Abstract
Archived samples from a previously unreported 1958 Stanley Miller electric discharge experiment containing hydrogen sulfide (H2S) were recently discovered and analyzed using high-performance liquid chromatography and time-of-flight mass spectrometry. We report here the detection and quantification of primary amine-containing compounds in the original sample residues, which were produced via spark discharge using a gaseous mixture of H2S, CH4, NH3, and CO2. A total of 23 amino acids and 4 amines, including 7 organosulfur compounds, were detected in these samples. The major amino acids with chiral centers are racemic within the accuracy of the measurements, indicating that they are not contaminants introduced during sample storage. This experiment marks the first synthesis of sulfur amino acids from spark discharge experiments designed to imitate primordial environments. The relative yield of some amino acids, in particular the isomers of aminobutyric acid, are the highest ever found in a spark discharge experiment. The simulated primordial conditions used by Miller may serve as a model for early volcanic plume chemistry and provide insight to the possible roles such plumes may have played in abiotic organic synthesis. Additionally, the overall abundances of the synthesized amino acids in the presence of H2S are very similar to the abundances found in some carbonaceous meteorites, suggesting that H2S may have played an important role in prebiotic reactions in early solar system environments.
Keywords: prebiotic chemistry, volcano plume chemistry, carbonaceous chondrites
Although the laboratory-based synthesis of biological compounds using a variety of energy sources and simple reagents had been studied by several researchers in the late 19th and early 20th centuries (see discussion in ref. 1), these experiments were carried out in order to understand the assimilation of carbon dioxide and nitrogen in plants. It is now widely recognized that the first efficient abiotic synthesis of organic compounds under simulated primitive Earth conditions in the context of the origin of life were the classic experiments done by Stanley Miller in the 1950s (2, 3). Miller used a reducing gas mixture composed of H2, H2O, CH4, and NH3, which at the time was believed to be representative of the primitive terrestrial atmosphere (4). Many geoscientists today favor an early atmosphere that was likely weakly reducing, containing N2, CO2 (5), H2O, CO and lesser amounts of more reduced species such as H2S, CH4, and H2 (6). However, reducing conditions may have been prevalent on the Earth locally or transiently; for example, in the vicinity of volcanic plumes (4, 7, 8, 9) and on other solar system bodies (e.g., protosolar nebula, ancient Mars, Titan, etc.). Even with a weakly reducing or neutral atmosphere, recent research indicates that significant yields of amino acids can still be synthesized (6, 10).
Following the discovery of an archived set of samples from Miller’s early experiments, analyses were undertaken to better understand the diversity of compounds produced from electric discharges acting on reducing gas mixtures (7). In a previous study, preserved dried samples produced by Miller using a lesser-known volcanic apparatus were found to contain a wide variety of amino acids and amines, including ornithine, homoserine, methylamine, and ethylamine, many of which had not been reported previously in spark discharge experiments (7). The volcanic apparatus differed from Miller’s classic apparatus in that it utilized an aspirator that injected steam into the electric discharge chamber, simulating a volcanic eruption (1). These results, combined with the findings that aqueous aerosols in the presence of spark discharges effectively produce abiotic organic compounds (11, 12), verified how readily prebiotic organics could have been synthesized on the primeval Earth in localized volcanic environments rich in lightning and steam (13, 14).
Additional preserved samples from an experiment conducted in 1958 were also found in Miller’s archived collection (15). These samples had been generated using a mixture of CH4, NH3, H2S and CO2. The original dried residues from the experiment had been collected, cataloged, and stored by Miller, but for unknown reasons their analysis was apparently never carried out and reported. The paper chromatography methods that Miller used in the 1950s were only capable of detecting a few amino acids and were unable to provide substantial quantitative data relative to today’s techniques. Current analytical techniques are much more sensitive and selective, and are capable of precisely quantifying a much larger range of amino acids and their enantiomeric abundances.
Although an experiment using H2S as a component of the reduced gas mixture and a spark discharge apparatus configured according to Miller’s original design can be readily carried out, the unique opportunity to investigate samples prepared by the pioneer in abiotic synthesis using state-of-the-art analytical methods is of considerable historic interest. In the results presented here, we have examined the original 1958 samples using modern analytical techniques and report on the quantitative distribution of two- to six-carbon amino acids and one- to two-carbon amines.
Results
A diverse array of primary amine compounds were identified using high-performance liquid chromatography–UV fluorescence detection (HPLC-UVFD) (Fig. 1). The compounds detected using HPLC-UVFD were independently verified using ultraperformance liquid chromatography–UV fluorescence detection time-of-flight mass spectrometry (UPLC-UVFD/ToF-MS). Coelution of some compounds with identical retention times yields a small, but nonnegligible loss in quantitative accuracy when calculating compound abundances using only UV fluorescence chromatograms. However, detection by ToF-MS can overcome coelution interferences, provided there are not unique compounds with both identical masses and chromatographic retention times. Consequently, the mass spectrometry data were used to provide a more accurate estimate of the concentrations of the target compounds identified.
