Abstract
Cancer stem-like cells (CSCs) and tumor-initiating cells (TICs) are a small population of cancer cells that share three properties: tumor initiating ability, self-renewal, and differentiation. These properties suggest that CSCs/TICs are essential for tumor maintenance, recurrence, and distant metastasis. Here, we show that cytotoxic T lymphocytes (CTLs) specific for the tumor-associated antigen CEP55 can efficiently recognize colon CSCs/TICs both in vitro and in vivo. Using Hoechst 33342 dye staining, we isolated CSCs/TICs as side population (SP) cells from colon cancer cell lines SW480, HT29, and HCT15. The SP cells expressed high levels of the stem cell markers SOX2, POU5F1, LGR5, and ALDH1A1 and showed resistance to chemotherapeutic agents such as irinotecan or etoposide.To evaluate the susceptibility of SP cells to CTLs, we used CTL clone 41, which is specific for the CEP55-derived antigenic peptide Cep55/c10orf3_193 (10) (VYVKGLLAKI). The SP cells expressed HLA class I and CEP55 at the same level as the main population cells. The SP cells were susceptible to CTL clone 41 at the same level as main population cells. Furthermore, adoptive transfer of CTL clone 41 inhibited tumor growth of SW480 SP cells in vivo. These observations suggest that Cep55/c10orf3_193(10) peptide-based cancer vaccine therapy or adoptive cell transfer of the CTL clone is a possible approach for targeting chemotherapy-resistant colon CSCs/TICs.
Colon cancer is one of the most common malignancies worldwide. With recent progress in treatment, the prognosis has improved to some extent. In advanced disease, however, the prognosis remains unfavorable, because of recurrence, distant metastasis, and resistance to treatment. Thus, novel treatment modalities are needed.
Cancers contain morphologically heterogeneous populations. This fact has led to the cancer stem cell theory,1 the idea that cancers are composed of several types of cells, and that only a small population of cancer cells that can regenerate cancer tissues, much as normal tissue can be regenerated only by a small population of stem-like cells. Recently, cancer stem-like cells and tumor-initiating cells (CSCs/TICs) have been isolated from various types of malignancies, including colon cancer.2–6 In colon cancer, CSCs/TICs can reinitiate tumors that resemble mother colon cancer tissues morphologically when transplanted into immunodeficient mice.3 Furthermore, these CSCs/TICs have higher tumorigenic potential than do non-CSCs/TICs. Previous reports have shown that CSCs/TICs are resistant to a variety of treatments, including chemotherapy and radiotherapy, with varied mechanisms of resistance, including high expression of drug transporters, relative cell cycle quiescence, high levels of DNA repair machinery, and resistance to apoptosis.7 These reports3–6 support the hypothesis that malignant cancers comprise heterogeneous populations that organize in a hierarchical differentiation model. The CSCs/TICs are located at the top of this hierarchy, and targeting CSCs/TICs is essential to achieve efficient effects for treatment of malignant diseases. Recently, some trials targeting CSCs/TICs have been reported for hematopoietic malignancies.8 Hedgehog signaling is essential for maintenance of myeloid leukemia stem cells, and inhibition of hedgehog signaling by cyclopamine is effective for imatinib-resistant myeloid leukemia.9 To date, however, no such CSC/TIC targeting approach has been reported for colon cancer.
In the present study, we evaluated the efficiency of CTL-based immunotherapy targeting colon CSCs/TICs. Using Hoechst 33342 dye, we isolated colon CSCs/TICs as side population (SP) cells from six colon cancer cell lines. The SP cells derived from SW480, HT29, and HCT15 showed higher tumorigenicity than did main population (MP) cells. On the other hand, SP cells from KM12LM, Lovo, and Colo320 did not show any increase in tumorigenicity, compared with MP cells. This suggests that SW480, HT29, and HCT15 SP cells (but not KM12LM, Lovo, and Colo320 SP cells) were enriched with CSCs/TICs. In RT-PCR analysis the SW480, HT29, and HCT15 SP cells showed a stem cell-like gene expression signature, including SOX2, POU5F1, LGR5, and ALDH1A1. Furthermore, these SP cells also showed resistance to chemotherapeutic agents, including irinotecan and etoposide. These observations support the idea that these SP cells had stem cell-like features. To assess the immunogenicity of SP cells, we evaluated the expression of HLA class I and of CEP55, which is a tumor-rejection antigen of breast and colon cancer.10,11 The SP cells expressed HLA class I (and also HLA-A24) at the same level as MP cells. The SP cells also expressed CEP55 messenger RNA (mRNA) at the same level as MP cells in RT-PCR. To confirm the susceptibility of SP cells to cytotoxic T lymphocytes (CTLs), we used CTL clone 41, which recognizes CEP55 in an HLA-A24-restricted manner.10 CTL clone 41 killed SW480, HT29, and HCT15 SP cells at the same level as it killed MP cells and presorted cells. These observations suggest that colon CSCs/TICs are also sensitive to CTLs, as non-CSC/TIC populations are. Furthermore, adoptive transfer of CTL clone 41 inhibited the tumor growth of SW480 SP cells in immunodeficient mice. These observations suggest that CTL-based colon cancer immunotherapy is efficient for colon CSCs/TICs. To our knowledge, the present study provides the first direct evidence that colon CSCs/TICs are susceptible to CTLs and thus opens possibilities for future applications in immunotherapy using CSC/TIC-specific vaccines.
