Abstract
Infections have become as important an event as acute rejection post-transplant for long-term allograft survival. Less invasive biomarkers tested so far predict risk for one event or the other, not both.
We prospectively tested blood and urine monthly for twelve months post-transplant from children receiving a kidney transplant. The indoleamine 2,3 dioxygenase (IDO) enzyme pathway was assessed by mass spectrometry assays using the ratio of product L-kynurenine (kyn) to substrate tryptophan (trp). Kyn/trp ratios and blood CD4 T-cell ATP levels were correlated with acute rejection or major infection events or stable group (no events) in the next 30 days.
The 25 subjects experienced 6 discrete episodes of acute rejection in 5 subjects and 16 discrete events of major infection in 14 subjects (7 BK viruria, 6 cytomegaloviremia, 1 Epstein-Barr and cytomegaloviremia, 2 transplant pyelonephritis). Mean serum kyn/trp ratios were significantly elevated in the group that experienced acute rejection (p = 0.02).Within-subject analyses revealed that over time, urine kyn/trp ratios showed an increase (p = 0.01) and blood CD4-ATP levels showed a decrease (p = 0.007) prior to a major infection event.
These pilot results suggest that a panel of biomarkers together can predict over- or under-immunosuppression, but need independent validation.
Keywords: Transplantation, immune monitoring, rejection, infection, pediatrics, kidney, IDO, Immuknow
Introduction
Organ transplantation is currently the treatment of choice for most patients with end-stage organ disease of the kidney, liver, lung or heart. Acute cellular or antibody-mediated rejection used to be the biggest problem affecting graft survival. With newer more potent medications, the risk of acute rejection has been reduced, though still present. With the newer medications, infections have emerged as a serious problem, which also affect graft survival (1, 2). Thus, both consequences are undesirable.
Currently, transplant professionals do not have any good way to assess if a given patient is under-or over-immunosuppressed at a given point in time. Drug levels, singly or in combination, do not predict total or “net” state of immunosuppression well for a variety of reasons. For some drugs such as mycophenolate mofetil, levels do not correlate with immunosuppressive activity. For steroids, no methods are commercially available to measure drug levels. For injectable induction agents, length of immunosuppression is variable. How to combine the effects of tacrolimus levels, mycophenolate level/dose, steroid dose and residual effect of induction agents is unknown.
The development of less invasive diagnostic methods that provide good prediction of acute rejection or infection risk is sorely needed in transplantation and perhaps in all diseases where extrinsic immunosuppression is used. Many biomarker immune molecules have been tested in organ transplantation for acute rejection prediction, such as FasL, Granzyme B, FoxP3, IP-10 and fractalkine (3-5), but none has been tested adequately to predict both extremes of immunosuppression. The FDA-approved and commercially available Immuknow® assay (6) measures intracellular CD4 T-cell ATP levels; low levels are associated with a 9-fold higher relative risk for subsequent infection (7). However, the test did not predict risk for acute rejection very well. The other postulated less-invasive tests of global immunosuppression are serial viral PCR monitoring, such as peripheral blood CMV or EBV monitoring or urine BK virus testing, all of which would predict over-immunosuppression (8). The natural history of these viruses is for viremia to precede clinical disease, such that earlier detection can be used to prevent progression to clinical disease by lowering of immunosuppression. Batal et al (9) demonstrated that ImmuKnow CD4 ATP levels were significantly lower in kidney transplant recipients with higher urinary BK virus load. These results suggested that lower CD4 ATP levels correlate with active viral replication.
The complexity of the immune system may be such that no one molecule can adequately quantify the overall activity of the immune system. Therefore, a panel of tests, representing both extremes of immunosuppression and adjusting for confounding etiologies, may provide the best discrimination.
Tryptophan (trp) is the rarest of the essential amino acids and is necessary for protein synthesis (10-14). It is catabolized by two separate enzymes, indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). Active IDO catalyses the initial and rate-limiting step of trp oxidative catabolism with multiple further intermediaries, collectively referred to as kynurenines. IDO activity has conventionally been represented as a ratio of L-kynurenine (kyn) to trp. IDO expression is inducible by inflammatory cytokines, particularly interferon-γ (IFN-γ), in multiple cell types, many of which are relevant to transplantation (15-17). IDO has been documented to be critically involved in establishing immune tolerance in pregnant mice upon their fetuses, or inducing T-cell unresponsiveness (18-20). In a prior study in adult kidney transplant recipients, Brandacher et al. demonstrated that blood and urinary kyn/trp ratios were elevated above baseline during acute rejection episodes (21). Ratios in blood increased, from 55.1 ± 39.6 μmol/mmol in patients with stable graft function, to 114 ± 44.5 μmol/mmol in patients with acute rejection. Similar increases in urinary ratios with acute rejection were demonstrated.