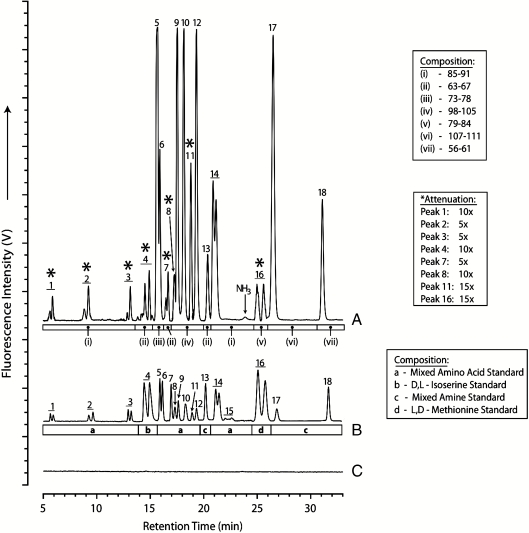
Fig. 1.
Five- to 33-min HPLC-UVFD chromatograms of 1-min OPA/NAC derivatized aliquots of: (A) original H2S spark discharge experimental samples from Stanley Miller’s archived collection, (B) a composite amino acid and amine standard trace, and (C) a reagent blank. Amino acids were identified based on retention times compared to standard runs. Peak identifications: 1 = D,L-Asp; 2 = L,D-Glu; 3 = D,L-Ser; 4 = D,L-Isoser; 5 = Gly; 6 = β-Ala; 7 = γ-ABA; 8 = β-AIB; 9 = D-Ala; 10 = L-Ala + D-β-ABA; 11 = L-β-ABA; 12 = α-AIB; 13 = ethanolamine; 14 = D,L-α-ABA; 15 = D,L-Isoval; 16 = L,D-Met; 17 = methylamine; 18 = ethylamine. The chromatogram displayed in A is a composite of several sample chromatograms of varying dilutions and is intended to demonstrate the diversity of products that were detected in these spark discharge residues. Thus, peak height/areas are not indicative of relative abundances. The composite trace (A) was created from seven separate runs labeled on the figure with their attenuations given (relative to glycine) and correspond to Miller’s labeled residues as follows: (i) 85–91, (ii) 63–67, (iii) 73–78, (iv) 98–105, (v) 79–84, (vi) 107–111, and (vii) 56–61. Those peaks in trace A that have been attenuated are marked with an asterisk above the number of the peak in question. The standard composite trace B was created from four standard runs as shown below the trace. A procedural blank for comparison was not found in the sample set saved by Miller, so trace C is a laboratory analytical solvent blank.
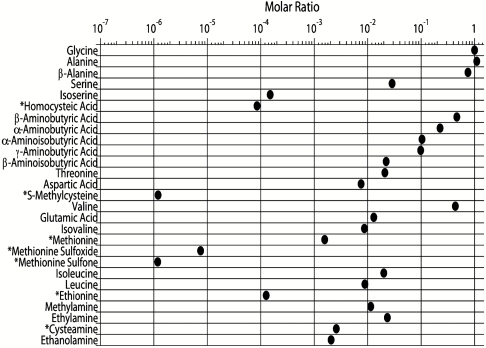
A total of 23 amino acids and 4 amines were identified in the H2S-containing experiment, including 6 sulfur-containing amino acids and 1 sulfur-containing amine (Fig. 2). The experiment produced a large variety of amino acids, including one- and two-carbon amines and two- to six-carbon amino acids. Protein amino acids, such as alanine and methionine, as well as nonprotein amino acids, including isoserine, isovaline, and β-aminobutyric acid were found to be racemic (D/L = 1 ± 10%) within experimental error, indicating they were produced during the experiment and are not terrestrial contaminants introduced during storage and subsequent processing. However, aspartic and glutamic acids as well as serine, which are present in relatively low abundances, are enriched in the L-enantiomer, indicating that trace amounts of contamination of some amino acids may be present. The detection of compounds containing secondary amino groups, such as sarcosine and proline, was not possible as o-phthaldialdehyde/N-acetyl-L-cysteine (OPA/NAC), the fluorescent agent used to label amino group–containing compounds in this study, only derivatizes primary amino groups.
Fig. 2.
Molar ratios (relative to glycine = 1) of amino acids and amines detected using UPLC-UVFD/ToF-MS. Sulfur-containing organic compounds are marked with an asterisk.
