Abstract
Vascular adhesion protein-1 (VAP-1) contributes to inflammatory and angiogenic diseases, including cancer and age-related macular degeneration. It is expressed in blood vessels and contributes to inflammatory leukocyte recruitment. The cytokines IL-1β and vascular endothelial growth factor A (VEGF-A) modulate angiogenesis, lymphangiogenesis, and leukocyte infiltration. The lymphatic endothelium expresses intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, which facilitate leukocyte transmigration into the lymphatic vessels. However, whether lymphatics express VAP-1 and whether they contribute to cytokine-dependent lymph- and angiogenesis are unknown. We investigated the role of VAP-1 in IL-1β– and VEGF-A–induced lymph- and angiogenesis using the established corneal micropocket assay. IL-1β increased VAP-1 expression in the inflamed cornea. Our in vivo molecular imaging revealed significantly higher VAP-1 expression in neovasculature than in the preexisting vessels. VAP-1 was expressed in blood but not lymphatic vessels in vivo. IL-1β–induced M2 macrophage infiltration and lymph- and angiogenesis were blocked by VAP-1 inhibition. In contrast, VEGF-A–induced lymph- and angiogenesis were unaffected by VAP-1 inhibition. Our results indicate a key role for VAP-1 in lymph- and angiogenesis-related macrophage recruitment. VAP-1 might become a new target for treatment of inflammatory lymph- and angiogenic diseases, including cancer.
Vascular adhesion protein-1 (VAP-1) is encoded by the AOC3 gene. VAP-1 is expressed in pericytes and vascular endothelium and is involved in leukocyte extravasation to inflamed tissues.1 Recently, we reported the expression of VAP-1 in the human eye2 and the critical role it plays in ocular inflammatory diseases, such as uveitis,3 age-related macular degeneration,4 and diabetic retinopathy.5
IL-1β, a multipotent cytokine, is critically involved in the acute inflammatory response, activation and chemotaxis of inflammatory and antigen-presenting cells, up-regulation of adhesion molecules, and neovascularization.6 IL-1β induces lymph- and angiogenesis and leukocyte infiltration.7–9 Macrophage activation and infiltration are prerequisites for IL-1β–induced lymph- and angiogenesis.7,8
Leukocyte-endothelial interaction and accumulation into inflammatory sites are accomplished by adhesion molecules, such as integrins.10 Leukocyte recruitment is critical for lymph- and angiogenesis.8,11,12 VAP-1 inhibition reduces pathological angiogenesis by diminishing macrophage infiltration5 but is not involved in physiologic vessel formation.13 VAP-1 contributes to tumor angiogenesis but not lymphangiogenesis through its impact on leukocytes recruitment.13
Recent work shows that polarization of mononuclear phagocytes into classically activated (M1) and alternatively activated (M2) macrophages is a decisive factor in various diseases and that M2 macrophages promote angiogenesis.14 M2 macrophage differentiation is induced by IL-4.14 Generally, M2 macrophages are found in noninflamed tissues, with the exception of tumors, where they contribute to inflammatory angiogenesis and to the tumor's evasion from immunity.14 However, whether M2 macrophages contribute to inflammatory angiogenesis in nontumor conditions is unknown.
During inflammation, leukocytes and plasma extravasate into the extracellular matrix, requiring drainage of excess interstitial fluid and debris. Thus, it appears logical that lymphangiogenesis is induced by inflammation. During lymphangiogenesis, lymphatic endothelial cells proliferate and grow into the stroma. The extravasated leukocytes enter lymphatic vessels and traffic into the lymph nodes.15 Intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1) on the lymphatic endothelium contribute to leukocyte migration into lymphatic vessels.16 However, whether VAP-1 is expressed in lymphatics and whether it contributes to lymphangiogenesis is unknown.2,5,17,18
Materials and Methods
Corneal Micropocket Assay in Mice
Male, 6- to 10-week-old BALB/cN mice (BALB/c; Taconic, Hudson, NY) were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). Polyhydroxyethylmethacrylic pellets (0.3 μL, P3932; Sigma Chemical Co., St. Louis, MO) containing 30 ng of IL-1β (401-ML; R&D Systems, Minneapolis, MN) or 200 ng of vascular endothelial growth factor A (VEGF-A) (293-VE; R&D Systems) were prepared and implanted into the corneas. IL-1β or VEGF-A pellets were positioned approximately 1 mm from the corneal limbus. After implantation, bacitracin ophthalmic ointment (E. Fougera & Co., Melville, NY) was applied to each eye to prevent infection. A specific VAP-1 inhibitor, U-V002 (0.3 mg/kg) (R-Tech Ueno, Tokyo, Japan), was injected i.p. daily for 5 days after implantation. On day 6 after implantation, digital images of the corneal vessels were obtained using OpenLab software version 2.2.5 (Improvision Inc., Lexington, MA) with standardized illumination and contrast.
