Abstract
Hepatic lipase (HL) is a lipolytic enzyme that contributes to the regulation of plasma triglyceride (TG) levels. Elevated TG levels may increase the risk of developing coronary heart disease, and studies suggest that mutations in the HL gene may be associated with elevated TG levels and increased risk of coronary heart disease. Hepatic lipase facilitates the clearance of TG from the very low density lipoprotein (VLDL) pool, and this function is governed by the composition and quality of high density lipoprotein (HDL) particles. In humans, HL is a liver resident enzyme regulated by factors that release it from the liver and activate it in the bloodstream. HDL regulates the release of HL from the liver and HDL structure controls HL transport and activation in the circulation. Alterations in HDL-apolipoprotein composition can perturb HL function by inhibiting the release and activation of the enzyme. HDL structure may therefore affect plasma TG levels and coronary heart disease risk.
Triglycerides and Heart Disease
Elevated plasma triglyceride (TG) levels have been viewed as a risk factor for coronary heart disease (CHD) for more than a decade.1,2 Plasma TG levels are regulated by both synthesis and degradation of both very low density lipoprotein (VLDL) and chylomicron particles. The clearance of TG-rich lipoproteins from the circulation is controlled by the actions of lipoprotein lipase (LPL) and hepatic lipase (HL) and by the interlipoprotein exchange of TG by cholesteryl ester transfer protein. Lipoprotein lipase is the predominant TG lipase and is responsible for hydrolyzing TG in chylomicrons and VLDL, whereas HL is both a phospholipase and a TG lipase and plays an important role in HDL metabolism and in the conversion of VLDL to LDL.3 Single nucleotide polymorphisms (SNPs) in the HL gene (LIPC) have been shown to associate with plasma lipid concentrations and increased CHD risk.4,5 Hepatic lipase deficiency is a result of relatively rare LIPC mutations that give rise to a loss in circulating HL activity (due to impaired secretion or inactive enzyme) and cause an increase in TG-rich HDL and VLDL remnants and increased CHD risk.1,6 The common SNPs have a variety of functional consequences. SNPs in the LIPC gene can be associated with both increased or decreased plasma levels of HDL cholesterol and with varied levels of CHD risk.7,8 Thus, unique SNPs may consequently confer both pro- and anti-atherogenic phenotypic consequences. This may explain why larger and more comprehensive studies have not observed an association between LIPC mutations and CHD risk.9 Variable phenotypes may be due in part to secondary factors, such as environment, lifestyle, and hormone levels,10 but depend primarily on the functional consequences of SNPs on HL activity. SNPs in the LIPC gene may directly affect the TG-hydrolytic ability of HL and may indirectly affect HL by affecting the metabolism of HDL and its ability to control the function of HL in the circulation.