Materials and Methods
Cell Lines
Colon adenocarcinoma cell lines SW480 (HLA-A*0201/2402), HCT15 (HLA-A*0201/2402), HT29 (HLA-A1/24), Lovo, and Colo320 were kind gifts of Dr. K. Imai (Sapporo, Japan), and KM12LM was a kind gift of Dr. K. Itoh (Kurume, Japan). All cell lines except K562 were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA). K562 was cultured in RPMI-1640 (Sigma-Aldrich) supplemented with 10% fetal bovine serum. HCT15-B2M, a stable transfectant of HCT15 cells with B2M (β2 microglobulin) cDNA, was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 10 μg/mL puromycin (Sigma-Aldrich).11
Side Population Analysis
Side population analysis was performed as described previously, with some modifications.12 Trypsinized cultured cells were washed with PBS and were resuspended at 37°C in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum. After 10 minutes preincubation, the cells were labeled with Hoechst 33342 dye (Lonza, Walkersville, MD) for 90 minutes at concentrations of 3.75 μg/mL for Colo320, 5 μg/mL for SW480 and Lovo, 7.5 μg/mL for HT29 and KM12LM, and 10 μg/mL for HCT15, with or without verapamil (Sigma-Aldrich), which is an inhibitor of ABC transporters, at concentrations of 50 μmol/L for SW480, HCT15, and Colo320, 75 μmol/L for Lovo, and 100 μmol/L for HT29. Cells were counterstained with 1 μg/mL propidium iodide to label dead cells. Next, 1 × 106 viable cells were analyzed and sorted using a BD FACSAria II fluorescence-activated cell sorting system (BD Biosciences, Franklin Lakes, NJ). The Hoechst dye was excited at 355 nm, and its fluorescence was measured at two wavelengths using optical filters 405 DF20 [450/20 nm band-pass filter O (Hoechst Blue)] and 635LP [635 nm long-pass edge filter (Hoechst Red)]. Propidium iodide labeling was measured through a 630/BP30 filter for discrimination of dead cells.
Xenograft Model
The SP cells, MP cells, and presorted cells from colon cancer cell lines were mixed 1:1 by volume with Matrigel (BD Biosciences) and were injected subcutaneously into the backs of female 4- to 8-week-old nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice. Tumor size in cubic millimeters was assessed weekly with calipers and was calculated as Tumor Size = (Longest Diameter × Shortest Diameter2)/2.
RT-PCR Analysis of SP and MP Cells
RT-PCR analysis was performed as described previously.10 Total RNAs were isolated from both SP cells and MP cells using an RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized from 2 μg of total RNA by reverse transcription using SuperScript III reverse transcriptase (Invitrogen). The PCR amplification was performed in 20 μL of PCR mixture containing 1 μL of cDNA mixture, 0.5 μL of Taq DNA polymerase (Qiagen) and 4 pmol of primers. The PCR mixture was initially incubated at 98°C for 2 minutes, followed by 30 cycles of denaturation at 98°C for 15 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds. The following primer pairs were used for RT-PCR analysis (forward and reverse, respectively): 5′-CATGATGGAGACGGAGCTGA-3′ and 5′-ACCCCGCTCGCCATGCTATT-3′ for SOX2, with an expected PCR product size of 410 bp; 5′-TGGAGAAGGAGAAGCTGGAGCAAAA-3′ and 5′-GGCAGATGGTCGTTTGGCTGAATA-3′ for POU5F1, with an expected PCR product size of 163 bp; 5′-CTCTTCCTCAAACCGTCTGC-3′ and 5′-GATCGGAGGCTAAGCAACTG-3′ for LGR5, with an expected PCR product size of 181 bp; 5′-TGTTAGCTGATGCCGACTTG-3′ and 5′-TTCTTAGCCCGCTCAACACT-3′ for ALDH1A1, with an expected PCR product size of 154 bp; 5′-TGAGTTTGCCATCACAGAGC-3′ and 5′-TTGCTTGCTGGTGCATTAAC-3′ for CEP55, with an expected PCR product size of 521 bp; and 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), with an expected product size of 452 bp. GAPDH was used as an internal control.
Quantitative Real-Time PCR Analysis
Quantitative real-time PCR was performed using an ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Primers and probes were designed by the manufacturer (TaqMan gene expression assays; Applied Biosystems). Thermal cycling was performed using 40 cycles of 95°C for 15 seconds followed by 60°C for 1 minute. Each experiment was done in triplicate, with normalization to the GAPDH gene as an internal control.
Flow Cytometric Analysis and Monoclonal Antibodies
Cells were incubated with mouse monoclonal antibodies at saturation concentration for 30 minutes on ice, washed with PBS, and stained with a polyclonal goat anti-mouse antibody coupled with fluorescein isothiocyanate for 30 minutes. Samples were analyzed using a BD FACSCalibur flow cytometry system (Becton Dickinson, Mountain View, CA). Anti-pan HLA class I (W6/32) and anti-HLA-A24 monoclonal antibodies (C7709A2.6 hybridoma, a kind gift from Dr. P.G. Coulie, Brussels, Belgium) were prepared from hybridomas.
Survival Studies for Etoposide and Irinotecan
We isolated SP and MP cells of SW480 and HCT15 and seeded them into 96-well culture plates at 1 × 104 cells per well for each population of cells. The cells in both populations were treated with etoposide (1 and 5 μg/mL) or irinotecan (40 and 400 μg/mL for SW480, 10 and 100 μg/mL for HCT15). After 72 hours of exposure to the chemotherapeutic agents, viability of the cells was determined using the SOD assay kit WST-1, which was performed according to the manufacturer's protocol (Dojindo Molecular Technologies, Kumamoto, Japan; Rockville, MD).
Cytotoxicity Assay for SP Cells with CTL Clone 41
We had previously established CTL clone 41, which recognizes an HLA-A24 restricted antigenic peptide (VYVKGLLAKI) termed Cep55/c10orf3_193(10), from an HLA-A24-positive breast cancer patient's peripheral blood mononuclear cells.8 The lytic activity of CTL clone 41 for SP cells, MP cells, and presorted cells was evaluated by 51Cr release assay. Briefly, SP cells, MP cells and presorted cells were labeled with 100 μCi of 51Cr for 1 hour at 37°C, washed four times with PBS, and resuspended in AIM-V medium (Invitrogen). The 51Cr-labeled target cells (2000 cells/well) were then incubated with various numbers of effector cells for 6 hours at 37°C in 96-well culture plates. Radioactivity of the culture supernatant was measured with a gamma counter. The percentage of cytotoxicity was calculated as follows: % Specific Lysis = (Experimental Release − Spontaneous Release) × 100/(Maximum Release − Spontaneous Release). Target cells were treated with 100 units/mL interferon-γ for 48 hours before the assay.