Whole-Mount Immunofluorescence
The animals' eyes were enucleated and fixed with 4% paraformaldehyde for 30 minutes at 4°C. For whole-mount preparation, the corneas were microsurgically exposed by removing other portions of the eye. Radial cuts were then made in the cornea. Tissues were washed with PBS three times for 5 minutes and then placed in methanol for 20 minutes. Tissues were incubated overnight at 4°C with anti-mouse CD31 monoclonal antibody (5 μg/mL, 550274; BD Pharmingen, San Diego, CA) and anti-mouse LYVE-1 antibody (4 μg/mL, 103-PA50AG; RELIATech GmbH, Braunschweig, Germany) diluted in PBS containing 10% goat serum and 1% Triton X-100. Tissues were washed four times for 20 minutes in PBS followed by incubation with AlexaFluor488 goat anti-rat IgG (20 μg/mL, A11006; Invitrogen) and AlexaFluor647 goat anti-rabbit IgG (20 μg/mL, A21244; Invitrogen) overnight at 4°C. Corneal flat mounts were prepared on glass slides using a mounting medium (TA-030-FM, Mountant Permafluor; Lab Vision Corporation, Fremont, CA). The flat mounts were examined by fluorescence microscopy and digital images were recorded using OpenLab software (version 2.2.5; Improvision Inc.) with standardized illumination and contrast. Lymph- and angiogenesis were quantitatively analyzed using Scion Image software (version 4.0.2; Scion Corp., Frederick MD).
Preparation of Conjugated Microspheres
Carboxylated fluorescent or nonfluorescent microspheres (MSs; 2 μm, Polysciences Inc., Warrington, PA) were covalently conjugated to protein G (Sigma), using a carbodiimide-coupling kit (Polysciences Inc.).19–23 Anti-mouse VAP-1 antibody (HM1094; Hycult Biotechnology b.v., Uden, the Netherlands) or control goat IgG (AB-108-C; R&D Systems) was incubated with the MSs at 0.4 mg/mL overnight at room temperature. The MSs were washed in PBS with 1% bovine serum albumin before use in vivo. A total of 6 × 107 fluorescent MSs were injected in each mouse.
Immunohistochemistry
The eyes were harvested and snap-frozen in optimal cutting temperature compound (Sakura Finetechnical, Torrance, CA). Sections (10 μm) were prepared, air-dried, and fixed in ice-cold acetone for 10 minutes. The sections were blocked with 3% nonfat dried milk bovine working solution (M7409; Sigma) and stained with anti-mouse CD11b (1:50, 550282; BD Pharmingen), anti-mouse Gr-1 (1:100, 553123; BD Pharmingen), anti-mouse F4/80 (1:100, MCA497R; Serotec, Raleigh, NC), anti-mouse CD206 monoclonal antibody (2 μg/mL, ab8918; Abcam, Cambridge, MA), anti-mouse CD31 monoclonal antibody (1:50, 550274; BD Pharmingen), anti-mouse LYVE-1 antibody (1 μg/mL, 103-PA50AG; RELIATech GmbH), anti–IL-4 antibody (0.1 μg/mL, ab11524; Abcam), and anti-VAP-1 antibody (4 μg/mL, sc-13743; Santa Cruz Biotechnology, Santa Cruz, CA) or anti–VAP-1 antibody (2 μg/mL, HM1094; Hycult Biotechnology b.v.). After an overnight incubation, sections were washed and stained for 20 minutes with Alexa Fluor488 goat anti-rabbit IgG (10 μg/mL, A11034; Invitrogen), Alexa Fluor488 donkey anti-goat IgG (10 μg/mL, A-11055; Invitrogen), and Alexa Fluor647 goat anti-rabbit IgG (10 μg/mL, A21244; Invitrogen).
Cell Culture
Human umbilical vein endothelial cells (HUVECs) (C-12200; PromoCell, Heidelberg, Germany) or human lymphatic endothelial cells (HLECs) (2500; ScienCell Research Laboratories, Carlsbad, CA) were routinely cultured in endothelial cell growth medium-2 KIT (C-22111; PromoCell) or supplemented with 2% fetal bovine serum (FBS) endothelial cell medium (1001; ScienCell), respectively, in a humidified incubator under 5% CO2 at 37°C.
Western Blot Analysis
At day 3 after 30 ng IL-1β implantation, conjunctiva and cornea were harvested and lysed in a mammalian cell lysis kit (MCL1; Sigma Chemical Co.). Conjunctiva, cornea, and heart in normal BALB/c mice were also harvested and lysed. After rinsing with ice-cold PBS, the cells were lysed in a mammalian cell lysis kit. Lysates were subjected to SDS polyacrylamide gel electrophoresis and transferred to Immobilon membranes (Millipore, Bedford, MA). Blots were incubated with anti–VAP-1 (1:200, sc-28642; Santa Cruz Biotechnology) or anti–β-tubulin (1:1000, ab11308; Abcam) and visualized with a secondary antibody coupled to horseradish peroxidase (Amersham) and enhanced chemiluminescence system.
Flow Cytometry
After culture for 12 hours in 1% FBS medium, HUVECs or HLECs were stimulated with 1 ng/mL of IL-1β or 20 ng/mL of VEGF-A for 24 hours at 37°C. The cells were harvested with 0.05% trypsin and washed with PBS. Subconfluent HUVECs or HLECs were harvested with 0.05% trypsin and washed with PBS. To check LYVE-1 expression, cells were resuspended in PBS at a concentration of 107 cells/mL. Subsequently, the cell suspension was incubated with anti-human VAP-1 monoclonal antibodies (2 μg/mL, ab36993; Hycult Biotechnology) or anti-human LYVE-1 antibodies (10 μg/mL, HM2213; Abcam) for 15 minutes at room temperature. Cells were then washed twice with PBS and analyzed in a flow cytometer. As a control, a nonimmune monoclonal antibody was used at the same concentration.
Statistical Analysis
All values are expressed as mean ± SEM. Data were analyzed by Student's t-test. Differences between the experimental groups were considered statistically significant or highly significant, when P < 0.05 or P < 0.01, respectively.