Hepatic Lipase and the Liver
Hepatic lipase is synthesized and secreted by the liver and binds to heparan sulfate proteoglycans (HSPG) on the cell surface of hepatocytes and endothelial cells.11,12 It has been known for more than 50 years that HSPG-bound lipases can be released into the bloodstream by heparin. Hahn showed in 1943 that intravenous heparin stimulates TG hydrolytic activity in lipemic serum.13 Although lipase activity is normally undetectable in human plasma, infusion of heparin increases both HL and LPL mass and activity in the bloodstream.14 Postheparin HL activity measurements have been used to reflect the functional levels of HL in an individual and are indirectly measured by subtracting NaCl-sensitive LPL activity from total postheparin lipase activity. Postheparin HL activity measurements are often elevated in hyperlipidemic patients and have been linked to an increased risk for developing CHD.15,16 This has led to the suggestion that HL may be a pro-atherogenic enzyme.17,18 High postheparin HL activity may also be related to CHD risk as an indicator of reduced lipolytic function. Increased postheparin HL activity may represent an elevated storage pool of inactive HL in the liver, which results from defective release and activation of the enzyme.19–22 Cell surface HSPG-bound HL is a catalytically inactive enzyme, and HDL functions to mobilize and activate this liver-resident pool of HL.19
Displacement of HSPG-Bound HL
In humans, HL is found primarily in association with cell-surface HSPG on hepatocytes and endothelial cells of the liver, and it is therefore considered to be a liver resident enzyme.23 Specific residues in the HL protein regulate the association of HL with HSPG.24,25 Mapping studies using peptide arrays have identified two HL-heparin binding domains, one at the N-terminus (R310, K312, K314, R315) and another at the C-terminus (R473, K474, R476).25 In rodents, HL is also synthesized in the liver, but predominantly is found circulating in the bloodstream.26,27 Murine HL appears to be more readily displaced from cell-surface HSPG, because of differences in the C-terminal amino acid composition of the enzyme.27 Human HL can be released or liberated from cell-surface HSPG by both heparin and HDL. Some findings suggest that heparin interacts directly with the TG lipases and/or competes for binding sites on cell-surface HSPG.28 Findings from other studies indicate that heparin can act through protein kinase and calcium signaling pathways to stimulate HL release.29 HDL-dependent HL displacement is regulated by interactions between HL and HDL and is affected by both the lipid and the apolipoprotein composition of HDL.20–22
HDL composition directly affects the displacement of HL from cell-surface HSPG.20,21 Ramsamy et al20 showed that different subclasses of HDL have unique abilities to displace HL. The larger, more buoyant HDL2 fractions were more effective at displacing HSPG-bound HL than the smaller, dense HDL3 fractions. Rouhani et al21 showed that the various lipids in HDL have unique effects on HL displacement. Increases in HDL-TG and phospholipid content directly inhibited HL displacement from the cell surface, whereas changes in the other lipid components of HDL had little effect on HL release. More recently, HDL and serum isolated from postprandial subjects were shown to promote increased HL displacement, relative to samples from fasted subjects.22 The study showed that, even though postprandial HDL is TG-enriched, the lipoprotein is deficient in ApoE and is more effective than fasting HDL at binding to and displacing cell-surface HL.22
Hepatic lipase displacement appears to be controlled by HDL apolipoproteins and is stimulated by the ApoA-II content of HDL.21 ApoA-II increases the release of HL from HSPG by enhancing the association of HL with HDL, and this increased association promotes an inhibition of HL activity.30,31 Conversely, HL displacement is inhibited by HDL-ApoE.22 Young et al22 showed that HDL isolated from female subjects was significantly better at displacing HL from cell surface HSPG, relative to HDL from male subjects. HDL isolated from the plasma of women also contained less ApoE, compared with that isolated from men. The study identified an inverse relationship between HDL-ApoE content and the amount of circulating HL in the bloodstream.22 Increased ApoE content on HDL results in decreased HL release (Figure 1). Treatment of HDL with monoclonal ApoE antibodies, directed against epitopes in the glutamic acid-enriched N-terminus of ApoE, resulted in greater HL displacement,22 which suggests that the binding of HL to HDL may be sensitive to ApoE-dependent electrostatic properties of the lipoprotein. Other work has shown that HL activity is also dependent on electrostatic events that regulate the association of HL with HDL.31,32
Figure 1.
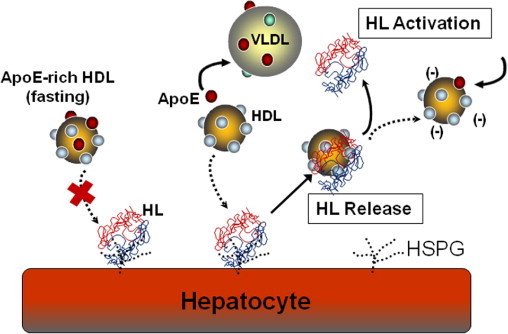
HDL regulates the release and activation of hepatic lipase (HL). The liver is a storage depot for catalytically inactive HL that is anchored to cell-surface heparan sulfate proteoglycans (HSPG). HDL binds to HL and releases the enzyme into the circulation. Fasting, ApoE-rich HDL is ineffective at releasing HL from cell surface HSPG. During a postprandial response, HDL loses ApoE to VLDL and the ApoE-deficient HDL is more efficient at releasing HL from the cell surface. HDL compositional changes can then release HDL-bound HL and activate the catalytic activity of the circulating enzyme. HDL therefore plays an important role in the mobilization and activation of HL.