The samples produced in Miller’s 1958 experiment mark the earliest synthesis of sulfur amino acids from spark discharge experiments simulating primordial Earth conditions. It is important to note that cysteine was not detected in the samples analyzed here. However, many samples did contain cysteamine and homocysteic acid, which are degradation products of cysteine and homocysteine, respectively (15). The presence of these sulfur compounds suggests that cysteine was initially present in the samples, but over time the cysteine was degraded by oxidation, among other mechanisms, because these samples were not stored under anoxic conditions. Methionine sulfoxide and methionine sulfone, which are both oxidation products of methionine, were also found in these samples. These species are evidence that Miller’s H2S samples likely originally contained a greater quantity of methionine than what was detected in this study. Further analyses of the sulfur chemistry observed in Miller’s H2S samples are detailed elsewhere (15).
Discussion
Modern analytical methods are significantly more sensitive than those used by Miller in the 1950s. Miller used paper chromatography with ninhydrin detection and the mixed melting-point determination of derivatives to identify and quantify organic compounds synthesized in his early experiments. Such techniques would have been able to detect micromolar abundances of a fairly limited range of organic compounds (3). However, the analytical instrumentation used in this study is up to 10 orders of magnitude more sensitive and can detect a much wider diversity of organic compounds than the techniques available in the 1950s (2, 3).
Other efforts have been made to generate organic compounds from simulated early atmospheres containing H2S, most of which were conducted after Miller’s 1958 experiment. A spark discharge was passed through a mixture of CH4, H2O, NH3, and H2S, but no sulfur amino acids were detected (16). A mixture of H2, CH4, NH3, H2O, and H2S was subjected to a spark discharge, and the detection of cysteine, cystine, and possibly methionine was reported (17). Hydrogen sulfide was used as a photosensitizer for long-wave UV radiation to generate alanine, glycine, serine, glutamic acid, aspartic acid, and cystine from a CH4, C2H6, NH3, and H2S gas mixture (18, 19). Significant yields of methionine were also reported from the action of an electric discharge on CH4, H2O, H2S, NH3, and N2 (20).
Volcanoes are major modern natural sources of atmospheric H2S (21–23). Much of the atmospheric sulfur on the early Earth was also likely derived from volcanism and may have allowed H2S to become a significant sulfur species in the primitive atmosphere (4). Hydrogen sulfide has likely been outgassed from the Earth continuously throughout its history (24). Early volcanic eruptions may have injected reduced gases into a local atmosphere subject to lightning discharges, which frequently appear during volcanic eruptions today (25, 26). If the volcanic emissions were overall reducing, H2S would have been the major sulfur species (18).
When reduced gases, including CH4, H2S and NH3, are emitted from a volcano into a lightning-rich atmosphere, hydrogen cyanide, ethylene, and acetylene can be generated (27), all of which can serve as intermediates for the formation of more complex prebiotic organic compounds (28). Once generated, biomolecular precursors could have rained out and accumulated in tidal areas where they may have reacted further.
Although models suggest that the early atmosphere was not highly reducing on a global scale, it was proposed that H2 mixing ratios in the early atmosphere may have exceeded 30% throughout the thermosphere (8). These model calculations (8) assumed low early Earth exobase temperatures, a volcanic outgassing rate five times greater than what is observed today, and a solar UV radiation flux more than twice the current flux. However, these calculations (8) only accounted for the absorption of UV light by H2 and omitted the effect of the Earth’s magnetic field, which can enhance the escape of H2 via nonthermal mechanisms such as charge exchange and interaction with the solar wind (29). Thus, a significant H2 mixing ratio throughout the early atmosphere may have been unlikely.
Despite the rapid escape of H2 from the early atmosphere, volcanic eruptions may have supplied significant quantities of reduced gases (30) to localized regions where lightning events were common (31). Although the chemical composition of early volcanic plumes is unknown, they may have been composed of both reduced and oxidized gases (5), similar to the mixture that Miller used to synthesize the samples studied here. We propose that Miller’s 1958 study could thus serve as a model for the chemistry that may have occurred in early volcanic plumes. The presence of species that may have been commonly emitted by early volcanic eruptions, including H2S and CO2, in conjunction with lightning, could have provided a powerful mechanism to drive the synthesis of numerous prebiotic biomolecules.
Comparison to Other Spark Discharge Studies.
