B-siRNA (n = 10 cells), and neurons cotransfected with 2B-siRNA and WT-resistant NR2B* (n = 4) or chimeric-resistant NR2B-CTA* (n = 7). Error bars represent SEM.
Together, these results indicate that the intracellular C-termini portion of NR2 subunits control the rate of synaptogenesis and spine growth. Interaction of CaMKII with NR2B C terminus is necessary for synapse formation and maturation, presumably by allowing synaptic plasticity to proceed and stabilize the connection.
Discussion
Hippocampal CA1 pyramidal neurons in rat organotypic slices undergo a rapid process of synaptogenesis between 4–5 dic and 7–8 dic (equivalent to p10–11 and p13–14), a process that is stabilized by 11–12 dic (equivalent to p17–18). During the first week in culture, spines are highly mobile with a larger number of new spines appearing than the number of spines retracting, producing a net increase in the overall number of synapses. In general, spine motility decreases significantly after 11–12 dic, in agreement with previous reports that indicate that in vivo spine motility decreases with age (22).
During the period studied, expression of the NR2A subunit begins and is followed by gradual incorporation into synapses in hippocampus and other brain regions (5). NR2A incorporation into synapses replaces NR2B-containing receptors (25) and reduces CaMKII-mediated synaptic potentiation (8, 9, 15) because of the lack of interaction between CaMKII and NR2A and possible inhibitory effects of the C terminus of NR2A (8, 9). Our results show that the balance of NR2A and NR2B in the synapse strongly affects the number of synapses formed. Increasing the expression of NR2A decreases the number of synapses, suggesting that NR2A prevents the process of synaptogenesis. Conversely, expression of NR2B does not affect the number of synapses (this study), or synaptic responses mediated by NMDARs or AMPARs (8).
Previous reports indicate that overexpression of NR2A at an age when NR2B normally dominates NMDARs decreases the amplitude of NMDAR and AMPAR-mediated EPSCs (8). Our results indicate that this reduction of glutamatergic synaptic transmission following early NR2A expression is due to a reduction in the number of synapses. In addition, NR2A and NR2B-CTA cause spines to have smaller volumes. Spine volume has been positively correlated with the strength of AMPAR-mediated synaptic transmission (27); thus, the small volume of these spines suggests that the synapses present have not undergone synaptic potentiation, as this process increases both AMPAR content and spine volume (28). This result is consistent with the observation that NR2A reduces LTP (8, 9, 15).
However, expression of NR2B increases spine motility via increasing spine additions and retractions, as well as filopodia motility. It has been suggested that the role of filopodia is to establish synaptic connections (4, 23, 29). Thus, we conclude that NR2B induces spinogenesis/synaptogenesis by providing greater structural plasticity that allows the neuron to find and establish appropriate synaptic contacts.
Another possibility is that spine motility is regulated by spine density. Several lines of evidence suggest this is not the case. (i) Spine density at 7–8 dic and 11–12 dic is the same (Fig. 1); however, spine motility is significantly lower at 11–12 dic than at 7–8 dic. (ii) Expression of NR2B does not alter synapse density; however, it clearly increases spine motility. (iii) Manipulations that decrease synapse density (early expression of NR2A and knockdown of NR2B) do not increase spine motility as expected if synapse density determines spine motility and we take into account the basic observation that low synapse density correlates with high spine motility, as in Fig. 1.
In addition to the ionotropic function of NMDARs, it has been proposed that NMDARs play a structural role in synaptic and spine stabilization (10, 30). Our results show that the C terminus of NR2 subunits confers a different role to each subunit in the process of synapse formation and stabilization. The C terminus of NR2A is sufficient to prevent synapse formation, presumably by decreasing motility and blocking induction of plasticity. In contrast, the C terminus of NR2B is sufficient to induce normal synapse formation. NR2B knockout mice exhibit a reduction in globular actin and in the density of dendritic spines (30), consistent with a role for NR2B in the organization of postsynaptic macromolecular complexes and spine formation.
