Abstract
Food anticipatory behavior (FAA) is induced by limiting access to food for a few hours daily. Animals anticipate this scheduled meal event even without the suprachiasmatic nucleus (SCN), the biological clock. Consequently, a food-entrained oscillator has been proposed to be responsible for meal time estimation. Recent studies suggested the dorsomedial hypothalamus (DMH) as the site for this food-entrained oscillator, which has led to considerable controversy in the literature. Herein we demonstrate by means of c-Fos immunohistochemistry that the neuronal activity of the suprachiasmatic nucleus (SCN), which signals the rest phase in nocturnal animals, is reduced when animals anticipate the scheduled food and, simultaneously, neuronal activity within the DMH increases. Using retrograde tracing and confocal analysis, we show that inhibition of SCN neuronal activity is the consequence of activation of GABA-containing neurons in the DMH that project to the SCN. Next, we show that DMH lesions result in a loss or diminution of FAA, simultaneous with increased activity in the SCN. A subsequent lesion of the SCN restored FAA. We conclude that in intact animals, FAA may only occur when the DMH inhibits the activity of the SCN, thus permitting locomotor activity. As a result, FAA originates from a neuronal network comprising an interaction between the DMH and SCN. Moreover, this study shows that the DMH–SCN interaction may serve as an intrahypothalamic system to gate activity instead of rest overriding circadian predetermined temporal patterns.
Physiology and behavior of all mammals is organized in an alternating pattern of rest and activity cycles, whereby the endogenous and light-induced daily neuronal activity of the suprachiasmatic nucleus (SCN) signals rest in nocturnal rodents and activity in diurnal primates, such as humans (1, 2). Restricting food access to a short and predictable episode during the rest phase changes this behavioral pattern, such that an animal becomes active and for up to several hours anticipates the upcoming feeding event. This food anticipatory activity (FAA) is even exhibited without the known circadian oscillator, the SCN (3), and thus may rely on a different circadian pacemaker.
In search of the location of this so-called “food entrained oscillator” (FEO), two recent studies have claimed that its position is within the dorsomedial nucleus of the hypothalamus (DMH) (4, 5). The designation of the DMH as master clock for food entrainment is, however, controversial because some groups reported unimpaired FAA despite large lesions of the DMH (6, 7); others have shown that lesions of the DMH disturb and diminish the intensity of FAA (6). The possible participation of other brain structures in FAA is evident from studies that demonstrate modulation of neuronal activity and induction of clock-gene rhythmicity in hypothalamic and limbic structures by feeding schedules (8–11). Thus, it has been suggested that FAA depends on a multioscillatory system comprised of a complex and redundant neuronal network in which even the participation of clock genes does not seem essential (12, 13). In the present study, we therefore examined the hypothalamic circuitry that is involved in FAA by using a combination of behavior with neuronal tracing, neuronal activity markers, and specific lesioning techniques. Because daytime neuronal activity of the SCN and light-induced neuronal activity of the SCN are known to inhibit locomotor activity in nocturnal rodents (14, 15), we hypothesized that during food restriction, to allow FAA during the light phase, the activity and influence of the SCN should be reduced. Because the DMH is one of the structures with the most c-Fos activity during FAA (5, 8), we explored the contribution of the DMH–SCN interaction toward FAA in the rat as nocturnal rodent. We show that the DMH has GABAergic projections to the SCN that are active during FAA and reduce the activity of the SCN. We show that a lesion of the DMH decreases that inhibition and leads to a highly active SCN, leading to reduction or disappearance of FAA. The disappearance of FAA following a lesion of the DMH and its reappearance in the same animal following a lesion of the SCN supports the notion that it is indeed the SCN that inhibits locomotor activity, thereby preventing FAA during the rest phase in DMH-lesioned animals.
Consequently the present results show that FAA is modulated by the interaction of the SCN and DMH and suggest that FAA is generated by a neuronal network, of which the SCN and the DMH are two components.
Results and Discussion
DMH–SCN Interaction.