In this study, we developed a mass spectrometry assay for kyn and trp and then hypothesized that a combination of serum kyn/trp ratios plus CD4-ATP levels, in absence of markers of significant fibrosis, would provide better prediction of infection versus rejection risk than either test alone.
Methods
A) Patient populations and samples
From July 2008 till June 2010, we prospectively and longitudinally tested blood and urine samples from children monthly within the first 12 months post-kidney transplant. This study was approved by the University of Florida Institutional Review Board. Clinical data collected included recipient and donor age/sex/race, donor source, delayed graft function presence or not, concomitant medications and clinical events. Data on serum and urine kyn/trp levels and ratios, blood CD4 ATP levels, trough tacrolimus and mycophenolate levels were correlated with occurrence of acute rejection event (rejection group) or major infection (infection group) event or no event (stable group) in the next 30 days from sample collection. Major infection event was defined as CM viremia, EB viremia, BK viruria (above our local lab cutoff), transplant pyelonephritis (fever > 38.5C + pyuria + positive urine culture for a single organism > 100,000 colonies/ml) or fever with culture proven bacteremia. All acute rejection events were biopsy proven.
B) Immunosuppression and viral studies
As part of our standard of care, we already perform Immuknow® assay blood CD4-ATP level testing and viral PCR monitoring (peripheral blood EBV and CMV and urinary BK virus) on a monthly basis in the first 12 months post-transplant. All PCR assays were performed at our local Shands Hospital Clinical Laboratories and used the same in-house methodology for each PCR amplification. Our current immunosuppression protocol, active since July 2007, includes a 3-day induction course of rabbit anti-thymocyte globulin and intravenous steroids, followed by oral maintenance tacrolimus and mycophenolate mofetil. Oral maintenance steroids beyond day 3 were reserved for specific situations. Standard anti-infective prophylaxis included trimethoprim-sulfamethoxazole three times a week for 6 months, anti-fungal prophylaxis (nystatin or clotrimazole) for 3 months and anti-viral prophylaxis with valganciclovir for 6 months.
C) L-kynurenine and tryptophan measurement
Serum and urine L-tryptophan and L-kynurenine (Sigma, St Louis, MO) were measured from batched samples stored at -80C by HPLC tandem mass spectrometry using a Thermo TSQ Quantum Ultra spectrometer (Thermo, San Jose, CA). The assay had a limit of quantitation for both species of 5 ng/mL with linear dynamic range from 5 ng/mL to 40,000 ng/mL for trp and 5 ng/mL to 10,000 ng/mL for kyn (typically providing R2 values equal to or better than 0.999 and relative coefficients of variation below 10%). Calibration standards and quality control standards for trp and kyn were prepared in albumin at 8 specified concentrations for the serum assay and in HPLC water for the urinary assay. IDO activity was expressed as the ratio of kyn/trp*100. To 100 μL of calibration standard, QC, serum or urine, 100 μL of internal standard solution containing tryptophan D3 (4000 ng/mL, C,D,N isotopes, Quebec, Canada) and kynurenine D4 (400 ng/mL, gift) in 5 mM ammonium acetate was added and the samples were mixed. Next, 20 μL of ice cold 2.4 M perchloric acid was added and mixed for 5 min to precipitate the proteins and then stored at 4 C for 10 min. The samples were then centrifuged at 20,800 g for 10 min at 15C and the supernatant was then transferred to a microcentrifuge filter tube (Costar Spin × 0.22 μm nylon, Fisher Scientific) and centrifuged again under the same conditions. The filtered solutions were then transferred to autosampler vials with inserts for LC/MS/MS analysis. Precipitated and filtered samples were injected (10 μL) onto a Betabasic C18 HPLC column (150 × 2.1 mm × 5 um, Thermo) equipped with a similar guard column maintained at 22 C. Analytes were eluted using a gradient method at 500 μL//min with A=0.1% formic acid in water and B=0.1% formic acid in acetonitrile, both HPLC grade (Fisher Scientific, Fairlawn, NJ). The gradient method was as follows: 0-0.5 min hold at 95% A, 0.5-5.8 min linear increase to 65% B, 5.8-6.2 min hold a 65% B, 6.2-6.4 min, increase to 95% A and hold for 6.2 min to equilibrate the column. The first 1.5 min was diverted to waste to keep the source clean. The mass spectrometer was operated in selected reaction monitoring mode (SRM) monitoring kyn, m/z 209 to m/z 192 (CE 18) and 146 (CE 7) and kynD4, m/z 213 to m/z 196 (CE 21) and 150 (CE 10) and trp, m/z 205 to m/z 146 (CE 17) and trpD3, m/z 208 to m/z 147 (CE 18). Kyn and kynD4 eluted at 2.25 min, while trp and trpD3 eluted at 2.94 min. The full-width-at-half-max was set to 0.7 and the width for each SRM transition was 0.1 amu with collision gas pressure at 1.5 Torr. Ion source conditions were as follows: heated-electrospray ionization 300 C, 800 V with sheath gas at 40 and aux gas at 20 arbitrary units and ion sweep gas at 1 arb. The heated transfer tube was maintained at 350 C.
