Author Summary
Eukaryotic cells possess various internal compartments that organize cellular metabolism, energy production, and the distribution of protein molecules within the cell and to the cell surface. For the most part, these compartments, or organelles, are linked by an assembly line called the secretion pathway that coordinates the manufacture of proteins, their subsequent shipment to each organelle, and their eventual export from the cell. However, not all proteins travel on the same assembly line from organelle to organelle. A particularly murky area is the transport of proteins to the peroxisome, an organelle responsible for the metabolism of certain alcohols and hydrocarbons. Here, we describe a method for detecting the formation of small membrane-enclosed packets, known as vesicles, within cells. We use this technique to reveal how two proteins found in the peroxisomal membrane, Pex3p and Pep15, exit a large organelle known as the endoplasmic reticulum (ER) in these vesicles and make their way—independently of the secretion pathway—to the peroxisome.
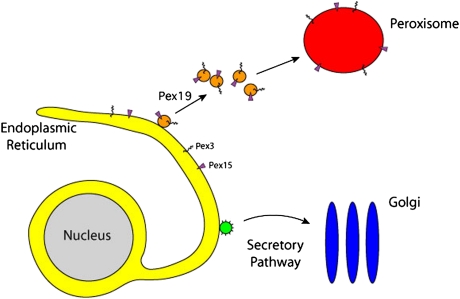
The secretion pathway has inspired decades of research and debate in the cell biology community and, consequently, many details of the process are now well known: deep within the cell, secretory proteins are assembled—one amino acid at a time—by ribosomes, which are RNA–protein machines that are organized on the surface of a large internal organelle called the ER. Proteins are transported from the ER in small vesicles that bud from the ER surface and are carried to the next station in the secretion pathway: the Golgi complex. From the Golgi, new vesicles are created to deliver secretory cargo to the cell surface (Fig. 1).
Fig. 1.
The manufacture and shipment of proteins within a cell. Together, the ER (yellow) and the membrane surrounding the nucleus (yellow) constitute an assembly line for the manufacture of protein molecules that are distributed to different destinations in animal, plant, and fungal cells. Protein molecules destined for export are captured into small vesicles (green), delivered to another station called the Golgi apparatus (blue), and then delivered to the cell surface. A new type of vesicle (orange), described in our study, carries membrane proteins (Pex15 and Pex3) to the peroxisome (red). The Pex19 protein is required to transport proteins from the ER to the peroxisome but is not necessary for secretion.
However, researchers know that the secretion pathway is not the only means of protein manufacture and localization. In 2005, a pivotal study published in Cell (1) presented evidence that at least one peroxisomal membrane protein, Pex3p, originates in the ER and then moves by some unknown process to the peroxisome, guided in part by a cytoplasmic protein called Pex19p, which has no role in protein secretion. In our current study, we build on this groundbreaking work by revealing how Pex3p and another peroxisomal membrane protein, Pep15, are transported from the ER to the peroxisome using a mechanism that acts independently of the secretion pathway.
We began by fusing the Pex15 gene to a part of a gene-encoding opsin, a retinal protein responsible for detecting photons. Opsin is a membrane protein made in the ER, where it is marked by the attachment of sugar molecules—a tell-tale sign of proteins assembled by the secretion pathway. Interestingly, these sugar molecules are not found on peroxisomal proteins. We find that by stitching the Pex15p protein to the portion of opsin that normally acquires sugar, the resulting hybrid protein retains these sugar molecules even after it travels to and functions in the peroxisome. This result affirms the role of the ER in the manufacture of Pex15, a peroxisomal membrane protein.
However, how do peroxisomal membrane proteins move out of the ER? To address this question, we devised a method to detect the formation of budded vesicles in a preparation of broken yeast cells. Previously, we described a gentle method for breaking cells while preserving the ER membrane in large, nearly intact envelopes. When these large membranes are mixed with a source of energy (ATP) and water-soluble cytoplasmic proteins, the resulting small, budded secretory vesicles can be physically separated from large membranes by sedimentation in a laboratory centrifuge (2). The molecular requirements for the formation of the small vesicles can then be established by isolating the specific soluble cytoplasmic proteins responsible for vesicle pinching (3).
Here, we used this approach to detect the action of soluble cytoplasmic proteins as they pinch peroxisomal membrane proteins into vesicles, which bud from the ER. Our findings revealed that two different peroxisomal proteins, Pex3p and Pex15p, are collected in a vesicle that is distinct from the membrane and that carries secretory proteins from the ER. Although soluble cytoplasmic proteins are required to pinch both vesicle types, we found that the two budding events do not share common protein and energy requirements. For example, Pex19p is not needed to form secretory vesicles; however, this protein is not sufficient to bud preperoxisomal vesicles. Thus, additional proteins provided by the soluble cytoplasmic fraction remain to be identified.
The results of our investigation reveal that at least two peroxisomal membrane proteins leave the ER in small, membrane-bound vesicles en route to the peroxisome. The budding process can be studied using isolated water-soluble proteins and membranes; thus, it should be possible to identify the unknown cytoplasmic proteins that collaborate with Pex19p to produce preperoxisomal vesicles. The biochemical approach we describe is an effective strategy for exploring the mechanisms of complex biological processes.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See full research article on page 21523 in issue 50 of volume 107.
Cite this Author Summary as: PNAS 10.1073/pnas.1103526108.
References
- 1.Hoepfner D, Schildknegt E, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122:85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Rexach M, Schekman R. Distinct biochemical requirements for the budding, targeting, and fusion of ER-derived transport vesicles. J Cell Biol. 1991;114:219–229. doi: 10.1083/jcb.114.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlowe C, et al. COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]