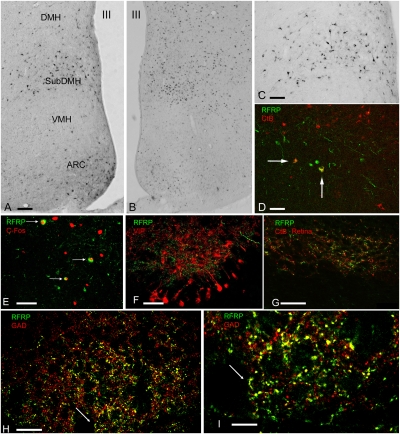
We used rats as our experimental animal and initially explored neuronal activation during FAA by means of c-Fos immunohistochemistry in the hypothalamus. We used c-Fos as a neuronal marker to investigate which brain areas are activated during FAA in a restricted food protocol. C-Fos is generally accepted as a marker for neuronal activity based on the early experiments of Morgan and Curran (16, 17); the limitation of this technique is that certain neuronal systems may not show c-Fos resulting from their activation. We observed, in agreement with earlier studies (18, 19), that together with increased c-Fos in the ventral DMH, neurons in the ventral part of the SCN decreased their activity (Fig. S1). Following our hypothesis that at least one brain structure that is involved in food anticipation needs to interact with the SCN to inhibit its activity, we placed the neuronal tracer cholera toxin subunit B (CtB) into the SCN (n = 24). These injections, especially when placed in the ventral SCN (n = 4) (Fig. S2A), revealed retrograde-labeled neurons in several hypothalamic areas, including the DMH (Fig. 1A). We observed that the CtB-positive neurons were present in the same area of the DMH that showed c-Fos activation during food restriction (Fig. 1 A and B). The next observation was that the distribution of the neurons within the DMH projecting to the SCN was similar to those neurons in the DMH that express Arg-Phe-Amide-related peptide (Fig. 1C), one of the five active mammalian restricted-food amide peptides (RFRP). This observation was further investigated using injection of the tracer CtB in the SCN, resulting in retrograde-labeled neurons in the DMH containing RFRP (Fig. 1D). Because RFRP peptides are postulated to have a feeding-related functionality (20–22), we examined and demonstrated by means of double-labeling immunofluorescence and confocal microscopical analysis that some neurons with c-Fos labeling during FAA are also positive for RFRP (Fig. 1E). In addition, neurons were observed that demonstrated both c-Fos and CtB, which indicates that restricted food activated neurons of the DMH projection to the SCN (Fig. S2B). Further confocal microscopy analysis showed that RFRP fibers terminate exclusively in the ventral SCN. RFRP fibers target vasoactive-intestinal peptide (VIP) and gastrin-releasing peptide neurons that are known to receive retinal input (Fig. 1F). Vasopressin neurons did not receive any input from RFRP fibers. This observation was confirmed by labeling projections from the retina to the SCN, via injecting CtB in the vitreous of the eye. This experiment showed that the distribution of RFRP fibers in the SCN was observed coincident with the fibers arriving from the retina (Fig. 1G). Both observations suggest that the projections of the DMH to the SCN may interact with light input that is arriving from the retina and may modulate the activity of the SCN in that area.
Fig. 1.
RFRP neurons in the DMH project to the retinorecipient region of the SCN, contain the enzyme for the inhibitory neurotransmitter GABA (GAD), and are activated during food anticipation. (A) Retrograde-labeled neurons in the ventral area of the DMH after an injection of CtB into the SCN. These neurons projecting to the SCN are present in the same ventral area of the DMH that is activated during food anticipation. The distribution of RFRP neurons in the ventral area of the DMH in A coincides with the area expressing c-Fos during FAA in B and coincides with the location of RFRP neurons in the DMH (C). (D) Retrograde-labeled neurons in the DMH after an injection of CtB (red) into the SCN, double-labeled for RFRP (green). (E) Confocal image of RFRP (green) -labeled neurons colocalizing with c-Fos (red) after food restriction. (F) RFRP (green) innervation of the SCN in the area of VIP (red) cellbodies; G shows that this RFRP (green) input is closely associated with retinal fibers (red). H and I illustrate that in the SCN these RFRP (green) fibers colocalize with GAD (red), as is illustrated by the yellow color of green RFRP and red GAD fibers. The arrow indicates the same area in H and I. (Scale bars: A and B, 100 μm; C–F, 50 μm; G and H, 25 μm; I, 10 μm.)
DMH Inhibits SCN Neuronal Activity During FAA.
In view of the projections of the RFRP neurons to the ventral SCN (Fig. 1D), and in view of the evidence that many neurons of the DMH contain the inhibitory amino acid GABA (23), we examined whether the RFRP projections from the DMH to the SCN may contain GABA. Therefore, the presence of the enzyme for the synthesis of GABA, glutamate decarboxylase (GAD), was examined in RFRP-positive fibers terminating in the SCN. Confocal microscopic analysis revealed that RFRP fibers terminating in the SCN contained GAD, indicating an inhibitory role of this DMH input to the SCN (Fig. 1 H and I).