D: Serum and urine TGF-β1 and CTGF measurment
Serum and urine TGF-β1 concentrations were determined by using by quantitative Emax ® ImmunoAssay System (Promega # G7590) according to the manufacturer's instructions. Using rabbit polyclonal anti-TGF-β1 antibody, this system is designed to detect biologically active TGFβ1 in an antibody sandwich format. The primary antibody was used as 1:1000 diluted, whereas, the secondary antibody (goat anti-rabbit IgG-HRP conjugate) was used at 1:100 concentration. Similarly, serum and urine CTGF concentrations were determined by using commercially available ELISA kit (Antigenix America Inc.# RHF461CKC) according to the manufacturer's instructions briefly described here. Using flat-bottom 96 well ELISA plate primary antibody (rabbit anti-human-CTGF (0.25ug/ml) was coated by adding 100ul/well (diluted PBS) and left overnight at room temperature. After four washes, coating buffer was applied for one hour. After removing the buffer, the let plates were let dry for an hour. Samples were applied and left for 90 minutes. After six washes with PBST streptavidin-HRP secondary antibody was applied at 1:1000 dilutions and left for 30 minutes at room temperature. After six washes with PBST, tetramethyl-benzidine followed by 1N HCL were applied and optical density was determined at 450 nm.
E: Statistical analyses
Statistical analyses were performed using GraphPad 5.0 (San Diego, CA, USA) or SAS 9.2 (Cary, NC, USA). Data and estimates for inferential purposes are expressed with mean and standard errors (SE) of estimation or proportions and expressed to 2 significant digits. Consistent with all prior studies of immune biomarkers (3, 4, 21, 22), we first correlated each sample to events occurring in the next 30 days, since a given patient may be under-immunosuppressed in one month and over-immunosuppressed in another month, such that both acute rejection and infection events may occur in the same subject at different time points. Mean values of the biomarkers between the three groups (stable, rejection or infection) were therefore compared by ANOVA. These methods presume that there is no within-subject association. If this assumption is false, the analysis will tend to underestimate the true variability of its estimates, have p-values below what they should be, and have confidence intervals narrower than they should be. We present these analyses because of this established tradition, but we also ran diagnostics on the assumptions behind this analysis.
Since longitudinal samples from patients may also be subject to individual subject variability, we also conducted two types of analyses that control in differing ways for within subject variability: a) mixed model repeated measures analysis, using an autocorrelated structure to account for the timing of the repeated measures for a subject (differential weighting of number of observations from a given patient in a given state); b) mean of means analysis (weighs all subjects equally but restricts the analysis to those subjects with observations in more than one state). To the best of our knowledge, these types of analyses have not been performed in prior biomarker longitudinal studies in transplantation. Results from each type of analyses are presented separately.
Finally, note that each P-value is presented without overall error control, such as a Bonferroni correction. This is consistent with pilot studies, where minimizing the overall false positive rate would undermine the false negative rate. Any significant result would need independent confirmation before treating it as conclusive evidence.
Results
A) Study population and outcome events
The study enrolled 26 consecutive subjects, of whom 1 enrolled subject was subsequently excluded as this subject lived very far away and decided to be followed locally only, with no study samples provided. The demographics of the 25 subjects who comprised the final study group are presented in Table 1. At the initiation of the study, some patients were already in the later part of the first 12 month window. At time of this analysis, some of the most recent subjects have not yet completed 12 months post-transplant. There were 6 discrete episodes of acute rejection in 5 subjects and 16 discrete events of major infection in 14 subjects (7 BK viruria, 6 CM viremia, 1 EB viremia, 2 transplant pyelonephritis). The one subject with EB viremia presented directly at 4 months post-transplant with simultaneous CM and EB viremia and PTLD. Three of the seven BK viruria subjects also developed BK viremia, one of whom also had evidence of BK virus associated nephropathy on biopsy. No patient experienced fever + bacteremia in the study period. No patient had simultaneous acute rejection and acute infection.