Winn Assay
SW480 SP cells were mixed with CTL clone 41 at a ratio of 1 SP cell to 10 CTL cells. The resulting mixture (200 μL with 1 × 106 CTL clone 41 and 1 × 105 SP cells) was injected subcutaneously into the backs of NOD/SCID mice. A control group of five mice was injected with SP cells alone. Tumor size was assessed weekly.
CTL Adoptive Transfer
NOD/SCID mice were inoculated subcutaneously on the back with 1 × 103 SW480 SP cells. Three weeks later, when the tumor started to be palpable, 5 × 104 Cep55/c10orf3_193(10)-specific CTL clone cells or PBS was injected intravenously. The same adoptive transfer procedure was performed 4 weeks after inoculation with SP cells. Tumor size was assessed weekly.
Statistical Analysis
In the xenograft model, survival studies using chemotherapeutic agents, cytotoxicity assay, Winn assay, and adoptive transfer model, the data were analyzed using the Mann-Whitney U-test, with P < 0.05 conferring statistical significance.
Results
Isolation of Colon CSCs/TICs as SP Cells
Several methods to isolate colon cancer CSCs/TICs has been reported, including cell surface markers such as CD44 or PROM1 (CD133), SP cells, and the Aldefluor assay.3–6,13 In the present study, we isolated colon CSCs/TICs using SP cell analysis. Several colon cancer cell lines were dyed with Hoechst 33342 and then analyzed with a BD FACSAria II flow cytometer as described under Materials and Methods. Side population cells could be detected in all six colon cancer cell lines analyzed (ie, SW480, HT29, HCT15, Colo320, Lovo, and KM12LM) (Figure 1, A and B). The frequency of SP cells ranged from 3.3% for SW480 to 11.1% for HCT15 cells. All these SP cells were specifically inhibited by verapamil, as has been shown previously,14 suggesting that these SP cells were specific for ABC transporter expression. Because previous studies showed that some colon cancer SP cells were not enriched with a CSC/TIC population,15 it was essential to confirm the presence of CSCs/TICs in SP cells for further analysis. We inoculated these SP cells subcutaneously into the back of immunodeficient NOD/SCID mice using serial dilution. The SP cells derived from SW480, HCT15, and HT29 showed higher tumor initiating ability, compared with MP cells (Table 1). Furthermore, SW480, HT29, and HCT15 SP cells showed faster tumor growth, compared with MP cells (Figure 1, C and D), suggesting the presence of CSCs/TICs in these SP cells. In contrast, the SP cells derived from Colo320, Lovo, and KM12LM did not show any difference in tumorigenicity or tumor growth, compared with MP cells. We therefore restricted further analysis to the SW480, HT29, and HCT15 SP cells as colon cancer CSCs/TICs.
Figure 1.
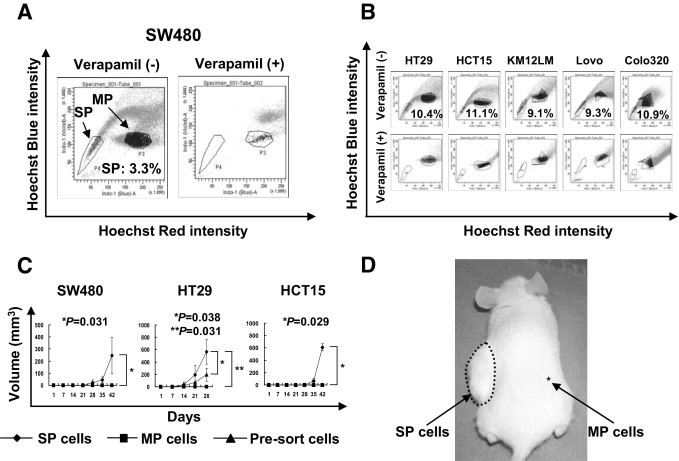
Isolation of colon CSCs/TICs from colon cancer cell lines and tumor growth of the SP cells. A: Colon cancer cell line SW480 was stained with Hoechst 33342 dye as described under Materials and Methods, with or without verapamil. Stained cells were analyzed using a BD FACSAria II fluorescence-activated cell sorting system. Frequency of SP cells was 3.3%. B: Colon cancer cell lines (HT29, HCT15, KM12LM, Lovo, and Colo320) were stained with Hoechst 33342 dye with or without verapamil. Stained cells were analyzed using a BD FACSAria II system. Frequencies of SP cells ranged from 9.1% for KM12LM cells to 11.1% for HCT15 cells. C: SP cells, MP cells, and presorted cells of colon cancer cell lines SW480, HT29, and HCT15 were inoculated subcutaneously into the backs of NOD/SCID mice (1 × 103 cells injected). Data are reported as means ± SD. P values indicate differences between cell types according to a Mann-Whitney U-test. D: Representative tumor growth in NOD/SCID mice at the SP cell injection site (1 × 103 cells injected). SP cells and MP cells were inoculated subcutaneously into the left and right side of the back, respectively.
Table 1.