To investigate the relevance of this DMH–SCN interaction for FAA in 30 naive animals, we first established a baseline of FAA by a restricted-food protocol. Because naive animals show a large variation in FAA, we only chose 24 animals that showed clear evidence of FAA. We divided these animals into three groups (n = 24): (i) a control group that received a (sham) lesion of the DMH; (ii) a group that received a bilateral electrolytic lesion of the DMH; and (iii) a group that received bilateral kainic acid lesions of DMH. After 20 d of the second restricted-food process, rats were killed at the moment of food arrival to analyze c-Fos immuno-reactivity in the brain. Because c-Fos protein takes between 60 and 90 min to be expressed after the activation of a neuron (24), this time point reflects the neuronal activation during FAA, at least 60 min before expected food arrival.
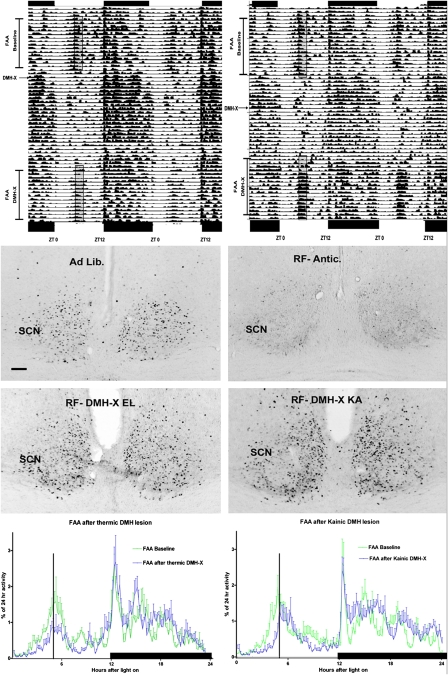
To compare the SCN neuronal activity in ad libitum-fed animals versus restricted food animals, we chose animals with the most accurate DMH lesion: four with kainic acid and four with thermic lesions versus four sham animals with the strongest anticipation. The size and site of the lesions was documented and the successful DMH lesions are presented in Fig. S3. Invariably, lesions that led to diminished or no anticipation covered the ventral and periventricular area of the DMH bilaterally, but lesions that did not affect FAA were unilateral, were outside the DMH area, or covered a larger area than the DMH itself (Figs. S3 and S4). The four animals from each lesion group with the most accurate DMH lesions are presented in Fig. 2 and Fig. S3. Well-lesioned animals did not show a significant FAA compared with their ad libitum activity, but sham-lesioned animals showed increased FAA before food arrival compared with their own ad libitum activity (P < 0.001) (Fig. S5). In addition, only the sham group showed a decreased c-Fos activity in the SCN compared with intact ad libitum animals (Fig. 2 and Fig. S5). In general, it was more difficult to obtain reduced FAA with electrolytic lesions than with kainic acid lesions; interestingly, the lesion of the DMH needed to be small to let the anticipation disappear, which in our hands was easier by a kainic acid lesion then by an electrolytic lesion. This finding could be because of the fact that an electrolytic lesion also destroys fibers of passage, for example of the SCN to nearby target structures, and that the kainic acid lesion does not kill all neurons in the injection site, as became clear of the c-Fos activation of some sparse neurons located in the lesion site (Fig. S6). The differential effects of both lesion strategies may explain why such controversy in the literature exists concerning the effect of the lesion of the DMH with respect to FAA (25, 26). In general, it can be stated that in studies demonstrating little or no diminishment on FAA after lesions of the DMH, the lesions encompassed the DMH completely, as well as parts of neighboring structures, thus often resulting in nearly arrhythmic animals (6, 7, 25). This loss of rhythmicity suggests that with such large lesions, the SCN is unable to impose its rhythmicity to areas such as the perifornical area, the ventro lateral hypothalamus, and the thalamus (27, 28). Large lesions may either destroy these areas or disrupt the fibers of the SCN passing to these areas, and consequently in this condition the SCN cannot prevent locomotor activity during the daytime. The latter may also explain why with kainic acid lesions in the DMH, it was easier to obtain a diminished FAA than with thermic lesions, and why in our hands small lesions were doing better than bigger ones. When we examined the c-Fos staining in the SCN during the restricted-food protocol, we observed that in the animals perfused at the time of food arrival, the c-Fos immunopositive profiles (which reflects the situation at least 1 h earlier) in both groups of DMH-X animals (n = 2 × 4) was as high as that in animals under ad libitum feeding conditions (n = 4). In contrast, only the SCN of sham food-anticipating animals (n = 4) showed a significant reduction of c-Fos (Fig.2 and Fig S5) compared with all other animal groups (P < 0.01). Furthermore, the same animals that endured a DMH lesion also showed a pronounced diminishment of RFRP innervation in the SCN (Fig. S7). These results support the hypothesis that lesioning the DMH results in removal of RFRP/GABA neurons and diminishes the inhibitory input to the SCN.