Table 1. Study subject demographic characteristics (n= 25).
| Parameter | Mean (SE) or n (%) |
|---|---|
| Recipient age | 12.71 (0.74) |
| Recipient sex male | 16 (64%) |
| Recipient race | |
| Caucasian | 13 (52%) |
| African-American | 8 (32%) |
| Other | 4 (16%) |
| Deceased donor source | 6 (24%) |
| Delayed graft function | 7 (28%) |
From these 25 subjects, 106 blood and 104 urine samples were available for analysis of kyn/trp ratios. Blood CD4 ATP level results were available from 108 samples. Tacrolimus level and mycophenolic acid level results, done more frequently, were available from 134 and 113 samples, respectively, taken at or within one week of the kyn/trp and CD4-ATP blood samples.
B) Sample to event correlations
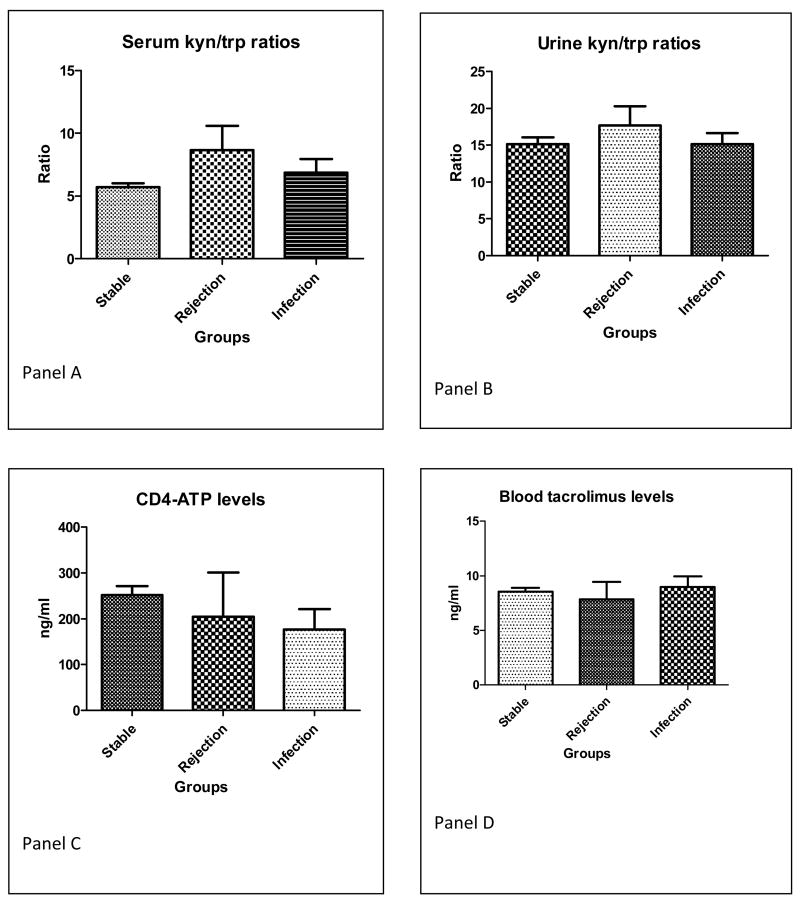
Serum kyn/trp ratios were significantly elevated in the group that experienced acute rejection within the next 30 days (mean ratio 8.658 ± SE 1.93; Figure 1A) compared to the other two groups (ratio 5.695 ± 0.29 in stable group and 6.844 ± 1.19 in major infection event group, p value = 0.02). In contrast, urine kyn/trp ratios were not significantly different among groups (mean 15.12 ± 0.93 stable group versus 17.67 ± 2.60 rejection group versus 15.12 ± 1.31 infection group, p value = 0.64; Figure 1B).
Figure 1.
A. Serum kyn/trp ratios across the three groups. (81 stable group, 9 rejection group, 16 infection group). p value = 0.02 by ANOVA across all three groups, significant difference is between stable vs rejection group by Bonferroni post-test
B. Urine kyn/trp ratios across the three groups (56 stable, 6 rejection, 8 infection group). p value = 0.27 by ANOVA across all three groups (NS)
C. Blood CD4 ATP levels (Immuknow assay) across the three groups (86 stable group, 4 rejection group, 18 infection group). P value = 0.27 by ANOVA across all three groups (NS)
D. Blood trough tacrolimus levels across the three groups. (104 stable, 7 rejection group, 23 infection group). P value = 0.78 by ANOVA across all three groups (NS).