Results
VAP-1 Inhibition Reduces IL-1β–Induced Corneal Angiogenesis
To investigate the role of VAP-1 in inflammatory angiogenesis, we implanted IL-1β in the mouse cornea and quantified the amount of angiogenesis at various time points after implantation. IL-1β induced substantial corneal angiogenesis compared with PBS-implanted controls (Figure 1A), in line with previous reports.8,9,24 Six days after implantation, the area of IL-1β–induced angiogenesis in corneas of VAP-1 inhibitor–treated mice was significantly smaller than in vehicle-treated controls (Figure 1, A and B).
Figure 1.
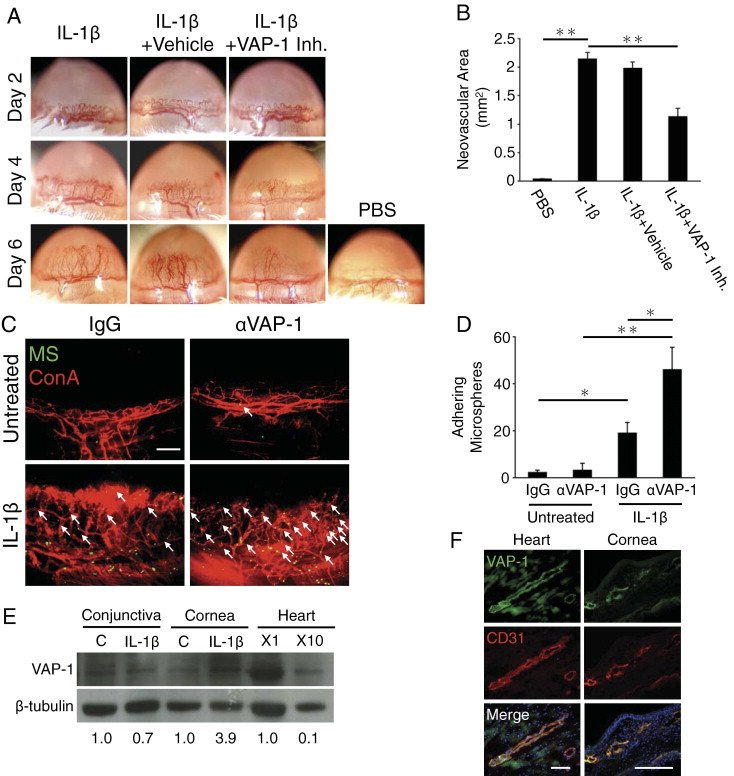
VAP-1 up-regulation and impact of its inhibition in IL-1β–induced angiogenesis. A: Photomicrographs of IL-1β–implanted corneas of BALB/c mice with and without VAP-1 inhibitor treatment 2, 4, and 6 days after implantation. B: Quantitation of the angiogenic area in IL-1β–implanted corneas on day 6 (n = 4 to 9). C: Molecular imaging of VAP-1 in normal and angiogenic corneal vessels. Anti–VAP-1 antibody-conjugated MSs (green) and rhodamin-conjugated concanavalin A (red). Arrows indicate MSs in vascular vessels. Scale bar = 200 μm. D: Quantitation of the number of VAP-1 antibody-conjugated MSs in corneal vessels of untreated and IL-1β–implanted eyes (day 4) (n = 4 to 8). E: Representative Western blots of untreated and IL-1β–implanted corneas (day 3) and heart (nondiluted and 10 times diluted samples) with antibodies of VAP-1 and β-tubulin. Numbers show the ratio of band intensity. F: Double immunostaining of heart and IL-1β–implanted corneal sections with antibodies of VAP-1 (green) and CD31 (red). Scale bar = 100 μm. *P < 0.05; **P < 0.01.
To examine whether IL-1β affects VAP-1 expression, we quantified VAP-1 in the newly growing corneal vessels using our in vivo molecular imaging technique. Quantification of the in vivo molecular imaging experiments revealed significantly higher VAP-1 expression in IL-1β–induced angiogenic vessels than in the preexisting vessels (Figure 1, C and D; see Supplemental Video at http://ajp.amjpathol.org).
To confirm our in vivo results, we examined VAP-1 expression with Western blotting and immunohistochemistry. The blots showed significant VAP-1 up-regulation in the corneas but not conjunctivas of the IL-1β–implanted eyes (Figure 1E). Immunohistochemistry with CD31 and VAP-1 antibodies showed VAP-1 expression in limbal and corneal angiogenic vessels and blood vessels in the heart, where the expression was shown previously (Figure 1F).25
VAP-1 Inhibition Blocks IL-1β–Induced Leukocyte Infiltration in the Cornea
To investigate the role of VAP-1 in cytokine-induced angiogenesis, we stained for and quantitated the number of leukocytes in the IL-1β–implanted corneas with or without VAP-1 inhibition. VAP-1 inhibition significantly reduced the number of CD11b(+) cells in the IL-1β–implanted corneas than in corneas without treatment (Figure 2, A and B). Interestingly, CD11b(+) cells accumulated around the limbus in VAP-1 inhibitor–treated eyes (Figure 2A). To examine the role of VAP-1 on granulocytes and macrophages, we stained for Gr-1 and F4/80, respectively. Both Gr-1(+) (Figure 2, C and D) and F4/80(+) (Figure 2, E and F) cell infiltration was inhibited by VAP-1 inhibition during IL-1β–induced inflammation.
Figure 2.