Fig. 2.
Diminished food anticipatory behavior after DMH lesion concurs with high neuronal activity in the SCN. (Top)Two double-plotted actograms of locomotor activity illustrate the initial baseline food anticipation (food presented in the time of the translucent rectangle) and the subsequent loss of this anticipatory activity after an electrolytic (Left) or neurotoxic (Right) lesion of the DMH. The activity 2 to 3 h preceding the translucent rectangle is taken as “food anticipatory activity.” (Middle) The neuronal activity by means of c-Fos staining in the SCN 5 h after light onset in an ad libitum control and in a sham restricted-food (RF) animal at the moment of food arrival. The DMH-lesioned animals electrolytic (RF-DMH-X EL) or kainic (RF-DMH-X KA) of the respective actograms above show as high c-Fos expression in the SCN of the ad libitum-fed animal. Only the sham-lesioned food-anticipating animal (RF-antic) showed less c-Fos in the SCN. The quantification of the number of c-Fos–positive neurons in the SCN and the quantification of the FAA for the 2 h before expected food arrival is given in Fig. S5. (Bottom) Waveform analysis of activity during the last 5 d of a restricted-food protocol of the two groups of DMH-X animals illustrating their 24-h activity in 10-min averages. The anticipatory activity to food (as percentage of total 24-h activity) after the lesion is diminished significantly. The vertical bar indicates the time (ZT5) of food delivery (5 h after light onset) and the horizontal black bar the dark period (see Fig. S5 for the quantitative analysis of FAA). (Scale bars, 100 μm.)
FAA Is Lost After Lesion of the DMH but Reappears After a Subsequent Lesion of the SCN.
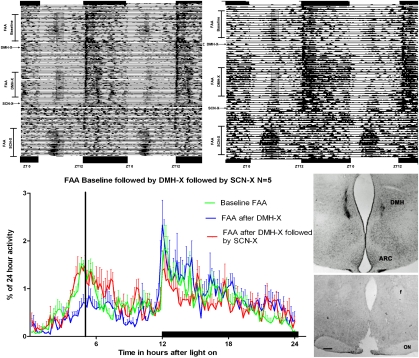
The fact that after a DMH lesion FAA disappears or is significantly diminished has led to the conclusion that the DMH may harbor the FEO (4). However, the present demonstration of an inhibitory input from the DMH to the SCN suggests that the DMH may inhibit neuronal activity of the SCN to permit FAA to occur. This emerging hypothesis was examined in a series of experiments, of which the first part is presented in the SI Materials and Methods and in Fig S8. In the following experiments the intensity of FAA was first established in intact rats under restricted-food conditions; this was followed by a bilateral lesion of the DMH (DMH-X) with kainic acid. Rats were allowed to recover for 3 wk and were then tested again under a restricted-food condition. DMH-X rats showed decreased or loss of FAA. Subsequently, the same animals received bilateral electrolytic lesions of the SCN (SCN-X). After recovery, the rats were again submitted to a period of restricted food. The subsequent SCN-X completely reversed the DMH-X pattern and resulted in animals (n = 5) that, despite their two hypothalamic lesions, strongly and significantly anticipated the scheduled meal time (Fig. 3 and analysis in Fig. S9). The results also showed that the SCN does not need to be completely lesioned; a partial lesion that results in a twofold increase of daytime activity was sufficient to reinstate the initial baseline FAA. This phenomenon cannot be explained by a possible recovery of function of the DMH because animals with double neurotoxic lesions of the DMH area show very little c-Fos, which is similar to single DMH-lesioned animals when killed at the moment of expected food arrival (Fig. S6). Moreover, surgery or retraining also does not explain the loss and reappearance of FAA because sham-lesioned animals or animals with lesions outside the SCN did not show this loss and subsequent gain of function. (See SI Materials and Methods and Figs. S10–S12 for more details.)
Fig. 3.
Loss and gain of food anticipation in the same animals bearing, first, a lesion of the DMH, followed by a lesion of the SCN. Both double-plotted actograms depict the activity of animals showing normal anticipatory activity just before the onset of meal delivery (translucent rectangle). This anticipatory activity is nearly completely lost after a kainic acid DMH lesion and returns when the same animal receives an additional SCN lesion. The waveform analysis illustrates the average activity of the same five animals in intact conditions under restricted-food conditions (green line) (the moment of food delivery depicted by the black vertical bar), and illustrates the loss of FAA after DMH-X (blue line) and its return after a successive SCN-X (red line). The right part of the figure shows the injection site of the kainic acid and the section of the hypothalamus showing the lesion of the SCN. The right actogram shows the result of a partial (>70%) lesioned SCN animal that still recovers FAA. See Fig. S9 for the quantification of the activity 3 h before food arrival. (Scale bar, 200 μm.)