Apolipoproteins are exchanged between HDL and the TG-rich lipoproteins, such as VLDL and intermediate density lipoprotein (IDL), during a postprandial lipemic event.33,34 Notably, ApoE and ApoCs are transferred from HDL to VLDL, where they act as lipolytic cofactors and receptor ligands.33 HDL is therefore a storage depot for ApoE in the fasting state. A few hours after a meal, when plasma TG levels are high, ApoE moves from HDL to the TG-rich lipoproteins.33,35 This decrease in HDL-ApoE content appears to initiate the mobilization of HL from the hepatocyte cell surface to the vascular compartment (Figure 1), where the enzyme can then act to hydrolyze circulating TG.22 At the end of the lipemic response, ApoE returns to the HDL pool and blocks the ability of HDL to release HL from the liver.
Regulation of HL Lipolytic Activity
In humans, there appear to be two inactive pools of HL, one that is HSPG-anchored in the liver and one that is HDL-bound and circulating in the bloodstream as an inactive enzyme. HDL therefore regulates HL activation in a two-step process, wherein HDL first binds and displaces HL from cell surface HSPG, and then the HDL dissociates and activates the circulating enzyme (Figure 1). Under fasting conditions, HL in the circulation appears to be catalytically inactive. Hepatic lipase activity can be detected in the plasma only after the enzyme is released from the liver by infusions of heparin.14 Although ApoA-I and HDL are also able to liberate HL from cell surface HSPG, the association of HL with HDL directly inhibits HL activity.19,31,32 Hepatic lipase is inactivated by its association with HDL particles containing both ApoA-I and ApoA-II.30,31,36
Hepatic lipase activity is stimulated by the dissociation of HL from HDL (Figure 1) and controlled by lipoprotein electrostatic properties.31,32 Enrichment of HDL or serum with free fatty acid or anionic phospholipids (such as phosphatidylinositol, phosphatidic acid, or phosphatidylserine) increases the net negative charge on HDL and stimulates VLDL-TG hydrolysis by HL.32 An increase in HDL net negative charge was shown to reduce the binding of HL to HDL and to increase HL hydrolytic activity for all lipoprotein substrates. Hepatic lipase activity is therefore inhibited by the electrostatic-dependent association of HL with HDL.19,32 ApoA-II has been shown to increase the association of HL with HDL and to directly inhibit TG hydrolytic activity.30,31,36 ApoE has the opposite effect. ApoE blocks the association of HL with HDL,22 but stimulates the HDL lipolytic activity of HL.37 Women have been shown to have reduced plasma ApoE levels and increased circulating HL, relative to men.22 Women also have a reduced postheparin HL activity, which has been thought to be a consequence of an inhibitory effect of estrogen on HL transcription.38 Reduced postheparin activity in women may therefore be in part a consequence of lower ApoE levels and reduced HL activation.22 ApoE has been shown to directly interact with ApoA-II39 and, as such, may block ApoA-II-dependent association of HL with HDL.31 ApoA-II may therefore control HL displacement and activation; its action is modulated by the amount of ApoE that can reside on the HDL particle surface.