Tumor Initiating Ability of Colon Cancer SP Cells
| Cell line (% SP cells) | Tumor initiating ability⁎ |
||
|---|---|---|---|
| 1 × 104† | 1 × 103† | 1 × 102† | |
| SW480 (3.3) | |||
| SP cells | 4/4 | 4/6 | 4/4 |
| MP cells | 2/4 | 3/5 | 0/4 |
| HT29 (10.4) | |||
| SP cells | 3/3 | 2/3 | 3/3 |
| MP cells | 3/3 | 0/3 | 0/3 |
| HCT15 (11.1) | |||
| SP cells | 3/3 | 3/4 | 3/3 |
| MP cells | 1/3 | 1/4 | 0/3 |
| Colo320 (10.9) | |||
| SP cells | 2/2 | 1/2 | 1/2 |
| MP cells | 2/2 | 2/2 | 1/2 |
| Lovo (9.3) | |||
| SP cells | 0/1 | 1/1 | 0/1 |
| MP cells | 1/1 | 0/1 | 0/1 |
| KM12LM (9.1) | |||
| SP cells | 1/2 | 2/2 | 1/1 |
| MP cells | 1/2 | 2/2 | 1/1 |
MP, main population; SP, side population.
Tumor initiating ability is expressed as the ratio of tumor-initiation to injection.
The tumor initiation abilities were evaluated at day 42 after injection of the indicated number of cells.
RT-PCR Analysis of Colon Cancer SP Cells
To examine the molecular properties of SP cells, we performed RT-PCR analysis. SOX2 and POU5F1 are representative markers for embryonal stem cells and CSCs/TICs.16 The SP cells derived from SW480, HT29, and HCT15 showed higher expression of both SOX2 and POU5F1, compared with MP cells (Figure 2A). ALDH1A1, a colon CSC/TIC marker,6 was expressed at a higher level in SP cells of HCT15 than in MP cells, but SP cells of SW480 and HT29 did not show any difference in comparison with MP cells. SW480 and HT29 SP cells also showed higher expression of LGR5, which is known as a normal colon stem cell marker.17 To confirm the expression of stem cell markers, we also performed real-time PCR. The SW480 SP cells expressed 90 times higher SOX2, 7 times higher POU5F1, 153 times higher LGR5, and 6.1 times higher ALDH1A1, compared with MP cells (Figure 2B). These findings indicate that these SP cells had molecular properties similar to those of embryonal stem cells.
Figure 2.
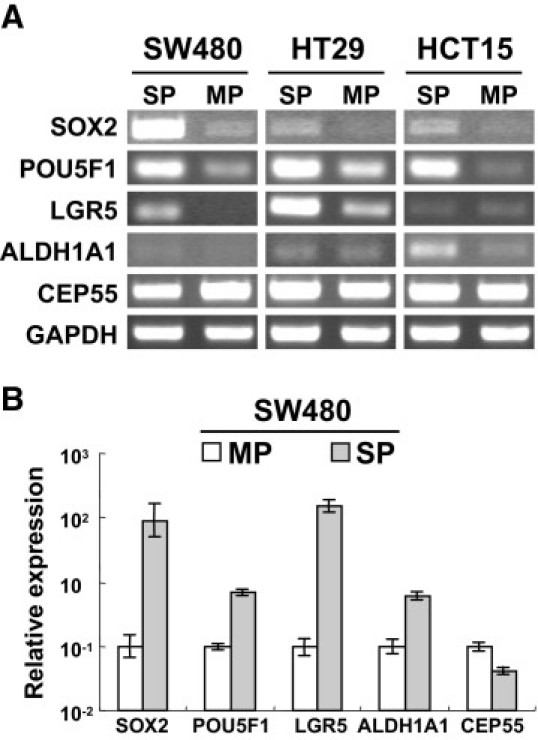
Expression of stem cell markers in SP and MP cells. A: mRNAs purified from SW480, HT29, and HCT15 SP and MP cells were analyzed by RT-PCR. B: mRNA purified from SW480 SP cells and MP cells were analyzed with real-time PCR. mRNA expression level is relative to MP cells. Data are reported as means ± SD.
Resistance to Chemotherapeutic Reagents
Although SP cells derived from liver cancer cell line HuH7 have showed resistance to chemotherapy,13 we know of no conclusive previous studies of such resistance in colon SP cells. We performed a cell survival study of colon cancer SP cells using the chemotherapeutic agents irinotecan and etoposide. The SW480 and HCT15 SP cells were more resistant to both irinotecan and etoposide than were MP cells (Figure 3, A and B). This finding is consistent with findings for CSCs/TICs derived from other organs.22,24
Figure 3.
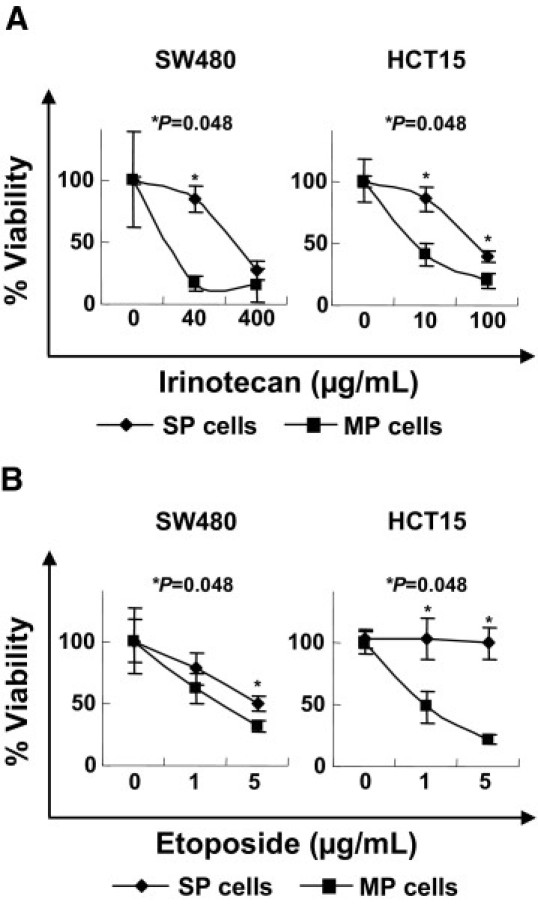
Sensitivity to chemotherapeutic agents. SP cells and MP cells derived from SW480 and HCT15 were incubated in the presence of irinotecan (CPT-11) (A) or etoposide (VP-16) (B) for 3 days. After incubation, the cell viabilities were measured by WST-1 assay. Data are reported as means ± SD. Differences between SP cells and MP cells were examined for statistical significance using the Mann-Whitney U-test.