Hunt for the FEO.
Since 1972 it has been recognized that the organization of circadian activity in locomotor, hormonal, or autonomic rhythms is the exclusive domain of the SCN because, after lesions confined to the SCN, all these overt rhythms disappeared (29, 30). When it was discovered that by giving food to an animal once a day during its rest phase, the animal could anticipate this event without the SCN (3, 31), a similar clock, the FEO, was proposed (32). Ever since then, scientists have tried to ascribe the location and function of such a FEO to a single structure in the brain by lesioning different areas and examining the effect on FAA (33). Recently, following the observation of a high activation of c-Fos in the DMH (8), it was suggested that the FEO resided in the DMH (4). Present results demonstrate that both the SCN and DMH are relevant but not indispensable for FAA, and that the DMH and SCN are just two of possibly many structures that modulate FAA. We propose that the FEO consists of a network of interacting brain structures that is initially driven by information from the periphery (Fig. S13).
DMH: Hypothalamic Center of Circadian and Metabolic Integration.
Early tracing studies examining the output of the DMH have shown that its projections are mainly limited to hypothalamic structures (34). This observation, together with data showing that the DMH receives an important input from the nucleus of the solitary tract, arcuate, and SCN (35–37), supports the view that the DMH is essential for the distribution of metabolic and circadian signals within the hypothalamus. The present results emphasize the important role of the DMH because they show that, under normal conditions, the DMH has the capacity to “silence” the SCN to allow locomotor activity at a moment of the day when such behavior is normally prevented by the activity of the SCN. This finding explains why DMH lesions could result in an absence of FAA.
SCN and FEO Multioscillatory Structures.
There is no doubt that FAA stems from activity within the brain, although the initiation of this activity may arise from peripheral oscillating or stimulating processes (38–40). All in all, the appearance of c-Fos or PERIOD immunoreactivity in many brain areas after food restriction and the failure to prevent FAA by lesioning different brain areas (13) suggests that the organization of the oscillatory nature of FAA should be within a system of interacting brain structures. Perhaps the most surprising outcome of the present study is that although the DMH was seen as an excellent candidate for distributing the signal of food entrainment, the present results show that even after DMH-SCN lesions, FAA is not diminished, and in some cases, even enhanced. The present experimental data clearly demonstrated that other structures are involved and are sufficient to induce FAA. In intact animals, the interaction between the SCN and the DMH may be essential for the expression of FAA, as is apparent following lesions of the DMH. These lesions, the resulting reduction of FAA, and the concomitant increased c-Fos expression in the SCN indicate that indeed the DMH inhibits the SCN to allow locomotor activity. Consequently, the earlier conclusion that the SCN does not play a role in FAA needs to be reconsidered (see also ref. 41). The present study demonstrates that the SCN will normally prevent FAA. In fact, the SCN participates by shaping a window for FAA, as is evident from animals with SCN lesions that show longer and more intense FAA than intact animals (41). The observation that FAA reappears after both DMH and SCN are lesioned cannot be explained as a recovery of function in the DMH, because in the kainic acid lesioned-DMH, induced decrease in c-Fos was visible in rats showing FAA after SCN lesion.
The arcuate nucleus is essential for metabolic integration and projects to many regions inside and outside the hypothalamus; it shows entrainment during food restriction and still shows c-Fos after DMH and SCN lesion. Thus, the arcuate nucleus seems a possible candidate within the network of structures for driving FAA (40). Other possible candidates are the nucleus accumbens, the paraventricular nucleus of the thalamus, and the cerebellum (9, 42). Clearly, more research needs to be done to define the nature of this food-entrained network. Recently it has been shown that the oscillatory property of the SCN is possibly a network property rather than the property of single neurons (43). We may therefore need to view in a similar manner the FEO as a neuronal network: its oscillatory capacity is neither derived nor dependent (as we have shown here) on one structure, but rather the interaction between the participating structures shapes, different aspects of FAA. We propose that the oscillations in this network are initiated, driven, and maintained by restricted-food schedules; without the continuous reinforcement of food restriction, the oscillation cannot be sustained for more than several days, probably because other inputs into the FEO system, for example the SCN and light-dark cycle, disturb and prevent the materialization of the oscillation. This finding could serve to explain why FAA is altered following lesions in some parts of the brain but maintained with lesions elsewhere (5). These observations may also provide an explanation as to why in early studies lesioning the ventro medial hypothalamus resulted in diminished FAA, which recovered in time (44, 45). We have not studied the effect of DMH lesion for periods longer than 6 wk, but it is possible that over time, the strength of other inhibitory inputs to the SCN [e.g., from the arcuate nucleus (46)] may increase and thus result in recovery of FAA consequent to an increase in inhibitory input to the SCN. Because we have observed a similar mechanism for arcuate–SCN interaction (47), we would like to propose that this is a general feature for hypothalamic structures, allowing them to impose adaptive changes depending on the requirements of the body.