In the circulation, HDL remains associated with HL, to keep the enzyme in an inactive state, until hydrolytic activity is required. A tight regulation of HL lipolytic activity by HDL would be expected, given that HL is a phospholipase and potentially lytic to cell membranes. The liberation of HL by HDL from the cell surface therefore primes HL for its hydrolytic function by releasing the anchored enzyme and enabling HL to gain access to circulating substrate. Increased lipase shuttling between substrate molecules has been shown to stimulate most interfacial lipolytic enzymes, including HL.40 Higher circulating levels of HDL-bound, inactive HL may be important to TG clearance. HDL isolated by sequential density ultracentrifugation from the plasma of female normolipidemic subjects was shown to contain significant HL mass.22 In contrast, HDL isolated from normolipidemic males and hyperlipidemic patients contains much less HL protein. An increased vascular pool of HL in women may therefore contribute to the reduced magnitude of postprandial lipemia that is often observed in women, relative to men.41 Increased HDL-bound HL in the circulation may also affect the remodeling of HDL, because HDL2 formation has been shown to be increased in subjects who can clear alimentary TG more rapidly.42
Regulation of HL Secretion from the Liver
Because HDL can liberate HL from the cell-surface HSPG, it follows that hepatic HDL secretion would be expected to affect the release of HL from the liver. This view has been confirmed in studies in primary human hepatocytes and HepG2 cells, which showed that factors that increase ApoA-I/HDL secretion from hepatocytes also increase HL secretion (Figure 2).43 Chatterjee et al43 showed that overexpression of ApoA-I in HepG2 cells directly stimulated HL release from the cell surface. Conversely, a knockdown of ApoA-I expression with siRNA decreased HL release into the medium. Therefore, newly secreted HDL may be able to bind and displace cell surface HL (Figure 2). Alternatively, HL may associate with ApoA-I/HDL complexes intracellularly and be cosecreted with HDL.
Figure 2.
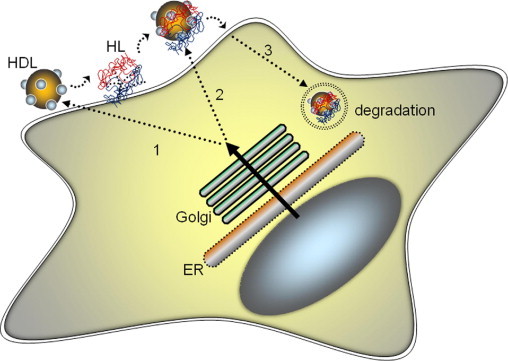
HDL and hepatic lipase (HL) secretion are coregulated. HDL secretion regulates HL release from the liver through three potential mechanisms: 1) newly secreted HDL binds and displaces cell surface HL, 2) HDL and HL associate intracellularly and are cosecreted, and/or 3) HDL and HL secretion are coregulated by plasma membrane reuptake and degradation pathways. ER, endoplasmic reticulum.
HDL and HL secretion may also be affected by membrane reuptake and degradative pathways. Treatment of HepG2 cells and primary human hepatocytes with compounds that block ApoA-I retroendocytosis, also affect HL release. Linoleic acid phospholipids (PL), such as dilinoleoylphosphatidylcholine, increase hepatic ApoA-I secretion by threefold and promote a twofold increase in HL release.43 Phospholipid treatment does not appear to affect HL transcription, because PLs have no effect on steady-state mRNA levels.43 Instead, PLs stimulate PPARα expression44 and inhibit membrane nucleotide signaling events on the cell surface to block retroendocytic degradative pathways.45 The data suggest that PLs block membrane recycling pathways that promote the reuptake and degradation of cell surface proteins such as HL (Figure 2). Hepatic lipase degradation has been shown to be rate-limiting to HL secretion, and associated with the dimerization of the enzyme.46 Doolittle and colleagues46 showed that, when HL does not form an active dimeric complex, large amounts of monomeric HL accumulate in the cell and are rapidly degraded. These investigators later showed that both the maturation and homodimerization of HL and lipoprotein lipase may be governed by a chaperone protein in the endoplasmic reticulum, called lipase maturation factor 1.47
Hepatic lipase may be secreted from hepatocytes as an inactive enzyme. Stimulants of HL secretion can increase HL mass in the hepatocyte medium by two-fold, but have no effect on HL activity in the medium.43 This characteristic, which may be important to the regulation of HL phospholipase activity, is due to an inhibitory effect of the specific species of HDL with which HL is associated in the medium. As in the circulation, HL in hepatocyte medium is primarily associated with larger HDL complexes containing both ApoA-I and ApoA-II. Phospholipid treatment increases the secretion of both ApoA-I and ApoA-II43,45 and, as shown by Boucher et al,31 the association of HL with ApoA-II-enriched HDL directly inhibits HL hydrolytic activity.