When analyzed separately, the mean levels of serum kyn or serum trp, not combined into a ratio, did not significantly differ among the 3 groups. For serum kyn (ng/mL), the values were: mean 2.95 ± 0.11 stable group versus 3.38 ± 0.78 rejection group versus 2.91 ± 0.30 infection group, p value = 0.61 (data not shown graphically). For serum trp (also ng/mL), the values were: mean 55.52 ± 1.93 stable group versus 53.60 ± 7.63 rejection group versus 53.09 ± 6.66 infection group, p value = 0.88 (data not shown graphically).
Blood CD4 ATP levels were not significantly different between the three groups (mean 251.4 ± 19.65 stable group versus 204.5 ± 96.48 rejection group versus 176.41 ± 45.45 infection group, p value 0.27; Figure 1C). Trough tacrolimus levels were not significantly different between the three groups (mean value 8.54 ± 0.34 stable group versus 7.85 ± 1.56 rejection group versus 8.95 ± 1.98 infection group, p value 0.78; Figure 1D). Trough mycophenolate levels, collected primarily to identify undetectable values (indicating possible non-compliance), were also not significantly different between groups (data not shown).
Caution is needed about interpreting these results, as our diagnostic analysis showed strong associations within subjects, making the assumption of non-association doubtful. Based on a one way ANOVA, the P-values for within- subject association were 0.0051, 0.020, and 0.0054 for serum kyn/trp ratio, urinary kyn/trp ratio, and CD4-ATP levels, respectively. All three R2 values slightly exceeded 40%, indicating that we estimate that subject explains at least 40% of the variation in the data.
C) Within subject analyses
We therefore proceeded with two different types of within-subject analyses. Using the mixed model repeated measures analysis, urine kyn/trp ratios in the infection state showed a significant difference from the non-infected, non-rejection state of +17 (± 5.27; p = 0.01; Table 2). In contrast, serum kyn/trp ratios and CD4-ATP levels did not show any significant differences in the infection state. All three biomarkers did not show any significant changes between stable state and acute rejection.
Table 2. Within-subject analyses.
| Analysis Type | Parameter | Acute rejection group Amount change (SE) P value |
Acute infection group Amount change (SE) p value |
|---|---|---|---|
| Mixed model | Serum kyn/trp ratio | + 0.62 (0.95) P value 0.56 |
-1.14 (0.89) P value 0.23 |
| Urine kyn/trp ratio | -1.84 (2.09) P value 0.44 |
- 17.3 (5.27) P value 0.011 |
|
| Serum CD4-ATP level | + 97.18 (73.58) P value 0.28 |
-68.94 (39.43) P value 0.11 |
|
| Mean of means | Serum kyn/trp ratio | + 1.68 (1.03) P value 0.20 |
- 1.12 (1.64) P value 0.51 |
| Urine kyn/trp ratio | -0.47 (1.57) P value 0.79 |
- 7.5 (3.8) P value 0.05 |
|
| Serum CD4-ATP level | +129.19 (94.68) P value 0.27 |
-110.70 (31.78) P value 0.0069 |
Amount change is stable group minus affected group
When using the mean of means procedure, restricting to those subjects in whom values are available in more than one state, the urine kyn/trp ratio showed a borderline significant rise of +7.5 (± 3.8; p value = 0.05) from stable state to major infection. In this analysis, the blood CD4-ATP level showed a significant drop of -110.69 points (± 31.77; p value 0.007) from stable state to major infection. In contrast, serum kyn/trp ratios levels did not show any significant changes within a given subject with respect to major infection. All three biomarkers did not show any significant changes within a given subject with respect to stable state and acute rejection.
D) Serum and Urine TGF-β and CTGF levels
Finally, we tested serum and urine samples for TGF-β and CTGF levels as surrogate markers of intragraft fibrosis, which may confound biomarker results. In almost all cases, serum and urine samples showed undetectable levels of TGF-β and CTGF (data not shown), consistent with the likely lack of significant fibrosis at the relatively early time points post-transplant of the study subjects.
Discussion
This analysis presents data from a pilot initial validation group of subjects for the biomarker panel under consideration as a panel to measure both sides of the immunosuppression spectrum. We were able to demonstrate an absolute increase in the serum kyn/trp ratio with subsequent acute rejection events and a within–subject increase in urine kyn/trp ratio or drop in CD4-ATP level with subsequent major infection event.