In conclusion, the present study shows that the FEO may depend on an oscillating neuronal network that consists of different cell groups in different locations in the brain with or without the active participation of clock genes (12, 42, 48) (Fig. S13). The present study does not identify all of the structures that may participate in food entrainment, but it is likely that in addition to a number of core structures, such as the SCN, DMH, the arcuate nucleus, nucleus of the solitary tract, and parabrachial nuleus (33), a large number of other brain structures may participate and provide input to this system. Moreover, this study shows that the DMH–SCN interaction may serve as an intrahypothalamic system modulating behavioral activation at hours when the SCN signals sleep and rest.
Materials and Methods
See SI Materials and Methods for details on animal handling and experimental procedures.
Experiment 1.
Analysis of c-Fos and RPRF in the DMH after FAA; relation with the SCN.
One week before the food restriction, animals (24) received a stereotaxic injection of CtB aimed to the SCN. At day 14 of the food-restriction protocol, animals were killed at the moment the food would normally have been presented and the brains were examined for the localization of c-Fos, CtB, and RPRF. Four animals had a successful injection into the ventral SCN.
Experiment 2.
Analysis of the RPRF innervation of the SCN.
Animals (n = 5) received an injection of CtB in the vitreous of the eye under brief ether anesthesia. One week after this injection, animals were perfused with paraformaldehyde and the SCN examined for RPRF and CtB to examine the relationship between RPRF innervation and the input of the retina. Other intact animals (n = 5) were perfused at ZT4 and analyzed simultaneously for RPRF, VIP, gastrin-releasing peptide, and vasopressin using different fluorescent markers to evaluate which cell bodies in the SCN received RPRF input. In addition, antibodies to RPRF and GAD (Santa Cruz) were used to visualize terminations of both neurotransmitters. Under high magnification by confocal microscopy, sections of the SCN of these animals were examined to identify possible colocalization of RPRF with GAD.
Experiment 3.
Food restriction in DMH-lesioned animals followed by analysis of neuronal activity in the SCN.
After the first food-restriction protocol, 24 animals were allowed food ad libitum for 3 d, after which 16 animals received a stereotaxic lesion aimed to the DMH either with kainic acid or with electrolytic lesioning. Eight animals received a sham injection into the DMH. Thereafter, the animals were allowed to recover for at least 2 wk before the second restricted-feeding protocol was initiated. On the last day of this protocol, animals were intracardially perfused under deep Nembutal anesthesia at the moment they normally received food. Brains were used for histological analysis and immunohistochemical staining for c-Fos in the SCN and DMH.
c-Fos count.
From each group, four animals were selected with high anticipation (Sham) or with the most accurate DMH lesion (electrolytic and kainic DMH-lesioned groups). Site and size of the lesions was established. See SI Materials and Methods and Figs. S3 and S4 for more details. Expression of c-Fos protein was explored in the region of the DMH and in the SCN. To quantify c-Fos–positive cells in the SCN, three representative sections (rostral, middle, and caudal) were selected. Images of selected sections were obtained at a 200× magnification using a computerized image system (Image-Pro plus 5.1; Media Cibernetic) attached to a Zeiss light microscope. Cells positive for c-Fos were counted bilaterally in the ventral and total SCN in the three selected sections with the image-processing program ImageJ (National Institutes of Health). To minimize the number of false-positives, background optic density was established for each section in a nearby region lacking c-Fos; stained nuclei that reached or surpassed 2× the background optic density were considered positive and were included, whereas cells under this staining threshold were discarded. A single examiner, who was blinded to treatment conditions, performed all counts. The mean of the number of c-Fos nuclei in the three sections was taken as the number of c-Fos in the SCN of that animal.
Experiment 4.
Food restriction in DMH- and SCN-lesioned animals.