Conclusion
An inverse relationship exists between blood TG and HDL levels. Low HDL levels are often associated with high TG in both men and women, and the combination of low HDL and high TG levels is related to an increased risk of developing CHD.48 HDL is a repository for regulatory apolipoproteins and alterations in HDL apolipoprotein composition can affect TG metabolism by influencing the function of both HL and LPL. Mutations in the LIPC gene may have a direct effect on HL function, or may indirectly affect lipolysis by causing reduced or dysfunctional HDL particles.7,8,10 Stimulants of hepatic HDL production may therefore act through cofactor pathways to stimulate lipolytic enzymes and enhance TG clearance.43 This may in part explain why drugs that increase HDL levels, such as the fibrates and niacin, also reduce plasma TG levels.49,50
Footnotes
Supported by the Heart and Stroke Foundation of Ontario and by a graduate scholarship from the Ontario Graduate Scholarship Program (OGS) (C.C.).
References
- 1.Davignon J., Cohn J.S. Triglycerides: a risk factor for coronary heart disease. Atherosclerosis. 1996;124(Suppl):S57–S64. doi: 10.1016/0021-9150(96)05858-3. [DOI] [PubMed] [Google Scholar]
- 2.Sarwar N., Sandhu M.S., Ricketts S.L., Butterworth A.S., Di Angelantonio E., Boekholdt S.M., Ouwehand W., Watkins H., Samani N.J., Saleheen D., Lawlor D., Reilly M.P., Hingorani A.D., Talmud P.J., Danesh J., Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg I.J. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 4.Zambon A., Deeb S.S., Pauletto P., Crepaldi G., Brunzell J.D. Hepatic lipase: a marker for cardiovascular disease risk and response to therapy. Curr Opin Lipidol. 2003;14:179–189. doi: 10.1097/00041433-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Baroni M.G., Berni A., Romeo S., Arca M., Tesorio T., Sorropago G., Di Mario U., Galton D.J. Genetic study of common variants at the Apo E, Apo AI, Apo CIII, Apo B, lipoprotein lipase (LPL), and hepatic lipase (LIPC) genes and coronary artery disease (CAD): variation in LIPC gene associates with clinical outcomes in patients with established CAD. BMC Med Genet. 2003;4:8–15. doi: 10.1186/1471-2350-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connelly P.W., Hegele R.A. Hepatic lipase deficiency. Crit Rev Clin Lab Sci. 1998;35:547–572. doi: 10.1080/10408369891234273. [DOI] [PubMed] [Google Scholar]
- 7.McCaskie P.A., Cadby G., Hung J., McQuillan B.M., Chapman C.M., Carter K.W., Thompson P.L., Palmer L.J., Beilby J.P. The C-480T hepatic lipase polymorphism is associated with HDL-C but not with risk of coronary heart disease. Clin Genet. 2006;70:114–121. doi: 10.1111/j.1399-0004.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 8.Hodoglugil U., Williamson D.W., Mahley R.W. Polymorphisms in the hepatic lipase gene affect plasma HDL-cholesterol levels in a Turkish population. J Lipid Res. 2010;51:422–430. doi: 10.1194/jlr.P001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teslovich T.M., Musunuru K., Smith A.V., Edmondson A.C., Stylianou I.M., Koseki M. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feitosa M.F., Myers R.H., Pankow J.S., Province M.A., Borecki I.B. LIPC variants in the promoter and intron 1 modify HDL-C levels in a sex-specific fashion. Atherosclerosis. 2009;204:171–177. doi: 10.1016/j.atherosclerosis.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen H., van Berkel T.J., Hülsmann W.C. Binding of liver lipase to parenchymal and non-parenchymal rat liver cells. Biochem Biophys Res Commun. 1978;85:148–152. doi: 10.1016/s0006-291x(78)80022-9. [DOI] [PubMed] [Google Scholar]