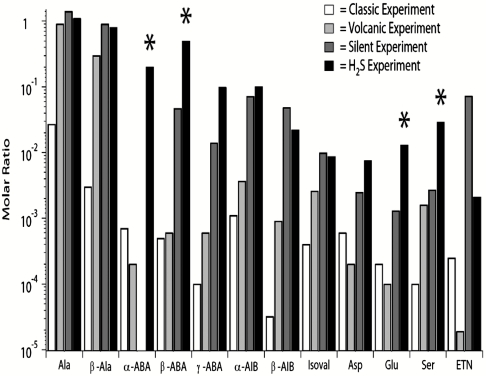
The yields of organic compounds in the H2S experiment were compared to the yields from three of Miller’s previously reported experiments, the original (2, 7), volcanic (3, 7), and silent discharge (3, 7) experiments. We found that the H2S samples analyzed here contained a greater relative abundance of many amino acids than the other three experiments (Fig. 3; experimental data used for the comparison were from ref. 7). The difference in nonprotein amino acid yields found in the H2S samples compared to those found in the other three experiments is remarkable. The combined yields of nonprotein amino acids, α-aminobutyric acid in particular, in the original, volcanic, and silent experiments were several hundred times smaller than what was detected in the H2S samples (Fig. 3). In addition to the sulfur-containing organic compounds, Miller’s unreported 1958 H2S experiment also generated amino acids such as threonine, leucine, and isoleucine, which were not detected in the other three discharge experiments.
Fig. 3.
Comparison of amino acid and amine molar ratios (relative to glycine = 1) found in Miller’s classic, volcanic, silent, and H2S electric discharge experiments. The H2S electric discharge data were obtained using UPLC-UVFD/ToF-MS. Data from the classic, volcanic, and silent discharge experiments were reported in ref. 7. Sulfur-containing compounds are not compared here because the classic, volcanic, and silent discharge experiments did not contain sulfur. Asterisks indicate compounds that are at least one order of magnitude more abundant relative to glycine in the H2S experimental samples than in all three other experiments. α-ABA was not detected in the silent discharge experiment. ETN, ethanolamine.
Comparison to Carbonaceous Chondrites.
In 1972, Miller and coworkers (32) showed that the relative abundances of amino acids produced when a spark discharge acts on H2, H2O, CH4, and NH3 were somewhat similar to those found in the Murchison carbonaceous chondrite (CC). It was thus suggested that the same type of synthetic pathways were responsible for generating the amino acids found in both the spark discharge experiment and the meteorite.
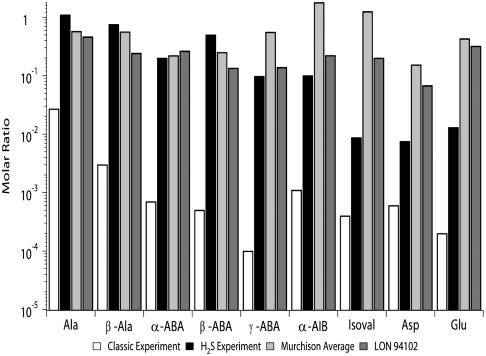
To further examine this surmise, we have compared the amino acid abundances from Miller’s H2S spark discharge experiment to those of CCs. The CCs chosen for comparison were two different samples of the CM2 Murchison meteorite (33–35) and the Antarctic CM2 CC Lonewolf Nunataks (LON) 94102 (33, 35). In addition to these studies, a comparison to Miller’s classic spark discharge experiment (2) was also included in order to compare the amino acid distributions in CCs and in spark discharge experiments with and without H2S (Fig. 4). The classic spark discharge results were included as opposed to the volcanic spark discharge or the silent discharge experimental results because the H2S experiment used the same apparatus as was used in the classic experiment. The classic spark discharge data used for this comparison were taken from ref. 7.
Fig. 4.
Comparison of amino acid molar ratios (relative to glycine = 1) found in Miller’s H2S and classic spark discharge experiments, and the Murchison USNM 5453, Murchison USNM 6650, and the LON 94102 meteorites. Data from the two Murchison meteorite samples were averaged and are presented here as “Murchison Average”. The H2S spark discharge experimental data were obtained in this study as reported above. The classic spark discharge experiment data were reported in ref. 7 and used similar analytical techniques as those used in this study. Literature data were used for the Murchison USNM 5453 (33), Murchison USNM 6650 (34, 35), and LON 94102 (33, 35) CC samples. Sulfur amino acids are not compared here because they were not detected in the CCs used for comparison. β-AIB, ethanolamine, and serine are not included in the comparison because serine was not detected in the Murchison USNM 5453 meteorite sample, and because both ethanolamine and β-AIB were not detected in any of the meteorites used in the comparison. The yield of β-AIB in the discharge experiments is lower than that of α-AIB and the α-, β-, and γ- isomers of aminobutyric acid (Fig. 2), which is consistent with its absence in the meteorite samples.
The yields of amino acids detected in Miller’s classic experiment, relative to glycine, are at least one order of magnitude smaller than those detected both in the H2S experiment and in the CCs, with the classic experiment being especially depleted in γ-aminobutyric acid relative to the other samples (Fig. 4). Amino acid abundances found in the CCs correlate much better with those found in the H2S experiment than with the classic experiment.