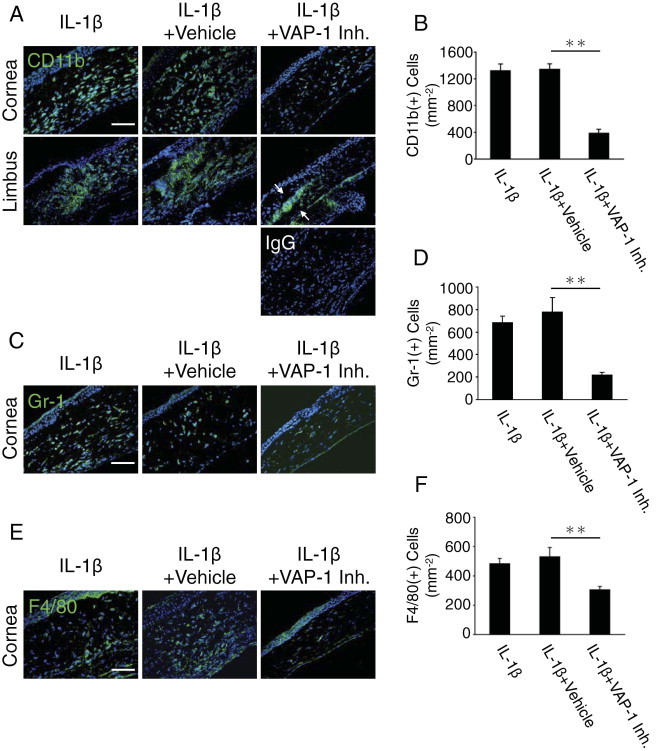
Impact of VAP-1 inhibition on leukocyte infiltration during IL-1β–induced angiogenesis. CD11b (A), Gr-1 (C), and F4/80 (E) staining (green) of IL-1β–implanted corneal sections with or without VAP-1 inhibitor. CD11b staining shows CD11b(+) leukocytes in cornea and limbal area. Arrows show accumulation of CD11b(+) cells around limbus. Quantitation of the number of CD11b (B), Gr-1 (D), and F4/80 (F)–positive leukocytes (n = 5 to 9). Scale bar = 50 μm. *P < 0.05; **P < 0.01.
VAP-1 Regulates IL-1β–Induced Lymphangiogenesis
To investigate, whether VAP-1 inhibition affects IL-1β–induced lymphangiogenesis, we stained lymphatic vessels with antibodies against the lymphatic endothelial marker LYVE-1 and quantified the lymphangiogenic areas in whole mounts. VAP-1 inhibition significantly reduced lymphangiogenesis compared with the vehicle-treated animals (Figure 3, A and B).
Figure 3.
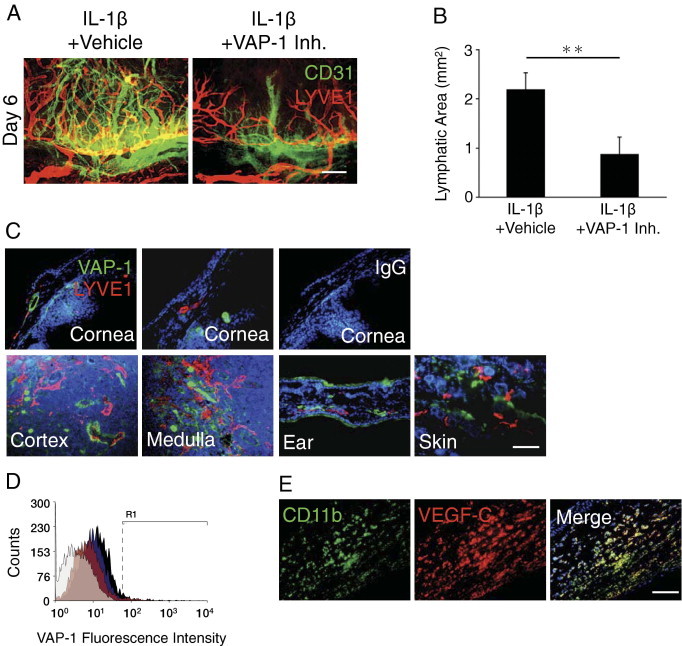
Impact of VAP-1 inhibition on IL-1β–induced lymphangiogenesis. A: Double staining of corneal flat mounts for angiogenic (CD31, green) and lymphangiogenic (LYVE-1, red) endothelium in IL-1β–implanted corneas of vehicle- or VAP-1 inhibitor–treated mice on day 6. Scale bar = 200 μm. B: Quantitative analysis of lymphangiogenesis in IL-1β–implanted corneas of vehicle- or VAP-1 inhibitor–treated mice on day 6 (n = 4 to 5). C: Double immunostaining of normal and IL-1β–implanted cornea, cortex, and medulla of cervical lymph node, ear and skin with antibodies against VAP-1 (green) and LYVE-1 (red). Scale bar = 50 μm. D: Flow cytometric analysis of VAP-1 expression in normal (black) and VEGF-A– (blue) or IL-1β–treated (red) HLECs. Isotype control staining, gray histogram. E: Double immunostaining of IL-1β–implanted cornea (day 3) with antibodies against CD11b (green) and VEGF-C (red). Scale bar = 50 μm. **P < 0.01.
To examine VAP-1 expression in the cornea, we immunostained untreated and IL-1β–implanted corneas for VAP-1. Surprisingly, we did not observe any VAP-1 staining in untreated or IL-1β–implanted corneal lymphatics (Figure 3C). To see, whether lymphatics in other tissues express VAP-1, similar to the expression in HLECs, we performed immunohistochemistry in the lymph node, ear, and dorsal skin; however, in none of these tissues did lymphatics show VAP-1(+) staining (Figure 3C).