- 12.Kuusi T., Nikklä E.A., Virtanen I., Kinnunen P.K. Localization of the heparin-releasable lipase in situ in the rat liver. Biochem J. 1979;181:245–246. doi: 10.1042/bj1810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn P.F. Abolishment of alimentary lipemia following injection of heparin. Science. 1943;98:19–20. doi: 10.1126/science.98.2531.19. [DOI] [PubMed] [Google Scholar]
- 14.Olivecrona T., Bengtsson-Olivecrona G., Ostergaard P., Liu G., Chevreuil O., Hultin M. New aspects on heparin and lipoprotein metabolism. Haemostasis. 1993;23(Suppl 1):150–160. doi: 10.1159/000216924. [DOI] [PubMed] [Google Scholar]
- 15.Kuusi T., Ehnholm C., Viikari J., Härkönen R., Vartiainen E., Puska P., Taskinen M.R. Postheparin plasma lipoprotein and hepatic lipase are determinants of hypo- and hyperalphalipoproteinemia. J Lipid Res. 1989;30:1117–1126. [PubMed] [Google Scholar]
- 16.Patsch J. Influence of lipolysis on chylomicron clearance and HDL cholesterol levels. Eur Heart J. 1998;19(Suppl H):H2–H6. [PubMed] [Google Scholar]
- 17.Santamarina-Fojo S., Haudenschild C., Amar M. The role of hepatic lipase in lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol. 1998;9:211–219. doi: 10.1097/00041433-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Jansen H., Verhoeven A.J., Sijbrands E.J. Hepatic lipase: a pro- or anti-atherogenic protein? J Lipid Res. 2002;43:1352–1362. doi: 10.1194/jlr.r200008-jlr200. [DOI] [PubMed] [Google Scholar]
- 19.Ramsamy T.A., Neville T.A., Chauhan B.M., Aggarwal D., Sparks D.L. Apolipoprotein A-I regulates lipid hydrolysis by hepatic lipase. J Biol Chem. 2000;275:33480–33486. doi: 10.1074/jbc.M005436200. [DOI] [PubMed] [Google Scholar]
- 20.Ramsamy T.A., Boucher J., Brown R.J., Yao Z., Sparks D.L. HDL regulates the displacement of hepatic lipase from cell surface proteoglycans and the hydrolysis of VLDL triacylglycerol. J Lipid Res. 2003;44:733–741. doi: 10.1194/jlr.M200339-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Rouhani N., Young E., Chatterjee C., Sparks D.L. HDL composition regulates displacement of cell surface-bound hepatic lipase. Lipids. 2008;43:793–804. doi: 10.1007/s11745-008-3214-1. [DOI] [PubMed] [Google Scholar]
- 22.Young E.K., Chatterjee C., Sparks D.L. HDL-ApoE content regulates the displacement of hepatic lipase from cell surface proteoglycans. Am J Pathol. 2009;175:448–457. doi: 10.2353/ajpath.2009.080989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanan D.A., Fan J., Bensadoun A., Taylor J.M. Hepatic lipase is abundant on both hepatocyte and endothelial cell surfaces in the liver. J Lipid Res. 1997;38:1002–1013. [PubMed] [Google Scholar]
- 24.Hill J.S., Yang D., Nikazy J., Curtiss L.K., Sparrow J.T., Wong H. Subdomain chimeras of hepatic lipase and lipoprotein lipase: Localization of heparin and cofactor binding. J Biol Chem. 1998;273:30979–30984. doi: 10.1074/jbc.273.47.30979. [DOI] [PubMed] [Google Scholar]