Expression of HLA and Tumor-Associated Antigens in SP Cells
Because CTLs recognize tumor-associated antigen (TAA)-derived antigenic peptides presented by HLA class I molecules, expression of HLA class I molecules is essential for activation of CTLs. Several types of malignancies have been reported to lose the expression of HLA class I molecules through various mechanisms and so escape CTL attack.18 We therefore evaluated the expression of HLA class I molecules and TAA. We assessed the differences of HLA class I and HLA-A24 expression between SP cells and MP cells by flow cytometry. Because ELISA study has revealed that HCT15 cells lack B2M because of gene mutations of B2M,19 we transduced wild-type B2M cDNA into HCT15 cells and so established HCT15-B2M cells. The SW480, HT29, and HCT15-B2M SP cells showed HLA class I and HLA-A24 expression at the same level as MP cells (Figure 4, A and B). Furthermore, we assessed the expression of one of the colon cancer TAAs, CEP55, by both RT-PCR and real-time PCR (Figure 2, A and B). Both SP cells and MP cells derived from SW480, HT29, and HCT15-B2M expressed CEP55 mRNA at the same level. These data raised the possibility that SP cells are also sensitive to CTLs specific for the CEP55-derived antigenic peptide. Because both SP cells and MP cells expressed CEP55 mRNA at the same level, this appeared to be an ideal target for comparing the susceptibilities of SP cells and MP cells to CTLs.
Figure 4.
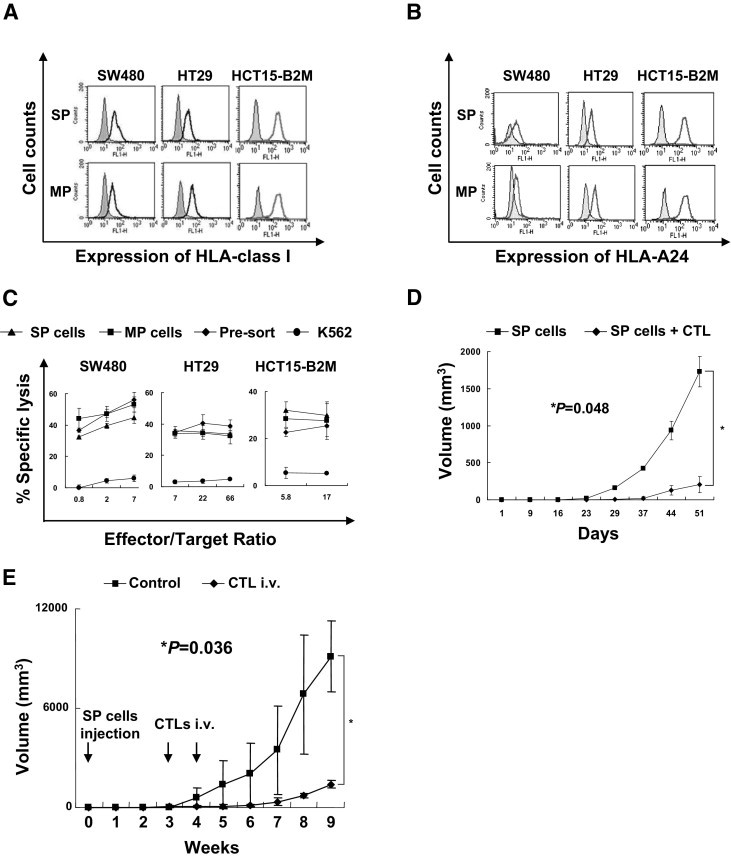
Immunogenicity of SP cells. A: Expression of HLA class I molecules. SW480, HT29, and HCT15-B2M SP cells and MP cells were stained with an anti-pan HLA class I monoclonal antibody (W6/32), then with a fluorescein isothiocyanate (FITC)-labeled secondary antibody. Stained cells were analyzed with a BD FACSCalibur cell sorting system. Solid profiles represent isotype control as the primary antibody. B: Expression of HLA-A24 molecule. SW480, HT29, and HCT15-B2M SP cells and MP cells were stained with an anti-HLA-A24 monoclonal antibody (C7709A2.6), then an FITC-labeled secondary antibody. Stained cells were analyzed with a FACSCalibur cell sorting system. Solid profiles represent isotype control as primary antibody. C: Cytotoxicity of CTL clone 41. A 51Cr release assay was performed using CTL clone 41 at several effector/target ratios. SW480, HT29, and HCT15-B2M SP cells, MP cells, and presorted cells were used for assay; K562 cells were used as a negative control. Data are reported as means ± SD. D: Winn assay. SW480 SP cells (1 × 105) were inoculated subcutaneously into the backs of NOD/SCID mice with or without CTL clone 41 cells (1 × 106). Data are reported as means ± SD. Differences between SP cells and SP cells + CTL were examined for statistical significance using the Mann-Whitney U-test. E: Adoptive transfer model. SW480 SP cells (1 × 103) were inoculated subcutaneously into the back of NOD/SCID mice. At 3 and 4 weeks later, CTL clone 41 or PBS was injected intravenously with or without CTL clone 41 cells (1 × 106). Data are reported as means ± SD. Differences were examined for statistical significance using the Mann-Whitney U-test.