Our results of serum kyn/trp ratios in relation to acute rejection are in accord with those of Brandacher et al. and Lahdou et al., who have previously demonstrated a rise in serum kyn/trp ratios in association with acute rejection events shortly thereafter, within 13 or 3 days, respectively (21, 23). However, it is pertinent to note that the apparent differences in the values presented in earlier studies relate to different ways of expressing individual ratios. For example, studies by Lahdou et al. (23) and Holmes et al. (24) utilized μmol units for both kyn and trp. In the study by Lahdou et al., kyn/trp ratios were represented without any transformation, such that kyn/trp ratios were presented as fractions, since kyn levels are much less than trp levels. In contrast, Brandacher et al. (21) used μmol units for kyn and mmol units for trp in their kyn/trp ratio, a thousand fold difference in trp units. In the present study, we converted ng/ml to μmol units and multiplied by 100 to arrive at our kyn/trp ratio. If our ratios are multiplied by 10, our units become identical to the units used in the study by Brandacher et al and serum results then look almost identical to that study.
Apart from differential expression of ratios, prior studies utilized a high performance liquid chromatography platform while we have utilized a tandem mass spectrometry technique, which is considered a more robust detection system. The Brandacher et al. study also restricted to events within the first 3 weeks post-transplant, while the study by Lahdou et al. tested retrospectively collected serum samples out to 6 months post-transplant. In contrast to the study by Brandacher et al., we were unable to replicate a significant rise in urine kyn/trp ratios in subjects going on to experience an acute rejection event. The reasons for this contrasting result are not clear, but may relate to the longer time interval studied by us, the potentially greater duration between sample collection to event, or the relatively low frequency of acute rejection events in our study.
To our knowledge, ours is the first study to evaluate in detail the change in kyn/trp ratios from serum or urine, in relation to major post-transplant infection events, out to one-year post-transplant. Holmes et al. (24) postulated that infections would be another major cause of increased IDO enzyme activity, since the enzyme is induced by interferon-γ, which is stimulated by viruses such as cytomegalovirus and Epstein-Barr virus. A growing body of literature has demonstrated increased IDO activity through interferon-γ mediated pathways in response to HIV infection (25-28). The more recent era of transplantation is characterized by reduced frequency of acute rejection events and emergence of major infections such as BK virus. Consistent with this trend, our study group exhibited a greater number of major infection events than acute rejection episodes. Of note, the viral infection events detected by PCR monitoring did not lead to full blown disease in most cases, perhaps because of reductions in immunosuppression made in response to these standard of care tests. Holmes et al. found that serum kyn levels were markedly raised, on the day of infection, in 5 patients with Gram negative bacterial infection or viral infections, in a study that was restricted to the first 3 weeks post-transplant (24). Kyn/trp ratios were not assessed in this study. The study by Brandacher et al. evaluated serum and urine kyn/trp ratios in a small group of 6 subjects with infection events (4 herpes simplex, 1 UTI, 1 sepsis). They were unable to detect any significant differences, probably due to a combination of small event number, time restriction to the first 21 days post-transplant only and 4 of the 6 infection events being relatively minor and localized.
Our study did not demonstrate lower mean levels of CD4-ATP in association with subsequent infection, as has been previously shown in a multi-center analysis (7). However, within subjects, we were able to detect a significant drop in CD4-ATP levels with subsequent infection, suggesting considerable intra-subject variability. We did not see a spike in CD4-ATP levels with active viremia as has been reported with EB viremia (29). Notably, only one subject in our study had EB viremia.
In studies of other biomarkers, fibrosis within the allograft could have represented a confounder variable. Two of the three prior studies of IDO enzyme activity post-transplantation were restricted to a very early time point within the first 3 weeks. Since we were looking to assess IDO enzyme activity out to 1 year, we prospectively measured serum TGF-β and CTGF levels as a surrogate marker of potential intragraft fibrosis. Prior studies have shown that, in serum, both TGF-β and CTGF levels rise in human kidney transplant recipients with documented chronic allograft nephropathy (30, 31).We do not perform protocol biopsies at our center, which would be the ideal measure of intragraft fibrosis. TGF-β may be affected by doses and levels of calcineurin inhibitors, so its absence may also reflect lower target tacrolimus levels. Nevertheless, our results of virtually undetectable serum TGF-β and CTGF levels suggest that fibrosis events were not significant in our subjects and would not represent any significant confounding.