For this initial experiment, a group of 24 animals received a kainic acid lesion or an electric lesion of the DMH, followed after recovery by a restricted-food protocol. Thereafter, the SCN was electrically lesioned, followed again by a restricted-food protocol. The results are presented in Fig. S8. However, because we realized that already intact animals may anticipate with a different intensity, we decided to first determine a baseline FAA for the following experiments. Thus, in 24 intact animals a baseline FAA was first established; thereafter, in 20 animals that showed clear FAA, the DMH was lesioned using kainic acid. Only those animals that showed a clear diminished FAA after the DMH lesion (12) were considered, as well DMH-lesioned, and were selected to continue for a bilateral lesion of the SCN. After interrupting the second food restriction, animals bearing a DMH lesion received a stereotaxic bilateral electrolytic lesion (1 min, 0.2 μ Amp) aimed at the SCN. Thereafter, rats were allowed to recover for at least 3 wk, during which the success of their SCN lesion was examined by inspection of the actograms. When animals had more than 30% of their 24-h activity in the light period they were considered SCN-lesioned. On the last day of this protocol, at the moment of food expectancy, animals were intracardially perfused under deep Nembutal anesthesia. Thereafter, the brains were used for histological analysis. The results of these animals are presented in Fig. 3 and Fig. S9.
Supplementary Material
Acknowledgments
This work is supported by the Consejo Nacional de Ciencia y Tecnologia, Grants 79797 and 82462, and Grants DGAPA-PAPIIT- UNAM IN-215038 and IN-203907.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.J.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015551108/-/DCSupplemental.
References
- 1.Schwartz WJ, Reppert SM, Eagan SM, Moore-Ede MC. In vivo metabolic activity of the suprachiasmatic nuclei: A comparative study. Brain Res. 1983;274:184–187. doi: 10.1016/0006-8993(83)90538-3. [DOI] [PubMed] [Google Scholar]
- 2.Houben T, Deboer T, van Oosterhout F, Meijer JH. Correlation with behavioral activity and rest implies circadian regulation by SCN neuronal activity levels. J Biol Rhythms. 2009;24:477–487. doi: 10.1177/0748730409349895. [DOI] [PubMed] [Google Scholar]
- 3.Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol. 1979;25:545–554. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- 4.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 6.Moriya T, et al. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci. 2009;29:1447–1460. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- 7.Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms. 2007;22:467–478. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- 8.Angeles-Castellanos M, Aguilar-Roblero R, Escobar C. c-Fos expression in hypothalamic nuclei of food-entrained rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R158–R165. doi: 10.1152/ajpregu.00216.2003. [DOI] [PubMed] [Google Scholar]
- 9.Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144:344–355. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 10.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci USA. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verwey M, Khoja Z, Stewart J, Amir S. Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience. 2007;147:277–285. doi: 10.1016/j.neuroscience.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci USA. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson AJ. Lesion studies targeting food-anticipatory activity. Eur J Neurosci. 2009;30:1658–1664. doi: 10.1111/j.1460-9568.2009.06961.x. [DOI] [PubMed] [Google Scholar]
- 14.Scheer FA, Pirovano C, Van Someren EJ, Buijs RM. Environmental light and suprachiasmatic nucleus interact in the regulation of body temperature. Neuroscience. 2005;132:465–477. doi: 10.1016/j.neuroscience.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Redlin U, Mrosovsky N. Masking of locomotor activity in hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;184:429–437. doi: 10.1007/s003590050342. [DOI] [PubMed] [Google Scholar]
- 16.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 17.Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- 18.Challet E, Jacob N, Vuillez P, Pévet P, Malan A. Fos-like immunoreactivity in the circadian timing system of calorie-restricted rats fed at dawn: Daily rhythms and light pulse-induced changes. Brain Res. 1997;770:228–236. doi: 10.1016/s0006-8993(97)00796-8. [DOI] [PubMed] [Google Scholar]
- 19.Escobar C, Martínez-Merlos MT, Angeles-Castellanos M, del Carmen Miñana M, Buijs RM. Unpredictable feeding schedules unmask a system for daily resetting of behavioural and metabolic food entrainment. Eur J Neurosci. 2007;26:2804–2814. doi: 10.1111/j.1460-9568.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- 20.Nicklous DM, Simansky KJ. Neuropeptide FF exerts pro- and anti-opioid actions in the parabrachial nucleus to modulate food intake. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1046–R1054. doi: 10.1152/ajpregu.00107.2003. [DOI] [PubMed] [Google Scholar]