It is apparent that relative concentrations of amino acids including alanine, β-alanine, α-aminoisobutyric acid, and the α-, β-, and γ-aminobutyric acid isomers may be more influenced by the presence of H2S during amino acid synthesis than amino acids such as glutamic and aspartic acids and isovaline (Fig. 4). The relative amino acid yields produced by the H2S spark discharge experiment and the amino acid abundances detected in the CCs compared here are intriguingly similar. This could be due to reactions involving H2S occurring either in the aqueous or gas phase. Other meteorite samples also show a fairly good correlation with respect to most alkyl amino acids found within these meteorites and the residues produced in the H2S experiment (Fig. S1).
Sulfur has been reported in carbonaceous meteorites, with much of it suggested as being in the form of H2S (36). A Curie point pyrolysis analysis revealed that H2S was a prominent gas phase species of the Murchison meteorite (37). Furthermore, H2S has been identified in the interstellar medium (38 and references therein). Based on the correlations between the presence of H2S and relative amino acid abundances in meteorite samples and Miller’s H2S samples, we conclude that hydrogen sulfide was likely present in the environment that gave rise to the CC amino acids, either during amino acid synthesis on the parent body, or during amino acid precursor synthesis in the solar nebula.
It is possible that H2S abundance may account for some of the variation in amino acids found in different CCs. For some CCs, such as MET 01070, SCO 06043, and GRO 95577, the comparison between meteorite amino acid abundances and those of the H2S experiment do not show as good a correlation, indicating H2S may not have played a major role in the synthesis of amino acids on some meteorite parent bodies.
Conclusions
In 1958, Stanley Miller tested the action of electric discharges on a simulated primitive Earth atmosphere of CH4, CO2, H2S, and NH3. Miller never reported the analysis of this experiment. These original sample residues have recently been discovered and analyzed using HPLC-UVFD and UPLC-UVFD/ToF-MS. The samples contained a large assortment of amino acids and amines, including numerous sulfur amino acids. Our results demonstrate the earliest example of the production of organosulfur compounds, including S-methylcysteine, methionine, ethionine, methionine sulfoxide, and methionine sulfone from a spark discharge experiment designed to mimic possible primitive Earth conditions. In most cases, the relative diversity and abundance of amino acids and amines generated by Miller’s H2S experiment exceeds the amino acid and amine yields from his previous spark discharge experiments (7).
Miller’s H2S experiment also synthesized amino acids in abundances strikingly similar to those found in various CCs. The observed similarities indicate that H2S may have been present during CC amino acid or amino acid precursor synthesis.
The gas mixture Miller tested in the experiment described here, which used both reduced and oxidized gases, may not have been ubiquitous throughout the primitive atmosphere. However, this mixture might have been prominent on a regional scale (for example, near volcanoes), where these gases may have played a vital role in the localized synthesis of some of the first terrestrial organic compounds. Our results suggest that a mixture of oxidized and reduced gases, including H2S, may have aided the synthesis of amino acids and amines on the primitive Earth and elsewhere.
Materials and Methods
Discovery of Vials and Miller’s Experimental Design.
The samples were produced using Miller’s classic apparatus design, detailed elsewhere (1), and were collected and preserved by Miller in sterilized vials (S.L. Miller, 1958, Laboratory Notebook 2, page 114, Serial number 655, MSS642, Box 25, Mandeville Collections, Geisel Library). Upon the discovery of Miller’s collection of preserved samples in 2007, the dried residues were carefully inventoried, cataloged, and stored at the Scripps Institution of Oceanography in La Jolla, CA. By referencing Miller’s original laboratory notebooks, the sample identities reported here were confirmed to have originated from experiments that he conducted at Columbia University in 1958.
In the 1958 experiment, Miller filled the 5-L classic apparatus (1) with a mixture of CH4, H2S, NH3, and CO2 at partial pressures of 258 mm Hg, 100 mm Hg, 250 mm Hg, and 87 mm Hg, respectively (S.L. Miller, 1958, Laboratory Notebook 2, page 114, Serial number 655, MSS642, Box 25, Mandeville Collections, Geisel Library). Additionally, the apparatus was filled with 300 mL of water, which was boiled continuously for 3 d, while a spark was continuously applied to the tungsten electrodes over the same time period using a Tesla coil to simulate lightning (S.L. Miller, 1958, Laboratory Notebook 2, page 114, Serial number 655, MSS642, Box 25, Mandeville Collections, Geisel Library). Details regarding the type and output of the Tesla coil that Miller used for his spark discharge experiments are provided in ref. 3.
Miller’s original laboratory notebook stated that sulfur had accumulated near or on the electrodes (S.L. Miller, 1958, Laboratory Notebook 2, page 114, Serial number 655, MSS642, Box 25, Mandeville Collections, Geisel Library). It is plausible that the interaction between H2S and the tungsten electrodes played a role in influencing the product ratios of amino acids not containing sulfur. This possibility warrants further investigation.