Flow cytometry showed 3.9% expression of VAP-1 in unstimulated HLECs, which expressed high levels of LYVE-1 (Figure 3D; see Supplemental Figure S1 at http://ajp.amjpathol.org). To examine, whether cytokines regulate VAP-1 expression in HLECs, we treated these cells with IL-1β and VEGF-A. However, neither IL-1β nor VEGF-A changed VAP-1 expression in HLECs (Figure 3D). Immunohistochemistry showed expression of the lymphangiogenic factor VEGF-C in CD11b(+) cells in IL-1β–implanted corneas (Figure 3E).
VAP-1 Inhibition Does Not Affect VEGF-A–Induced Lymph- and Angiogenesis
VEGF-A induces both lymph- and angiogenesis.23,26 To investigate the contribution of VAP-1 in VEGF-A–induced angiogenesis, we examined the expression of VAP-1 in VEGF-A–induced angiogenesis using our novel in vivo molecular imaging approach. The number of adhering anti–VAP-1 antibody-conjugated MSs was significantly higher than control MSs (Figure 4, A and B; see Supplemental Video at http://ajp.amjpathol.org), indicating VAP-1 expression in VEGF-A–induced angiogenesis.
Figure 4.
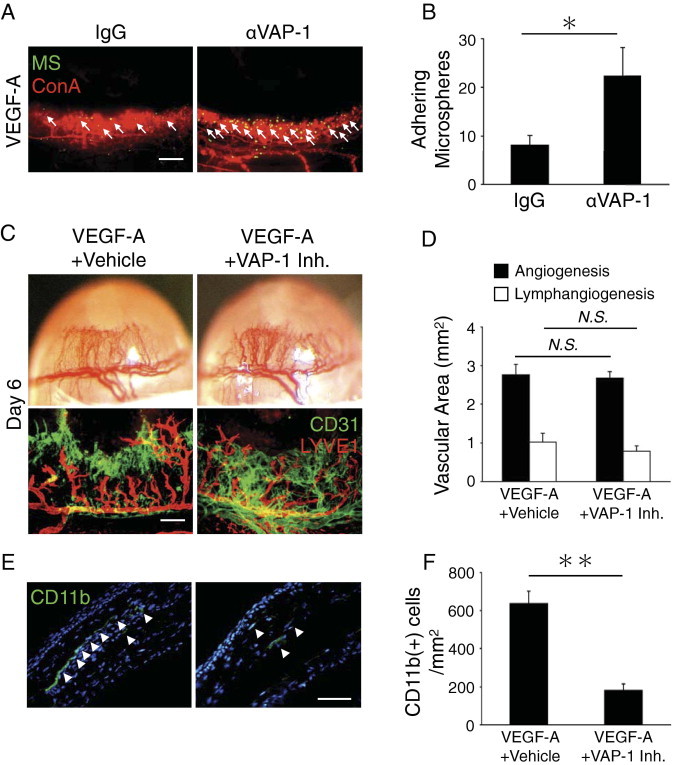
Impact of VAP-1 inhibition on VEGF-A–induced lymph- and angiogenesis. A: Anti–VAP-1 antibody-conjugated MSs (green) and rhodamin-conjugated concanavalin A (red). Arrows, MSs in blood vessels. Bar, 200 μm. B: Quantitation of the number of VAP-1 antibody-conjugated MSs in corneal vessels of untreated and VEGF-A–implanted eyes (day 4) (n = 7). C: Photomicrographs of VEGF-A–implanted corneas of BALB/c mice without and with the treatment of vehicle or VAP-1 inhibitor on day 6 after implantation. Double staining of corneal flat mounts for angiogenesis (CD31, green) and lymphangiogenesis (LYVE-1, red). Scale bar = 200 μm. D: Quantitative analysis of angiogenesis (black) and lymphangiogenesis (white) in VEGF-A–implanted BALB/c corneas on day 6 (n = 6 to 8). E: CD11b staining (green) of VEGF-A–implanted corneal sections with vehicle or VAP-1 inhibitor. Arrowheads, CD11b(+) leukocytes in the cornea. F: Quantitation of the number of CD11b-positive leukocytes (n = 13). Scale bar = 50 μm. *P < 0.05; **P < 0.01.
To study the role of VAP-1 in VEGF-A–induced lymph- and angiogenesis, we next implanted VEGF-A in the mouse corneas and quantified the amount of lymphatics and blood vessels with and without VAP-1 inhibition. Treatment with the VAP-1 inhibitor did not change the amount of VEGF-A–induced lymph- and angiogenesis (Figure 4, C and D). These data indicate that VAP-1 is not necessary for VEGF-A–induced lymph- and angiogenesis.
To investigate the role of VAP-1 in VEGF-A–induced leukocyte infiltration, we stained for CD11b(+) cells in VEGF-A–implanted corneas with vehicle or VAP-1 inhibitor treatment. VAP-1 inhibition significantly reduced the number of CD11b(+) leukocytes in VEGF-A–implanted corneas compared with vehicle-treated controls (Figure 4, E and F). These data indicate that VAP-1 contributes to VEGF-A–induced CD11b(+) leukocyte infiltration but not to lymph- and angiogenesis.