- 21.Murase T, Arima H, Kondo K, Oiso Y. Neuropeptide FF reduces food intake in rats. Peptides. 1996;17:353–354. doi: 10.1016/0196-9781(95)02137-x. [DOI] [PubMed] [Google Scholar]
- 22.Sunter D, Hewson AK, Lynam S, Dickson SL. Intracerebroventricular injection of neuropeptide FF, an opioid modulating neuropeptide, acutely reduces food intake and stimulates water intake in the rat. Neurosci Lett. 2001;313:145–148. doi: 10.1016/s0304-3940(01)02267-4. [DOI] [PubMed] [Google Scholar]
- 23.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332:123–143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- 24.Verbalis JG, Stricker EM, Robinson AG, Hoffman GE. Cholecystokinin activates C-fos expression in hypothalamic oxytocin and corticotropin-releasing hormone neurons. J Neuroendocrinol. 1991;3:205–213. doi: 10.1111/j.1365-2826.1991.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 25.Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistance of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- 26.Mistlberger RE, et al. Standards of evidence in chronobiology: Critical review of a report that restoration of Bmal1 expression in the dorsomedial hypothalamus is sufficient to restore circadian food anticipatory rhythms in Bmal1−/− mice. J Circadian Rhythms. 2009;7 doi: 10.1186/1740-3391-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi CX, et al. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes. 2009;58:1998–2005. doi: 10.2337/db09-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buijs RM, Hou YX, Shinn S, Renaud LP. Ultrastructural evidence for intra- and extranuclear projections of GABAergic neurons of the suprachiasmatic nucleus. J Comp Neurol. 1994;340:381–391. doi: 10.1002/cne.903400308. [DOI] [PubMed] [Google Scholar]
- 29.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 30.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197:398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- 32.Boulos Z, Rosenwasser AM, Terman M. Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res. 1980;1:39–65. doi: 10.1016/0166-4328(80)90045-5. [DOI] [PubMed] [Google Scholar]
- 33.Davidson AJ, Cappendijk SL, Stephan FK. Feeding-entrained circadian rhythms are attenuated by lesions of the parabrachial region in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1296–R1304. doi: 10.1152/ajpregu.2000.278.5.R1296. [DOI] [PubMed] [Google Scholar]
- 34.ter Horst GJ, Luiten PGM. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- 35.Ter Horst GJ, de Boer P, Luiten PGM, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31:785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 36.Buijs RM, Markman M, Nunes-Cardoso B, Hou YX, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: A light and electron microscopic study. J Comp Neurol. 1993;335:42–54. doi: 10.1002/cne.903350104. [DOI] [PubMed] [Google Scholar]
- 37.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blum ID, et al. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164:351–359. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Escobar C, Cailotto C, Angeles-Castellanos M, Delgado RS, Buijs RM. Peripheral oscillators: The driving force for food-anticipatory activity. Eur J Neurosci. 2009;30:1665–1675. doi: 10.1111/j.1460-9568.2009.06972.x. [DOI] [PubMed] [Google Scholar]
- 40.LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA. 2009;106:13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angeles-Castellanos M, Salgado-Delgado R, Rodriguez K, Buijs RM, Escobar C. The suprachiasmatic nucleus participates in food entrainment: A lesion study. Neuroscience. 2010;165:1115–1126. doi: 10.1016/j.neuroscience.2009.11.061. [DOI] [PubMed] [Google Scholar]
- 42.Mendoza J, Pévet P, Felder-Schmittbuhl MP, Bailly Y, Challet E. The cerebellum harbors a circadian oscillator involved in food anticipation. J Neurosci. 2010;30:1894–1904. doi: 10.1523/JNEUROSCI.5855-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci USA. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krieger DT. Ventromedial hypothalamic lesions abolish food-shifted circadian adrenal and temperature rhythmicity. Endocrinology. 1980;106:649–654. doi: 10.1210/endo-106-3-649. [DOI] [PubMed] [Google Scholar]
- 45.Honma S, Honma K-I, Nagasaka T, Hiroshige T. The ventromedial hypothalamic nucleus is not essential for the prefeeding corticosterone peak in rats under restricted daily feeding. Physiol Behav. 1987;39:211–215. doi: 10.1016/0031-9384(87)90011-4. [DOI] [PubMed] [Google Scholar]
- 46.Yi CX, et al. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology. 2006;147:283–294. doi: 10.1210/en.2005-1051. [DOI] [PubMed] [Google Scholar]
- 47.Yi CX, et al. A circulating ghrelin mimetic attenuates light-induced phase delay of mice and light-induced Fos expression in the suprachiasmatic nucleus of rats. Eur J Neurosci. 2008;27:1965–1972. doi: 10.1111/j.1460-9568.2008.06181.x. [DOI] [PubMed] [Google Scholar]
- 48.Pendergast JS, et al. Robust food anticipatory activity in BMAL1-deficient mice. PLoS ONE. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.