After isolation of the total amino acids by ion exchange chromatography, Miller further separated the individual samples into fractions by HCl-based cation exchange chromatography (3, 39). These fractions were evaporated to dryness, and the dried residues were stored and preserved as described above.
Chemicals and Reagents.
All reagents used in the analyses reported here were purchased from Fisher Scientific or Sigma-Aldrich. All tools used to handle the samples were thoroughly cleaned using doubly distilled water (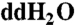 ) before wrapping them in aluminum foil, followed by subsequent overnight heating in air at 500 °C. Sample preparation for UPLC-UVFD/ToF-MS analyses was carried out in a similar manner except Millipore water (18.2 MΩ, < 3 ppb total organic carbon) was used instead of
) before wrapping them in aluminum foil, followed by subsequent overnight heating in air at 500 °C. Sample preparation for UPLC-UVFD/ToF-MS analyses was carried out in a similar manner except Millipore water (18.2 MΩ, < 3 ppb total organic carbon) was used instead of 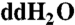 .
.
Stock amino acid solutions (approximately 10-3 M) were produced by mixing individual amino acid crystals (97–99% purity) with 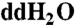 . Three reagents were prepared to derivatize the samples for analysis by HPLC-UVFD: (i) 0.4 M saturated sodium borate (pH 9.4), (ii) 0.05 M sodium acetate (pH 5.5), and (iii) OPA/NAC, a fluorescent reagent used to tag primary amino groups. These three reagents were prepared using methods detailed elsewhere (40).
. Three reagents were prepared to derivatize the samples for analysis by HPLC-UVFD: (i) 0.4 M saturated sodium borate (pH 9.4), (ii) 0.05 M sodium acetate (pH 5.5), and (iii) OPA/NAC, a fluorescent reagent used to tag primary amino groups. These three reagents were prepared using methods detailed elsewhere (40).
An ammonium formate buffer used for UPLC-UVFD/ToF-MS analyses was generated by NH4OH titration of 50-mM formic acid to pH 8. A 1-μM phenolphthalein solution in acetonitrile containing 0.1% formic acid was used to carry out mass calibrations of the time-of-flight mass spectrometer, which used an electrospray ionization source (35).
HPLC-UVFD.
Precolumn derivatization was performed as described in ref. 7. HPLC-UVFD analysis was conducted using a 250 mm × 4.6 mm, 100-Å pore size, 5-μm particle size Phenomenex Luna C-18, reverse-phase HPLC column. HPLC elution was monitored with a Shimadzu RF-535 Fluorescence HPLC detector with an excitation wavelength of 340 nm and an emission wavelength of 450 nm. Two buffer solutions were used for elution: (i) 0.05-M sodium acetate with 8% methanol (buffer A), and (ii) Fisher Optima grade methanol (buffer B). Buffer A was prepared by dissolving 13.6 g of sodium acetate trihydrate in 174 mL of methanol (Fisher Optima grade), followed by dilution to 2 L using 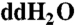 . The following gradient was used: 0–4 min, 0% B; 4–14 min, 0–37% B; 14–24 min, 37–42% B; 24–34 min, 42–60% B; 34–44 min, 60–0% B; 44–55 min, 0% B. The buffer flow rate was 1 mL/ min.
. The following gradient was used: 0–4 min, 0% B; 4–14 min, 0–37% B; 14–24 min, 37–42% B; 24–34 min, 42–60% B; 34–44 min, 60–0% B; 44–55 min, 0% B. The buffer flow rate was 1 mL/ min.
UPLC-UVFD/ToF-MS.
To compare to the results obtained using HPLC-UVFD and for mass spectral identification of products, portions of the sample residues were also analyzed by UPLC-UVFD/ToF-MS, as described elsewhere (7). Samples underwent the aforementioned preparation and derivatization protocols with the following modification. The samples were derivatized for 1 min, after which the reaction was quenched with 75 μL of 0.1-M hydrazine. The samples were then immediately placed into the LC-UVFD auto sampler and injected. For LC-UVFD, a Waters Acquity UPLC with tandem fluorescence detector was used. For ToF-MS a Waters LCT Premier time-of-flight mass spectrometer with positive electrospray ionization was used. Details of the ToF-MS settings and the amino acid quantification methods used for these analyses are reported elsewhere (34). The UPLC-UVFD/ToF-MS had a detection limit in the low femtomole range.
Supplementary Material
Acknowledgments.