VAP-1 Inhibition Affects IL-1β- but Not VEGF-A–Induced M2 Macrophage Infiltration
To further explore the contribution of VAP-1 in IL-1β– and VEGF-A–induced lymph- and angiogenesis, we investigated the effect of VAP-1 inhibition on M2 macrophages. We examined the presence of M2 macrophages in IL-1β– and VEGF-A–implanted corneas. We observed CD11b(+)CD206(+) leukocytes in IL-1β– and VEGF-A–implanted corneas (Figure 5, A and C). The number of CD11b(+)CD206(+) leukocytes in IL-1β–implanted corneas of VAP-1 inhibitor–treated mice was significantly lower than in vehicle-treated mice (Figure 5, A and B). However, the number of CD11b(+)CD206(+) leukocytes in VEGF-A–implanted corneas did not differ between VAP-1 inhibitor–treated and vehicle-treated animals (Figure 5, C and D). To examine, whether IL-4, the classic cytokine for macrophage phenotype differentiation, is expressed in inflammatory cytokine-induced angiogenesis, we performed immunostaining in IL-1β–implanted and control corneas. IL-4 expression was distinctly expressed in IL-1β–implanted corneas but not control. VAP-1 inhibition suppressed IL-4 expression in IL-1β–implanted corneas (Figure 5E).
Figure 5.
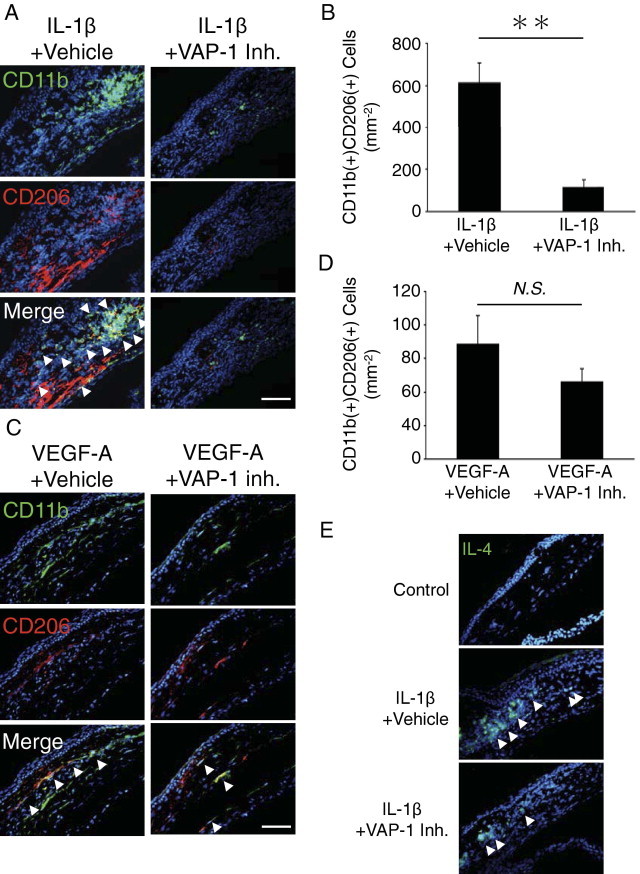
Impact of VAP-1 inhibition on M2 macrophages infiltration during lymph- and angiogenesis. A and C: Double immunostaining of IL-1β– (A) or VEGF-A– (C) implanted cornea with vehicle or VAP-1 inhibitor (day 3) with antibodies against CD11b (green) and CD206 (red). Arrowheads, double positive cells. Scale bar = 50 μm. B and D: Quantitation of the number of CD11b and CD206- double positive M2 macrophages in IL-1β– (B) or VEGF-A– (D) implanted cornea (n = 5 to 9). E: IL-4 staining (green) of control or IL-1β–implanted corneal sections with vehicle or VAP-1 inhibitor. Scale bar = 50 μm. **P < 0.01.
Discussion
VAP-1 is expressed in blood vessel endothelium in most organs and tissues.1 Here, we show VAP-1 expression in the resting and angiogenic corneal blood vessel endothelium. In the resting blood endothelium, VAP-1 is in intracellular vesicles, whereas in inflammation the molecule translocates onto the luminal surface in vivo.27 HUVECs express VAP-1 in the cytoplasm; however, in these cells VAP-1 does not translocate onto the surface after tumor necrosis factor-α stimulation.17 On the surface of cultured HLECs, we found slight expression of VAP-1 that remained unchanged with VEGF-A or IL-1β stimulation. Furthermore, VAP-1 is not expressed in lymphatics in vivo. These results indicate differential mechanisms for VAP-1 expression between blood and lymphatic vessels.
Our in vivo molecular imaging shows higher VAP-1 expression in angiogenic than normal blood vessels. This finding supports previous reports revealing the key role of VAP-1 in angiogenesis-related diseases, including cancer13 and age-related macular degeneration.5 To date, molecular diagnosis has not been introduced to patient care and need is great for in vivo molecular markers of angiogenic disease. Our results reveal that VAP-1 might become a useful diagnostic target for inflammatory and angiogenic diseases. Interestingly, VEGF-A–dependent lymph- and angiogenesis are unaffected by VAP-1 inhibition despite suppression of leukocyte infiltration with VAP-1 inhibition. Because macrophages are important for VEGF-A–induced angiogenesis,8 other factors beside macrophage infiltration and M2 macrophage population might be sufficient to induce VEGF-A–induced angiogenesis.