Susceptibility of CSCs/TICs to CTLs, in Vitro and in Vivo
We had previously established CTL clone 41, which is specific for the cancer-related, antigen-derived, HLA-A24-restricted peptide Cep55/c10orf3_193(10).10 CTL clone 41 recognized CEP55-positive and HLA-A24-positive cancer cells, as described previously.10,11 In the present study, we used a 51Cr release assay to examine whether CTL clone 41 can recognize SP cells. All SP cells derived from SW480, HT29, and HCT15-B2M showed susceptibility to CTL clone 41 at the same level as the MP cells and the presorted bulk cell lines (Figure 4C). This indicates that the colon CSCs/TICs were sensitive to CTLs.
To analyze the cytotoxic activity of the CTL clone in vivo, we performed a Winn assay. SW480 SP cells with or without CTL clone 41 were injected into the backs of NOD/SCID mice subcutaneously. CTL clone 41 significantly inhibited the tumorigenicity of SW480 SP cells (Figure 4D). Because CTLs were injected at the same time and place as the SP cells in the Winn assay, we could not rule out the possibility that SP cells were killed in vitro. We therefore used an adoptive transfer model, as described under Materials and Methods. SW480 SP cells were inoculated into the back of NOD/SCID mice subcutaneously. Three weeks later, after confirmation of palpable tumors, CTLs were injected intravenously. Tumors of CTL-injected mice were significantly inhibited in growth, compared with tumors of control mice (Figure 4E). These data indicate that CTLs could recognize CSCs/TICs both in vitro and in vivo.
Discussion
In the present study, we successfully isolated colon cancer CSCs/TICs as SP cells, using Hoechst 33342 staining. Side population cells were first described by Goodell et al,12 and CSCs/TICs of several types of malignancies were successfully isolated as SP cells in subsequent studies.14,20–24 Haraguchi et al13 isolated SP cells from gastrointestinal cancer cell lines; they reported the gene expression profiles and resistance to chemotherapeutic agents of SP cells derived from liver cancer cell line Huh7, but did not determine their tumorigenicity. Burkert et al15 found that SP cells derived from gastrointestinal cancers cell lines HT29, HGT101, Caco2, and HRA19a1.1 were not enriched with a CSC/TIC population. In the present study, we were able to isolate SP cells from all six colon cancer cell lines studied (SW480, HT29, HCT15, KM12LM, Lovo, and Colo320). However, in only three of the six cell lines did the SP cells show higher tumorigenicity than MP cells, suggesting that these SP cells were enriched with CSC/TIC populations. Thus, SP cells might not be the definitive phenotype of CSCs/TICs, and confirmation of tumorigenicity in immunodeficient mice is essential for validation of SP cells as a source of CSCs/TICs. In the present study, the SP cells derived from SW480, HCT15, and HT29 cells were confirmed to be enriched with CSCs/TICs. Furthermore, these SP cells expressed stem cell markers, including SOX2, POU5F1 and LGR5, at higher levels than MP cells, suggesting correspondence with CSCs/TICs. Thus, these SP cells would be a useful tool for analysis of colon CSCs/TICs.
In the present study, we evaluated the immunogenicity of colon CSCs/TICs. Colon cancer CSCs/TICs expressed HLA class I molecules, and also CEP55, which is one of the TAAs. Furthermore, colon CSCs/TICs expressed several other TAA-encoding genes (data not shown), including BIRC5 (encoding apoptosis inhibitor survivin), BIRC7 (encoding livin), WT1, CTAG1B (alias NY-ESO-1), and MAGEA4. As a novel finding, colon cancer CSCs/TICs were sensitive to CTLs both in vitro and in vivo. Recently, Todaro et al25 showed that colon CSCs/TICs were sensitive to γδT cells. Because both CTLs and γδT cells kill target cells through secretion of perforin (encoded by the PRF1 gene) and granzyme B (encoded by GZMB), these observations strongly suggest that CSCs/TICs are sensitive to PRF1- and GZMB-dependent apoptosis. Todaro et al26 had earlier reported that PROM1-positive (CD133+) colon cancer CSCs/TICs secrete IL-4 in an autocrine manner and upregulate the antiapoptotic proteins CFLAR (c-FLIP), BCL2L1 (Bcl-xL), and PEA15 (PED), thereby gaining resistance to chemotherapeutic agents. Saigusa et al27 reported that distant recurrence of rectal cancer after chemotherapy was related to the expression of CSC/TIC markers such as PROM1 (CD133), POU5F1 (Oct3/4), and SOX2. These reports support the idea that colon CSCs/TICs are resistant to apoptotic cell death. The fact that immunocytes induce apoptosis in their target cells raises the question of whether colon CSCs/TICs are also sensitive to immunotherapy.
In the present study, and in that of Todaro et al,25 colon CSCs/TICs were sensitive to perforin- and granzyme B-dependent apoptosis. Thus, both CTLs and γδT cells can be useful tools for colon CSC/TIC targeting therapy. However, because γδT cells do not recognize target cells in an antigen-specific manner, immunotherapy using γδT cells should also recognize the non-CSC/TIC population. Because the number of γδT cells is restricted in vivo, it may be in doubt whether γδT cell can recognize colon cancer CSCs/TICs in vivo efficiently. Recently, based on a large cohort study, Ogino et al28 reported that lymphocytic reaction to tumor was associated with longer survival of colorectal cancer patients. They did not analyze the subtypes of infiltrating lymphocytes; however, the findings from this large-scale study strongly support the notion that immune reaction to tumor cells is important for control of the disease.
Wei et al29 reported recently that glioma-derived CSCs/TICs suppressed T-cell proliferation and activation, and induced T-cell apoptosis through expression of costimulatory inhibitory molecule CD274 (B7-H1) and soluble LGALS3 (galectin-3); glioma CSCs/TICs enhance the induction of regulatory T cells. We also observed that SW480 SP cells express higher mRNA of the immunosuppressive cytokine IL-10 than MP cells (data not shown). Thus, colon CSCs/TICs may have immunosuppressive potential and so inhibit CTL induction. However, colon CSCs/TICs are efficiently killed by CTLs, and colon CSCs/TICs have no influence on the effector phase of CTLs. Thus, adoptive cell transfer of CSC/TIC-specific CTL clones, T-cell-receptor–induced T cells, or peptide vaccination accompanied by an anti-IL-10 monoclonal antibody might be an effective approach for eliminating colon CSCs/TICs.