- 25.Yu W., Hill J.S. Mapping the heparin-binding domain of human hepatic lipase. Biochem Biophys Res Commun. 2006;343:659–665. doi: 10.1016/j.bbrc.2006.02.175. [DOI] [PubMed] [Google Scholar]
- 26.Schoonderwoerd K., Verhoeven A.J.M., Jansen H. Rat liver contains a limited number of binding sites for hepatic lipase. Biochem J. 1994;302:717–722. doi: 10.1042/bj3020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown R.J., Schultz J.R., Ko K.W., Hill J.S., Ramsamy T.A., White A.L., Sparks D.L., Yao Z. The amino acid sequences of the carboxyl termini of human and mouse hepatic lipase influence cell surface association. J Lipid Res. 2003;44:1306–1314. doi: 10.1194/jlr.M200374-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Kolset S.O., Salmivirta M. Cell surface heparan sulfate proteoglycans and lipoprotein metabolism. Cell Mol Life Sci. 1999;56:857–870. doi: 10.1007/s000180050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tagashira H., Nakahigashi S., Kerakawati R., Motoyashiki T., Morita T. Involvement of Ca2+/calmodulin-dependent protein kinase II in heparin-stimulated release of hepatic lipase activity from rat hepatocytes. Biol Pharm Bull. 2005;28:409–412. doi: 10.1248/bpb.28.409. [DOI] [PubMed] [Google Scholar]
- 30.Mowri H.O., Patsch, Gotto A.M., Jr, Patsch W. Apolipoprotein A-II influences the substrate properties of human HDL2 and HDL3 for hepatic lipase. Arterioscler Thromb Vasc Biol. 1996;16:755–762. doi: 10.1161/01.atv.16.6.755. [DOI] [PubMed] [Google Scholar]
- 31.Boucher J., Ramsamy T.A., Braschi S., Sahoo D., Neville T.A., Sparks D.L. Apolipoprotein A-II regulates HDL stability and affects hepatic lipase association and activity. J Lipid Res. 2004;45:849–858. doi: 10.1194/jlr.M300431-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Boucher J.G., Nguyen T., Sparks D.L. Lipoprotein electrostatic properties regulate hepatic lipase association and activity. Biochem Cell Biol. 2007;85:696–708. doi: 10.1139/o07-137. [DOI] [PubMed] [Google Scholar]
- 33.Blum C.B. Dynamics of apolipoprotein E metabolism in humans. J Lipid Res. 1982;23:1308–1316. [PubMed] [Google Scholar]
- 34.Murdoch S.J., Breckenridge W.C. Influence of lipoprotein lipase and hepatic lipase on the transformation of VLDL and HDL during lipolysis of VLDL. Atherosclerosis. 1995;118:193–212. doi: 10.1016/0021-9150(95)05606-8. [DOI] [PubMed] [Google Scholar]
- 35.Murdoch S.J., Breckenridge W.C. Effect of lipid transfer proteins on lipoprotein lipase induced transformation of VLDL and HDL. Biochim Biophys Acta. 1996;1303:222–232. doi: 10.1016/0005-2760(96)00105-1. [DOI] [PubMed] [Google Scholar]
- 36.Mowri H.O., Patsch W., Smith L.C., Gotto A.M., JR, Patsch Different reactivities of high density lipoprotein2 subfractions with hepatic lipase. J Lipid Res. 1992;33:1269–1279. [PubMed] [Google Scholar]
- 37.Hime N.J., Drew K.J., Hahn C., Barter P.J., Rye K.A. Apolipoprotein E enhances hepatic lipase-mediated hydrolysis of reconstituted high-density lipoprotein phospholipid and triacylglycerol in an isoform-dependent manner. Biochemistry. 2004;43:12306–12314. doi: 10.1021/bi036305i. [DOI] [PubMed] [Google Scholar]