The important difference between the C termini of NR2 subunits seems to be the ability to interact with CaMKII. Disruption of NR2B interaction with CaMKII reduced the number of spines and the number of functional synapses. It is important to note that this effect was not as pronounced as the one exerted by WT NR2A, suggesting that the rapid kinetics of NR2A, and therefore less Ca+2 influx, may also contribute to the inability of NR2A to form new synapses. On the other hand, a mutant of NR2A with increased CAMKII binding capability restores spine density to control levels but does not increase the frequency of AMPAR-mediated mEPSCs. It is possible that those spines are “silent” (31, 32), suggesting that NR2A C-tail could prevent the addition of AMPA-type receptors and/or that the amount of Ca2+ influx is not enough to drive AMPARs to the synapse.
NR2B interaction with CaMKII seems to be important for structural plasticity leading to the formation of new spines and synaptic contacts. It has been described that CaMKII has the ability to bundle actin filaments (33), a process required to modify the structure of synaptic protrusions (34). When functional synaptic plasticity is induced, the presence of NR2B and CaMKII allows structural modification and the incorporation of AMPARs while simultaneously, NR2A-containing receptors are replacing NR2B-containing receptors. This replacement increases structural and functional stability, and presumably stabilizes synapses. Whether a fraction of NR2B/CaMKII must remain in the spine is not clear. Similarly, whether NR2A also contributes other structural elements that confer stability remains to be determined.
Materials and Methods
Cultured organotypic hippocampal slices were prepared from Sprague Dawley rats 6–7 d old and cultured as described (35). Slices were transfected with proteins of interest using biolistics 60–72 h prior to experiments, or 5–6 d for siRNA. AMPAR-mediated mEPSCs were recorded in the presence of 1 μM TTX and 100 μM PTX at −60 mV as described (9). For all experiments, an equivalent number of control nontransfected and transfected cells from the same slice were recorded. For imaging, slices were either fixed after 3 d of expression using 4% PFA and 4% sucrose or perfused with artificial cerebrospinal fluid (ACSF) for live imaging at 32–34 °C. All images were taken on apical dendrites of first or second branching order. Spines were defined as protrusions more than 0.5 μm and less than 3 μm long and with a round head. Filopodia were defined as protrusions over 3 μm in length, with no visible head. A Kolmogorov–Smirnov test was used to analyze cumulative distribution of IEI. All other analysis used mean ± SEM, and Student's t test. Significance was set at P ≤ 0.05 (see Table S1 for specific values). For constructs and details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Morgan Sheng for providing siRNA constructs, Ximena Opitz for technical assistance, and Dr. Rachel Wong for resources and expert advice. This study was funded by Public Health Service National Research Service Award T32 GM07270 from the National Institute of General Medical Sciences (to A.C.G.) and National Institutes of Health-National Institute of Neurological Disorders and Stroke Grant R01NS060756 (to A.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012676108/-/DCSupplemental.
References
- 1.Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: A review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 4.Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 9.Foster KA, et al. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010;30:2676–2685. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez VA, Ridenour DA, Sabatini BL. Distinct structural and ionotropic roles of NMDA receptors in controlling spine and synapse stability. J Neurosci. 2007;27:7365–7376. doi: 10.1523/JNEUROSCI.0956-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traynelis SF, et al. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii T, et al. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 13.Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Köhr G, et al. Intracellular domains of NMDA receptor subtypes are determinants for long-term potentiation induction. J Neurosci. 2003;23:10791–10799. doi: 10.1523/JNEUROSCI.23-34-10791.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, et al. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci. 2007;27:13843–13853. doi: 10.1523/JNEUROSCI.4486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 17.De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: Role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 19.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 20.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature. 1959;183:1592–1593. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- 22.Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci USA. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: Evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer G, et al. Ro 25-6981, a highly potent and selective blocker of N-methyl-d-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- 25.Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- 26.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 30.Akashi K, et al. NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J Neurosci. 2009;29:10869–10882. doi: 10.1523/JNEUROSCI.5531-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: Implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: A potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- 34.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: Connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opitz-Araya X, Barria A. Organotypic hippocampal slice cultures. J Vis Exp. 2011 doi: 10.3791/2462. 10.3791/2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.