In the present study, we observed that both colon CSCs/TICs and non-CSCs/TICs were sensitive to CEP55-specific CTLs at the same level. This finding seems reasonable, given that CSCs/TICs express CEP55 mRNA at the same level. Huge numbers of TAAs have already been reported,30,31 and the next challenge is to identify which TAAs would be the most suitable targets for cancer immunotherapy. According to the manner of expression in CSCs/TICs and non-CSCs/TICs, TAAs can be classified into three categories: i) CSC/TIC-specific antigens, such as SOX2 and ALDH1A1; ii) non-CSC/TIC-specific antigens; and iii) shared antigens, such as CEP55.32 The frequencies of colon CSCs/TICs are 1% to 10%, and in the present study these cells had 10- to 100-fold higher tumorigenicity than non-CSCs/TICs. It is likely, therefore, that 1% to 10% of colon CSC/TIC populations have almost the same tumorigenic potential as 90% to 99% of the non-CSC/TIC population. To achieve a complete cure of the disease, shared antigens seem to a be reasonable candidate strategy. In vivo, however, CTL numbers are limited. Given that 1 L of peripheral blood contains approximately 5 × 109 lymphocytes, there are approximately 5 × 108 CD8 T cells in 1 L of peripheral blood and approximately 3 × 109 CD8 cells in the total volume of peripheral blood in a human adult. If the CTL precursor frequency reaches 0.1% of CD8 T cells in a patient receiving peptide vaccination therapy, then the total peptide-specific CTLs can be calculated as 3 × 106 cells in whole blood. This is not an inconsiderable number. Visible tumors as large as 1 cm diameter contain 1 × 109 tumor cells, and the estimated effector/target ratio (E/T) in vivo is 0.003. This ratio may be too low to expect an anti-tumor effect in vivo. However, if we focus only on CSCs/TICs, then the effector/target ratio will be improved. For targeting CSCs/TICs with 1% frequency, the effector/target ratio is correspondingly improved (E/T = 0.3). Thus, focusing only on the CSC/TIC population with CSC/TIC-specific antigens seems to be a better approach for advanced cancer cases. For prevention of disease recurrence after treatment, the target cells are likely to be limited, so shared antigens might be a reasonable choice for cancer immunotherapy.
Recently, some research groups have reported that monoclonal antibodies for insulin-like growth factor-1 receptor (IGF-1R), δ-like 4 ligand (DLL4), and CD47 efficiently eliminate colon cancer and leukemia CSCs/TICs.33–36 These approaches are also fascinating, and a reasonable option for elimination of CSCs/TICs. An antibody is a relatively stable protein, but the half-life in peripheral blood is approximately 2 to 3 weeks, and therefore serial administration is needed to maintain the effects of the antibody. On the other hand, antigenic peptide vaccination can induce specific CTLs as memory cells in vivo, such that the specific immunity will last for several years. Thus, peptide vaccination therapy may also be useful for prevention of post-treatment cancer recurrence.
In conclusion, we report here the novel finding that colon cancer CSCs/TICs are as sensitive to CTLs as are non-CSCs/TICs, and that CEP55, a tumor-associated antigen, is a suitable antigen for targeting colon cancer CSCs/TICs.
Acknowledgments
We thank Drs. Kohzoh Imai, Kyogo Itoh, and Pierre G. Coulie for kindly providing cell lines.
Footnotes
Supported in part by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (N.S.) and by the Program for Developing the Supporting System for Upgrading Education and Research under the Ministry of Education, Culture, Sports, Science and Technology of Japan (N.S.).
CME Disclosure: None of the authors disclosed any relevant financial relationships.
References
- 1.Papailiou J., Bramis K.J., Gazouli M., Theodoropoulos G. Stem cells in colon cancer: A new era in cancer theory begins. Int J Colorectal Dis. 2011;26:1–11. doi: 10.1007/s00384-010-1022-6. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M.F., Dick J.E., Dirks P.B., Eaves C.J., Jamieson C.H., Jones D.L., Visvader J., Weissman I.L., Wahl G.M. Cancer stem cells—perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 3.Dalerba P., Dylla S.J., Park I.K., Liu R., Wang X., Cho R.W., Hoey T., Gurney A., Huang E.H., Simeone D.M., Shelton A.A., Parmiani G., Castelli C., Clarke M.F. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien C.A., Pollett A., Gallinger S., Dick J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 5.Ricci-Vitiani L., Lombardi D.G., Pilozzi E., Biffoni M., Todaro M., Peschle C., De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 6.Huang E.H., Hynes M.J., Zhang T., Ginestier C., Dontu G., Appelman H., Fields J.Z., Wicha M.S., Boman B.M. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean M., Fojo T., Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 8.Low J.A., de Sauvage F.J. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol. 2010;28:5321–5326. doi: 10.1200/JCO.2010.27.9943. [DOI] [PubMed] [Google Scholar]
- 9.Zhao C., Chen A., Jamieson C.H., Fereshteh M., Abrahamsson A., Blum J., Kwon H.Y., Kim J., Chute J.P., Rizzieri D., Munchhof M., VanArsdale T., Beachy P.A., Reya T. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [Erratum appeared in Nature 2009, 460:652] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoda S., Hirohashi Y., Torigoe T., Nakatsugawa M., Kiriyama K., Nakazawa E., Harada K., Takasu H., Tamura Y., Kamiguchi K., Asanuma H., Tsuruma T., Terui T., Ishitani K., Ohmura T., Wang Q., Greene M.I., Hasegawa T., Hirata K., Sato N. Cep55/c10orf3, a tumor antigen derived from a centrosome residing protein in breast carcinoma. J Immunother. 2009;32:474–485. doi: 10.1097/CJI.0b013e3181a1d109. [DOI] [PubMed] [Google Scholar]