Limitations of our pilot study include the relatively small sample size, the need for a separate validation group in future, and the absence of pre-transplant sample measures. However, prior studies by Lahdou et al. and Brandacher et al. have already demonstrated via pre-transplant samples that patients with end stage renal disease have higher serum kyn/trp ratios than normal controls, and that post-transplant recipients with early acute rejection have levels higher than those seen in end stage renal disease (21, 23). Neither we nor others have assessed changes in ratio with timing of meals, which may provide a trp load.
In summary, our pilot results suggest that less invasive immune monitoring may be able to detect a net state of immune suppression at either end of the spectrum. However, the results of this study do not possess sufficient discrimination ability for routine use in clinical practice. Further validation studies are needed to best define the combination of markers and time points that will provide the best prediction.
Acknowledgments
This study was supported in part by the Children's Miracle Network and the University of Florida General Clinical Research Center, through the National Institutes of Health (NIH) and National Center for Research Resources (NCRR) CTSA grant 1UL1RR029890. The authors thank acknowledge Jesse Gregory of UF and Per Ueland (University of Bergen) for donation of kynD4 and thank Ms. Amisha Mehta for assistance with the development of the mass spectrometry assays of IDO enzyme activity.
Abbreviations
- IDO
indoleamine 2,3 dioxygenase
- Trp
tryptophan
- Kyn
kynurenines
- CMV
cytomegalovirus
- EBV
Epstein-Barr virus
- BKV
BK virus
- FasL
Fas Ligand
- FoxP3
forkhead Box P3
- IP-10
Interferon gamma induced protein 10
- IFN-γ
interferon-gamma
- HPLC
high performance liquid chromatography
- LC
liquid chromatography
- MS
mass spectrometry
- SRM
selected reaction monitoring
- CE
collision energy
- TGF-β
transforming growth factor beta
- CTGF
connective tissue growth factor
- SE
standard error
- ANOVA
analysis of variance
- PTLD
post-transplant lymphoproliferative disorder
References
- 1.Dharnidharka VR, Caillard S, Agodoa LY, Abbott KC. Infection Frequency and Profile in Different Age Groups of Kidney Transplant Recipients. Transplantation. 2006 Jun 27;81(12):1662–7. doi: 10.1097/01.tp.0000226068.66819.37. [DOI] [PubMed] [Google Scholar]
- 2.Dharnidharka VR, Stablein DM, Harmon WE. Post-transplant infections now exceed acute rejection as cause for hospitalization: a report of the NAPRTCS. Am J Transplant. 2004 Mar;4(3):384–9. doi: 10.1111/j.1600-6143.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- 3.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005 Dec 1;353(22):2342–51. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 4.Peng W, Chen J, Jiang Y, Wu J, Shou Z, He Q, et al. Urinary fractalkine is a marker of acute rejection. Kidney Int. 2008 Dec;74(11):1454–60. doi: 10.1038/ki.2008.459. [DOI] [PubMed] [Google Scholar]
- 5.Strehlau J, Pavlakis M, Lipman M, Shapiro M, Vasconcellos L, Harmon W, et al. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci U S A. 1997;94(2):695–700. doi: 10.1073/pnas.94.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowalski R, Post D, Schneider MC, Britz J, Thomas J, Deierhoi M, et al. Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant. 2003 Apr;17(2):77–88. doi: 10.1034/j.1399-0012.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 7.Kowalski RJ, Post DR, Mannon RB, Sebastian A, Wright HI, Sigle G, et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation. 2006 Sep 15;82(5):663–8. doi: 10.1097/01.tp.0000234837.02126.70. [DOI] [PubMed] [Google Scholar]
- 8.Dharnidharka V, Gupta S. Viral immune monitoring for post-transplant lymphoproliferative disorder. Pediatr Transplant. 2009 Aug;13(5):521–3. doi: 10.1111/j.1399-3046.2009.01144.x. [DOI] [PubMed] [Google Scholar]
- 9.Batal I, Zeevi A, Heider A, Girnita A, Basu A, Tan H, et al. Measurements of global cell-mediated immunity in renal transplant recipients with BK virus reactivation. Am J Clin Pathol. 2008 Apr;129(4):587–91. doi: 10.1309/23YGPB1E758ECCFP. [DOI] [PubMed] [Google Scholar]
- 10.Lob S, Konigsrainer A. Role of IDO in organ transplantation: promises and difficulties. Int Rev Immunol. 2009;28(3-4):185–206. doi: 10.1080/08830180902989119. [DOI] [PubMed] [Google Scholar]