- 38.Deeb S.S., Zambon A., Carr M.C., Ayyobi A.F., Brunzell J.D. Hepatic lipase and dyslipidemia: interactions among genetic variants, obesity, gender, diet. J Lipid Res. 2003;44:1279–1286. doi: 10.1194/jlr.R200017-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Weisgraber K.H., Mahley R.W. Apoprotein (E–A-II) complex of human plasma lipoproteins: I. Characterization of this mixed disulfide and its identification in a high density lipoprotein subfraction. J Biol Chem. 1978;253:6281–6288. [PubMed] [Google Scholar]
- 40.Jain M.K., Berg O.G. The kinetics of interfacial catalysis by phospholipase A2 and regulation of interfacial activation: hopping versus scooting. Biochim Biophys Acta. 1989;1002:127–156. doi: 10.1016/0005-2760(89)90281-6. [DOI] [PubMed] [Google Scholar]
- 41.Couillard C., Bergeron N., Prud'homme D., Bergeron J., Tremblay A., Bouchard C., Mauriège P., Després J.P. Gender difference in postprandial lipemia: importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 1999;19:2448–2455. doi: 10.1161/01.atv.19.10.2448. [DOI] [PubMed] [Google Scholar]
- 42.Patsch, Karlin J.B., Scott L.W., Smith L.C., Gotto A.M., Jr Inverse relationship between blood levels of high density lipoprotein subfraction 2 and magnitude of postprandial lipemia. Proc Natl Acad Sci USA. 1983;80:1449–1453. doi: 10.1073/pnas.80.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee C., Young E.K., Pussegoda K.A., Twomey E.E., Pandey N.R., Sparks D.L. Hepatic high-density lipoprotein secretion regulates the mobilization of cell-surface hepatic lipase. Biochemistry. 2009;48:5994–6001. doi: 10.1021/bi802009e. [DOI] [PubMed] [Google Scholar]
- 44.Pandey N.R., Renwick J., Misquith A., Sokoll K., Sparks D.L. Linoleic acid-enriched phospholipids act through peroxisome proliferator-activated receptors alpha to stimulate hepatic apolipoprotein A-I secretion. Biochemistry. 2008;47:1579–1587. doi: 10.1021/bi702148f. [DOI] [PubMed] [Google Scholar]
- 45.Pandey N.R., Renwick J., Rabaa S., Misquith A., Kouri L., Twomey E., Sparks D.L. An induction in hepatic HDL secretion associated with reduced ATPase expression. Am J Pathol. 2009;175:1777–1787. doi: 10.2353/ajpath.2009.090082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben-Zeev O., Doolittle M.H. Maturation of hepatic lipase: Formation of functional enzyme in the endoplasmic reticulum is the rate-limiting step in its secretion. J Biol Chem. 2004;279:6171–6181. doi: 10.1074/jbc.M310051200. [DOI] [PubMed] [Google Scholar]
- 47.Doolittle M.H., Ehrhardt N., Peterfy M. Lipase maturation factor 1: structure and role in lipase folding and assembly. Curr Opin Lipidol. 2010;21:198–203. doi: 10.1097/MOL.0b013e32833854c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castelli W.P., Garrison R.J., Wilson P.W., Abbott R.D., Kalousdian S., Kannel W.B. Incidence of coronary heart disease and lipoprotein cholesterol levels: The Framingham Study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 49.Staels B., Dallongeville J., Auwerx J., Schoonjans K., Leitersdorf E., Fruchart J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 50.Kamanna V.S., Kashyap M.L. Nicotinic acid (niacin) receptor agonists: will they be useful therapeutic agents? Am J Cardiol. 2007;100:S53–S61. doi: 10.1016/j.amjcard.2007.09.080. [DOI] [PubMed] [Google Scholar]