Stanley L. Miller passed away on May 20, 2007. Because his research ushered in the era of experimental studies on the origin of life, we dedicate this work to his memory. We thank Jamie Elsila for GC-MS analyses of these residues and Facundo Fernandez and the reviewers for helpful comments. We deeply appreciate the Mandeville Special Collections in the University of California, San Diego Geisel Library for assistance with archiving and retrieving Miller’s original laboratory notebooks. We are grateful for funding support from the National Aeronautics and Space Administration Astrobiology Institute and the Goddard Center for Astrobiology. M.C. and H.J.C. acknowledge support from the National Aeronautics and Space Administration Postdoctoral Program administered by Oak Ridge Associated Universities. Assistance with data analysis and figure preparation (A.A.) was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration. J.L.B. and H.J.C. are also affiliated with the Center for Chemical Evolution at the Georgia Institute of Technology, which is jointly supported by the National Science Foundation and the National Aeronautics and Space Administration Astrobiology Program, under the National Science Foundation Grant CHE-1004570.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019191108/-/DCSupplemental.
References
- 1.Lazcano A, Bada JL. The 1953 Stanley L. Miller Experiment: Fifty Years of Prebiotic Organic Chemistry. Origins Life Evol Biosph. 2003;33:235–242. doi: 10.1023/a:1024807125069. [DOI] [PubMed] [Google Scholar]
- 2.Miller SL. A production of amino acids under possible primitive Earth conditions. Science. 1953;117:528–529. doi: 10.1126/science.117.3046.528. [DOI] [PubMed] [Google Scholar]
- 3.Miller SL. Production of some organic compounds under possible primitive Earth conditions. J Am Chem Soc. 1955;77:2351–2361. [Google Scholar]
- 4.Urey HC. On the early chemical history of the Earth and the origin of life. Proc Natl Acad Sci USA. 1952;38:351–363. doi: 10.1073/pnas.38.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasting JF. Earth’s early atmosphere. Science. 1993;259:920–926. doi: 10.1126/science.11536547. [DOI] [PubMed] [Google Scholar]
- 6.Cleaves HJ, Chalmers JH, Lazcano A, Miller SL, Bada JL. A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Origins Life Evol Biosph. 2008;38:105–115. doi: 10.1007/s11084-007-9120-3. [DOI] [PubMed] [Google Scholar]
- 7.Johnson AP, et al. The Miller volcanic spark discharge experiment. Science. 2008;322:404. doi: 10.1126/science.1161527. [DOI] [PubMed] [Google Scholar]
- 8.Tian F, Toon OB, Pavlov AA, De Sterck H. A hydrogen-rich early Earth atmosphere. Science. 2005;308:1014–1017. doi: 10.1126/science.1106983. [DOI] [PubMed] [Google Scholar]
- 9.Walker JCG, Brimblecombe P. Iron and sulfur in the pre-biologic ocean. Precambrian Res. 1985;28:205–222. doi: 10.1016/0301-9268(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 10.Plankensteiner K, Reiner H, Rode BM. Amino acids on the rampant primordial Earth: Electric discharges and the hot salty ocean. Mol Divers. 2006;10:3–7. doi: 10.1007/s11030-006-7009-0. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Bermejo M, Menor-Salván C, Osuna-Esteban S, Veintemillas-Verdaguer S. Prebiotic microreactors: A synthesis of purines and dihydroxy compounds in aqueous aerosol. Origins Life Evol Biosph. 2007;37:123–142. doi: 10.1007/s11084-006-9026-5. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Bermejo M, Menor-Salván C, Osuna-Esteban S, Veintemillas-Verdaguer S. The effects of ferrous and other ions on the abiotic formation of biomolecules using aqueous aerosols and spark discharges. Origins Life Evol Biosph. 2007;37:507–521. doi: 10.1007/s11084-007-9107-0. [DOI] [PubMed] [Google Scholar]
- 13.Navarro-González R, Molina MJ, Molina LT. Nitrogen fixation by volcanic lightning in the early Earth. Geophys Res Lett. 1998;25:3123–3126. [Google Scholar]
- 14.Navarro-González R, McKay CP, Nna Mvondo D. A possible nitrogen crisis for Archaean life due to reduced nitrogen fixation by lightning. Nature. 2001;412:61–64. doi: 10.1038/35083537. [DOI] [PubMed] [Google Scholar]
- 15.Parker ET, et al. Prebiotic synthesis of methionine and other sulfur-containing organic compounds on the primitive Earth: A contemporary reassessment of an unpublished 1958 Stanley Miller experiment. Origins Life Evol Biosph. 2010 doi: 10.1007/s11084-010-9228-8. 10.1007/s11084-010-9228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyns HK, Walter W, Meyer E. Model experiments on the formation of organic compounds in the atmosphere of simple gases by electrical discharges (Translated from German) Die Naturwissenschaften. 1957;44:385–389. [Google Scholar]