- 11.Jia L, Tian P, Ding C. Immunoregulatory effects of indoleamine 2, 3-dioxygenase in transplantation. Transpl Immunol. 2009 May;21(1):18–22. doi: 10.1016/j.trim.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Brandacher G, Margreiter R, Fuchs D. Clinical relevance of indoleamine 2,3-dioxygenase for alloimmunity and transplantation. Curr Opin Organ Transplant. 2008 Feb;13(1):10–5. doi: 10.1097/MOT.0b013e3282f3df26. [DOI] [PubMed] [Google Scholar]
- 13.Mulley WR, Nikolic-Paterson DJ. Indoleamine 2,3-dioxygenase in transplantation. Nephrology (Carlton) 2008 Jun;13(3):204–11. doi: 10.1111/j.1440-1797.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 14.King NJ, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007;39(12):2167–72. doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents of induction of indoleamine 2,3-dioxygenase in human peripheral blood monocytes. Biochem Biophys Res Commun. 1987 May 14;144(3):1147–53. doi: 10.1016/0006-291x(87)91431-8. [DOI] [PubMed] [Google Scholar]
- 16.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991 Aug;5(11):2516–22. [PubMed] [Google Scholar]
- 17.Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994 Sep;62(9):3705–11. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002 Dec;107(4):452–60. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999 May 3;189(9):1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998 Aug 21;281(5380):1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 21.Brandacher G, Cakar F, Winkler C, Schneeberger S, Obrist P, Bosmuller C, et al. Non-invasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney Int. 2007 Jan;71(1):60–7. doi: 10.1038/sj.ki.5002023. [DOI] [PubMed] [Google Scholar]
- 22.Dadhania D, Snopkowski C, Ding R, Muthukumar T, Lee J, Bang H, et al. Validation of noninvasive diagnosis of BK virus nephropathy and identification of prognostic biomarkers. Transplantation. 2010 Jul 27;90(2):189–97. doi: 10.1097/TP.0b013e3181e2a932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahdou I, Sadeghi M, Daniel V, Schenk M, Renner F, Weimer R, et al. Increased pretransplantation plasma kynurenine levels do not protect from but predict acute kidney allograft rejection. Hum Immunol. 2010 Aug 21; doi: 10.1016/j.humimm.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Holmes EW, Russell PM, Kinzler GJ, Reckard CR, Flanigan RC, Thompson KD, et al. Oxidative tryptophan metabolism in renal allograft recipients: increased kynurenine synthesis is associated with inflammation and OKT3 therapy. Cytokine. 1992 May;4(3):205–13. doi: 10.1016/1043-4666(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs D, Moller AA, Reibnegger G, Werner ER, Werner-Felmayer G, Dierich MP, et al. Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett. 1991 Jun;28(3):207–11. doi: 10.1016/0165-2478(91)90005-u. [DOI] [PubMed] [Google Scholar]
- 26.Schroecksnadel K, Winkler C, Werner ER, Sarcletti M, Romani N, Ebner S, et al. Interferon-gamma-mediated pathways and in vitro PBMC proliferation in HIV-infected patients. Biol Chem. 2009 Feb;390(2):115–23. doi: 10.1515/BC.2009.018. [DOI] [PubMed] [Google Scholar]
- 27.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010 May 19;2(32):32ra6. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray MF. Insights into therapy: tryptophan oxidation and HIV infection. Sci Transl Med. 2010 May 19;2(32):32ps23. doi: 10.1126/scitranslmed.3001082. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Youssef R, Baron PW, Sahney S, Weissman J, Baqai W, Franco E, et al. The impact of intercurrent EBV infection on ATP levels in CD4+ T cells of pediatric kidney transplant recipients. Pediatr Transplant. 2009 Nov;13(7):851–5. doi: 10.1111/j.1399-3046.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 30.Cheng O, Thuillier R, Sampson E, Schultz G, Ruiz P, Zhang X, et al. Connective tissue growth factor is a biomarker and mediator of kidney allograft fibrosis. Am J Transplant. 2006 Oct;6(10):2292–306. doi: 10.1111/j.1600-6143.2006.01493.x. [DOI] [PubMed] [Google Scholar]
- 31.Baczkowska T, Perkowska-Ptasinska A, Sadowska A, Lewandowski Z, Nowacka-Cieciura E, Cieciura T, et al. Serum TGF-beta1 correlates with chronic histopathological lesions in protocol biopsies of kidney allograft recipients. Transplant Proc. 2005 Mar;37(2):773–5. doi: 10.1016/j.transproceed.2005.01.030. [DOI] [PubMed] [Google Scholar]