- 11.Inoda S., Morita R., Hirohashi Y., Torigoe T., Asanuma H., Nakazawa E., Nakatsugawa M., Tamura Y., Kamiguchi K., Tsuruma T., Terui T., Ishitani K., Hashino S., Wang Q., Greene M.I., Hasegawa T., Hirata K., Asaka M., Sato N. The feasibility of Cep55/c10orf3 derived peptide vaccine therapy for colorectal carcinoma. Exp Mol Pathol. 2011;90:55–60. doi: 10.1016/j.yexmp.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Goodell M.A., Brose K., Paradis G., Conner A.S., Mulligan R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haraguchi N., Utsunomiya T., Inoue H., Tanaka F., Mimori K., Barnard G.F., Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 14.Murase M., Kano M., Tsukahara T., Takahashi A., Torigoe T., Kawaguchi S., Kimura S., Wada T., Uchihashi Y., Kondo T., Yamashita T., Sato N. Side population cells have the characteristics of cancer stem-like cells/cancer-initiating cells in bone sarcomas. Br J Cancer. 2009;101:1425–1432. doi: 10.1038/sj.bjc.6605330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkert J., Otto W.R., Wright N.A. Side populations of gastrointestinal cancers are not enriched in stem cells. J Pathol. 2008;214:564–573. doi: 10.1002/path.2307. [DOI] [PubMed] [Google Scholar]
- 16.Tysnes B.B. Tumor-initiating and -propagating cells: cells that we would like to identify and control. Neoplasia. 2010;12:506–515. doi: 10.1593/neo.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermeulen L., Todaro M., de Sousa Mello F., Sprick M.R., Kemper K., Perez Alea M., Richel D.J., Stassi G., Medema J.P. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campoli M., Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bicknell D.C., Rowan A., Bodmer W.F. Beta 2-microglobulin gene mutations: a study of established colorectal cell lines and fresh tumors. Proc Natl Acad Sci USA. 1994;91:4751–4755. doi: 10.1073/pnas.91.11.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo T., Setoguchi T., Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiba T., Kita K., Zheng Y.W., Yokosuka O., Saisho H., Iwama A., Nakauchi H., Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 22.Ho M.M., Ng A.V., Lam S., Hung J.Y. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 23.Mitsutake N., Iwao A., Nagai K., Namba H., Ohtsuru A., Saenko V., Yamashita S. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology. 2007;148:1797–1803. doi: 10.1210/en.2006-1553. [DOI] [PubMed] [Google Scholar]
- 24.Wang J., Guo L.P., Chen L.Z., Zeng Y.X., Lu S.H. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 25.Todaro M., D'Asaro M., Caccamo N., Iovino F., Francipane M.G., Meraviglia S., Orlando V., La Mendola C., Gulotta G., Salerno A., Dieli F., Stassi G. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 26.Todaro M., Alea M.P., Di Stefano A.B., Cammareri P., Vermeulen L., Iovino F., Tripodo C., Russo A., Gulotta G., Medema J.P., Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Saigusa S., Tanaka K., Toiyama Y., Yokoe T., Okugawa Y., Ioue Y., Miki C., Kusunoki M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16:3488–3498. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 28.Ogino S., Nosho K., Irahara N., Meyerhardt J.A., Baba Y., Shima K., Glickman J.N., Ferrone C.R., Mino-Kenudson M., Tanaka N., Dranoff G., Giovannucci E.L., Fuchs C.S. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei J., Barr J., Kong L.Y., Wang Y., Wu A., Sharma A.K., Gumin J., Henry V., Colman H., Sawaya R., Lang F.F., Heimberger A.B. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16:461–473. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Hirohashi Y., Torigoe T., Inoda S., Kobayasi J., Nakatsugawa M., Mori T., Hara I., Sato N. The functioning antigens: beyond just as the immunological targets. Cancer Sci. 2009;100:798–806. doi: 10.1111/j.1349-7006.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato N., Hirohashi Y., Tsukahara T., Kikuchi T., Sahara H., Kamiguchi K., Ichimiya S., Tamura Y., Torigoe T. Molecular pathological approaches to human tumor immunology. Pathol Int. 2009;59:205–217. doi: 10.1111/j.1440-1827.2009.02353.x. [Erratum appeared in Pathol Int 2009. 59:900] [DOI] [PubMed] [Google Scholar]
- 32.Hirohashi Y., Torigoe T., Inoda S., Takahashi A., Morita R., Nishizawa S., Tamura Y., Suzuki H., Toyota M., Sato N. Immune response against tumor antigens expressed on human cancer stem-like cells/tumor-initiating cells. Immunotherapy. 2010;2:201–211. doi: 10.2217/imt.10.10. [DOI] [PubMed] [Google Scholar]
- 33.Dallas N.A., Xia L., Fan F., Gray M.J., Gaur P., van Buren G., 2nd, Samuel S., Kim M.P., Lim S.J., Ellis L.M. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoey T., Yen W.C., Axelrod F., Basi J., Donigian L., Dylla S., Fitch-Bruhns M., Lazetic S., Park I.K., Sato A., Satyal S., Wang X., Clarke M.F., Lewicki J., Gurney A. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Majeti R., Chao M.P., Alizadeh A.A., Pang W.W., Jaiswal S., Gibbs K.D., Jr, van Rooijen N., Weissman I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaiswal S., Jamieson C.H., Pang W.W., Park C.Y., Chao M.P., Majeti R., Traver D., van Rooijen N., Weissman I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]