- 17.Lu H-K, et al. Formation of sulfur-containing amino acids by electric discharge in a reductive atmosphere. Chem Abstracts. 1960;54:4209–4210. [Google Scholar]
- 18.Sagan C, Khare BN. Long-wavelength ultraviolet photoproduction of amino acids on the primitive Earth. Science. 1971;173:417–420. doi: 10.1126/science.173.3995.417. [DOI] [PubMed] [Google Scholar]
- 19.Khare BN, Sagan C. Synthesis of cystine in simulated primitive conditions. Nature. 1971;232:577–579. doi: 10.1038/232577a0. [DOI] [PubMed] [Google Scholar]
- 20.Van Trump JE, Miller SL. Prebiotic synthesis of methionine. Science. 1972;178:859–860. doi: 10.1126/science.178.4063.859. [DOI] [PubMed] [Google Scholar]
- 21.Bates TS, Lamb BK, Guenther A, Dignon J, Stoiber RE. Sulfur emissions to the atmosphere from natural sources. J Atmos Chem. 1992;14:315–337. [Google Scholar]
- 22.Berner EK, Berner RA. Global Environment: Water, Air and Geochemical Cycles. Old Tappan, NJ: Prentice Hall; 1996. [Google Scholar]
- 23.Aiuppa A, et al. H2S fluxes from Mt. Etna, Stromboli, and Vulcano (Italy) and implications for the sulfur budget at volcanoes. Geochim Cosmochim Acta. 2005;69:1861–1871. [Google Scholar]
- 24.Wächtershäuser G. Before enzymes and templates: Theory of surface metabolism. Microbiol Rev. 1988;52:452–484. doi: 10.1128/mr.52.4.452-484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNutt SR, Davis CM. Lightning associated with the 1992 eruptions of Crater Peak, Mount Spurr Volcano, Alaska. J Volcanol Geotherm Res. 2000;102:45–65. [Google Scholar]
- 26.Thomas RJ, et al. Electrical activity during the 2006 Mount St. Augustine volcanic eruptions. Science. 2007;315:1097. doi: 10.1126/science.1136091. [DOI] [PubMed] [Google Scholar]
- 27.Segura A, Navarro-González R. Experimental simulation of early Martian volcanic lightning. Adv Space Res. 2001;27:201–206. doi: 10.1016/s0273-1177(01)00048-5. [DOI] [PubMed] [Google Scholar]
- 28.Miller SL, Cleaves HJ. In: Systems Biology: Genomics. Ritgoutsos I, Stephanopoulous G, editors. New York: Oxford Univ Press; 2007. pp. 3–56. [Google Scholar]
- 29.Catling DC. Comment on ‘‘A hydrogen-rich early Earth atmosphere’’. Science. 2006;311:38. doi: 10.1126/science.1118412. [DOI] [PubMed] [Google Scholar]
- 30.Wills C, Bada J. The Spark of Life: Darwin and the Primeval Soup. Cambridge, MA: Perseus Publishing; 2001. [Google Scholar]
- 31.Bada JL. How life began on Earth: A status report. Earth Planet Sci Lett. 2004;226:1–15. [Google Scholar]
- 32.Wolman Y, Haverland WJ, Miller SL. Nonprotein amino acids from spark discharges and their comparison with the Murchison meteorite amino acids. Proc Natl Acad Sci USA. 1972;69:809–811. doi: 10.1073/pnas.69.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glavin DP, Callahan MP, Dworkin JP, Elsila JE. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit Planet Sci. 2010;45:1948–1972. [Google Scholar]
- 34.Glavin DP, et al. Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography-time of flight-mass spectrometry. Meteorit Planet Sci. 2006;41:889–902. [Google Scholar]
- 35.Glavin DP, Dworkin JP. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc Natl Acad Sci USA. 2009;106:5487–5492. doi: 10.1073/pnas.0811618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urey HC. On the dissipation of gas and volatilized elements from protoplanets. Astrophys J Suppl. 1954;1:147–173. [Google Scholar]
- 37.Remusat L, Derenne S, Robert F, Knicker H. New pyrolytic and spectroscopic data on Orgueil and Murchison insoluble organic matter: A different origin than soluble? Geochim Cosmochim Acta. 2005;69:3919–3932. [Google Scholar]
- 38.Ziurys LM. The chemistry in circumstellar envelopes of evolved stars: Following the origin of the elements to the origin of life. Proc Natl Acad Sci USA. 2006;103:12274–12279. doi: 10.1073/pnas.0602277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christianson D, Wall JS, Dimler RJ, Senti FR. Separation and determination of quaternary nitrogen compounds and other nitrogenous substances by ion exchange chromatography application to analysis of corn extracts. Anal Chem. 1960;32:874–878. [Google Scholar]
- 40.Zhao M, Bada JL. Determination of α-dialkylamino acids and their enantiomers in geological samples by high-performance liquid chromatography after derivatization with a chiral adduct of o-phthaldialdehyde. J Chromatogr A. 1995;690:55–63. doi: 10.1016/0021-9673(94)00927-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.