Different diseases or different stages in angiogenic diseases show varying angiogenic phenotypes. Previous studies showed differences between IL-1β- and VEGF-A–dependent angiogenesis. For instance, monocytic chemotactic protein-1 (MCP-1) or cyclooxygenase-2 deficiency in mice inhibits IL-1β–induced but not VEGF-A–induced angiogenesis.8,24 To use the most effective molecular targeting therapy in angiogenic disorders, understanding details of the molecular mechanisms will be vital.
Anti–VAP-1 antibodies inhibit migration of lymphocytes, granulocytes, and macrophages into inflamed tissues in many animal models.1 CD11b(+) cells, especially macrophages, are important for IL-1β–induced lymph- and angiogenesis.7,8 VAP-1 inhibition blocks infiltration of leukocytes and specifically M2 macrophages in inflammatory cytokine–implanted corneas. A recent article also showed impaired leukocyte infiltration in cancer in VAP-1–deficient mice.13 Tumor lymphangiogenesis in VAP-1−/− mice does not differ from wild type.13 During tumor development and metastasis, cytokines such as VEGF-A and IL-1β are up-regulated.8 Our data show that VAP-1 blockade inhibits IL-1β–induced but not VEGF-A–induced lymphangiogenesis, indicating cytokine dependency of leukocyte-mediated lymphangiogenesis. Because macrophages are critical to growth factor–induced lymph- and angiogenesis, macrophage subtypes (eg, M1 or M2) might be differentially recruited through VAP-1, depending on the implanted cytokine. Lymphocytes secrete IL-4, which promotes M2 macrophage differentiation.14 VAP-1 regulates lymphocyte infiltration1 and might thus indirectly regulate macrophage polarization. Aside from IL-4, other factors might also contribute to macrophage polarization in inflammatory angiogenesis. For instance, IL-1β implantation in the cornea causes up-regulation of MCP-1.8 Recently, MCP-1 was shown to be involved in M2 polarization.28
Lymphatic endothelial ICAM-1 and VCAM-1 facilitate leukocyte reentry into the lymphatics.16 In contrast, VAP-1 is only expressed in blood vessels but not in lymphatics. Anti-inflammatory treatments that reduce ICAM-1 and VCAM-1 expression might interfere with the leukocytes entering the lymphatics, resulting in a deficient immune response and tissue clearance of cells. However, VAP-1 blockade would likely not reduce recruitment of the immune cells from tissues to the lymph nodes and the adaptive immune response.
Our in vivo molecular imaging reveals higher VAP-1 expression in angiogenic vessels, making this molecule an attractive target for early detection of angiogenic diseases. VAP-1 inhibition reduces IL-1β–induced M2 macrophage infiltration, making this molecule a key regulator of diseases, where macrophage polarization plays a role.
Footnotes
Supported by National Institutes of Health grant AI050775, the Malaysian Palm Oil Board, American Health Assistance Foundation, an overseas Research Fellowship Award from Bausch & Lomb, a Fellowship Award from the Japan Eye Bank Association, and Tear Film & Ocular Surface Society Young Investigator Fellowship (to S.N. under the mentorship of A.H.-M.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.01.011.
Supplementary data
LYVE-1 expression in HUVECs and HLECs. Flow cytometric analysis of LYVE-1 expression in untreated HUVECs and HLECs. LYVE-1 staining, black histogram; isotype control staining, gray histogram.
References
- 1.Jalkanen S., Salmi M. VAP-1 and CD73, endothelial cell surface enzymes in leukocyte extravasation. Arterioscler Thromb Vasc Biol. 2008;28:18–26. doi: 10.1161/ATVBAHA.107.153130. [DOI] [PubMed] [Google Scholar]
- 2.Almulki L., Noda K., Nakao S., Hisatomi T., Thomas K.L., Hafezi-Moghadam A. Localization of vascular adhesion protein-1 (VAP-1) in the human eye. Exp Eye Res. 2009;90:26–32. doi: 10.1016/j.exer.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noda K., Miyahara S., Nakazawa T., Almulki L., Nakao S., Hisatomi T., She H., Thomas K.L., Garland R.C., Miller J.W., Gragoudas E.S., Kawai Y., Mashima Y., Hafezi-Moghadam A. Inhibition of vascular adhesion protein-1 suppresses endotoxin-induced uveitis. FASEB J. 2008;22:1094–1103. doi: 10.1096/fj.07-9377com. [DOI] [PubMed] [Google Scholar]
- 4.Noda K., Nakao S., Zandi S., Engelstadter V., Mashima Y., Hafezi-Moghadam A. Vascular adhesion protein-1 regulates leukocyte transmigration rate in the retina during diabetes. Exp Eye Res. 2009;89:774–781. doi: 10.1016/j.exer.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noda K., She H., Nakazawa T., Hisatomi T., Nakao S., Almulki L., Zandi S., Miyahara S., Ito Y., Thomas K.L., Garland R.C., Miller J.W., Gragoudas E.S., Mashima Y., Hafezi-Moghadam A. Vascular adhesion protein-1 blockade suppresses choroidal neovascularization. FASEB J. 2008;22:2928–2935. doi: 10.1096/fj.07-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 7.Watari K., Nakao S., Fotovati A., Basaki Y., Hosoi F., Bereczky B., Higuchi R., Miyamoto T., Kuwano M., Ono M. Role of macrophages in inflammatory lymphangiogenesis: enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem Biophys Res Commun. 2008;377:826–831. doi: 10.1016/j.bbrc.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 8.Nakao S., Kuwano T., Tsutsumi-Miyahara C., Ueda S., Kimura Y.N., Hamano S., Sonoda K.H., Saijo Y., Nukiwa T., Strieter R.M., Ishibashi T., Kuwano M., Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakao S., Hata Y., Miura M., Noda K., Kimura Y.N., Kawahara S., Kita T., Hisatomi T., Nakazawa T., Jin Y., Dana M.R., Kuwano M., Ono M., Ishibashi T., Hafezi-Moghadam A. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am J Pathol. 2007;171:1058–1065. doi: 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafezi-Moghadam A., Thomas K.L., Prorock A.J., Huo Y., Ley K. L-selectin shedding regulates leukocyte recruitment. J Exp Med. 2001;193:863–872. doi: 10.1084/jem.193.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avraamides C.J., Garmy-Susini B., Varner J.A. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakao S., Kuwano T., Ishibashi T., Kuwano M., Ono M. Synergistic effect of TNF-alpha in soluble VCAM-1-induced angiogenesis through alpha 4 integrins. J Immunol. 2003;170:5704–5711. doi: 10.4049/jimmunol.170.11.5704. [DOI] [PubMed] [Google Scholar]
- 13.Marttila-Ichihara F., Auvinen K., Elima K., Jalkanen S., Salmi M. Vascular adhesion protein-1 enhances tumor growth by supporting recruitment of Gr-1+CD11b+ myeloid cells into tumors. Cancer Res. 2009;69:7875–7883. doi: 10.1158/0008-5472.CAN-09-1205. [DOI] [PubMed] [Google Scholar]
- 14.Sica A., Allavena P., Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Johnson L.A., Jackson D.G. Cell traffic and the lymphatic endothelium. Ann N Y Acad Sci. 2008;1131:119–133. doi: 10.1196/annals.1413.011. [DOI] [PubMed] [Google Scholar]
- 16.Johnson L.A., Clasper S., Holt A.P., Lalor P.F., Baban D., Jackson D.G. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmi M., Jalkanen S. Different forms of human vascular adhesion protein-1 (VAP-1) in blood vessels in vivo and in cultured endothelial cells: implications for lymphocyte-endothelial cell adhesion models. Eur J Immunol. 1995;25:2803–2812. doi: 10.1002/eji.1830251014. [DOI] [PubMed] [Google Scholar]
- 18.Jalkanen S., Karikoski M., Mercier N., Koskinen K., Henttinen T., Elima K., Salmivirta K., Salmi M. The oxidase activity of vascular adhesion protein-1 (VAP-1) induces endothelial E- and P-selectins and leukocyte binding. Blood. 2007;110:1864–1870. doi: 10.1182/blood-2007-01-069674. [DOI] [PubMed] [Google Scholar]
- 19.Hafezi-Moghadam A., Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J Exp Med. 1999;189:939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyahara S., Almulki L., Noda K., Nakazawa T., Hisatomi T., Nakao S., Thomas K.L., Schering A., Zandi S., Frimmel S., Tayyari F., Garland R.C., Miller J.W., Gragoudas E.S., Masli S., Hafezi-Moghadam A. In vivo imaging of endothelial injury in choriocapillaris during endotoxin-induced uveitis. FASEB J. 2008;22:1973–1980. doi: 10.1096/fj.07-096891. [DOI] [PubMed] [Google Scholar]
- 21.Sun D., Nakao S., Xie F., Zandi S., Scering A., Hafezi-Moghadam A. Superior sensitivity of novel molecular imaging probe: simultaneously targeting two types of endothelial injury markers. FASEB J. 2010;24:1532–1540. doi: 10.1096/fj.09-148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie F., Sun D., Schering A., Nakao S., Zandi S., Liu P., Hafezi-Moghadam A. Novel molecular imaging approach for subclinical detection of iritis and evaluation of therapeutic success. Am J Pathol. 2010;177:39–48. doi: 10.2353/ajpath.2010.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakao S., Zandi S., Hata Y., Kawahara S., Arita R., Schering A., Sun D., Melhorn M.I., Ito Y., Lara-Castillo N., Ishibashi T., Hafezi-Moghadam A. Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: an endogenous trapping mechanism links lymph- and angiogenesis. Blood. 2011;117:1081–1090. doi: 10.1182/blood-2010-02-267427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwano T., Nakao S., Yamamoto H., Tsuneyoshi M., Yamamoto T., Kuwano M., Ono M. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004;18:300–310. doi: 10.1096/fj.03-0473com. [DOI] [PubMed] [Google Scholar]
- 25.Salmi M., Kalimo K., Jalkanen S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med. 1993;178:2255–2260. doi: 10.1084/jem.178.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakao S., Maruyama K., Zandi S., Melhorn M.I., Taher M., Noda K., Nusayr E., Doetschman T., Hafezi-Moghadam A. Lymphangiogenesis and angiogenesis: concurrence and/or dependence?: Studies in inbred mouse strains. FASEB J. 2010;24:504–513. doi: 10.1096/fj.09-134056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bono P., Jalkanen S., Salmi M. Mouse vascular adhesion protein 1 is a sialoglycoprotein with enzymatic activity and is induced in diabetic insulitis. Am J Pathol. 1999;155:1613–1624. doi: 10.1016/S0002-9440(10)65477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roca H., Varsos Z.S., Sud S., Craig M.J., Ying C., Pienta K.J. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LYVE-1 expression in HUVECs and HLECs. Flow cytometric analysis of LYVE-1 expression in untreated HUVECs and HLECs. LYVE-1 staining, black histogram; isotype control staining, gray histogram.


