Abstract
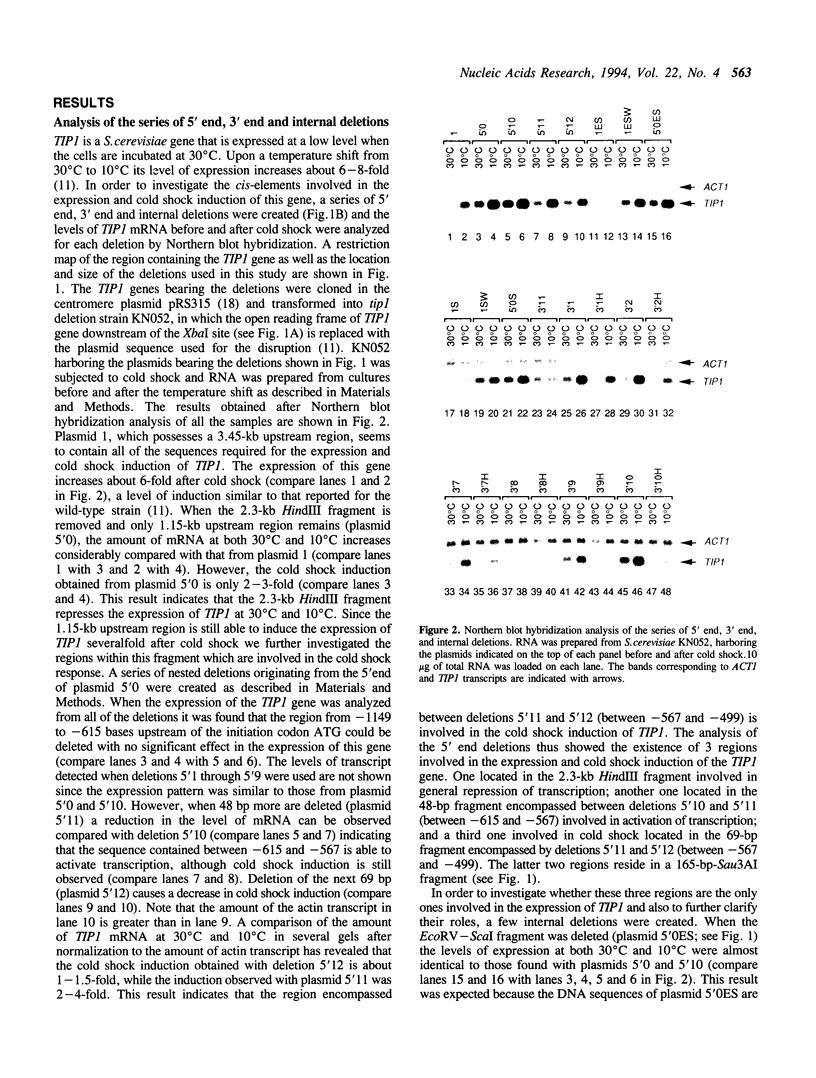
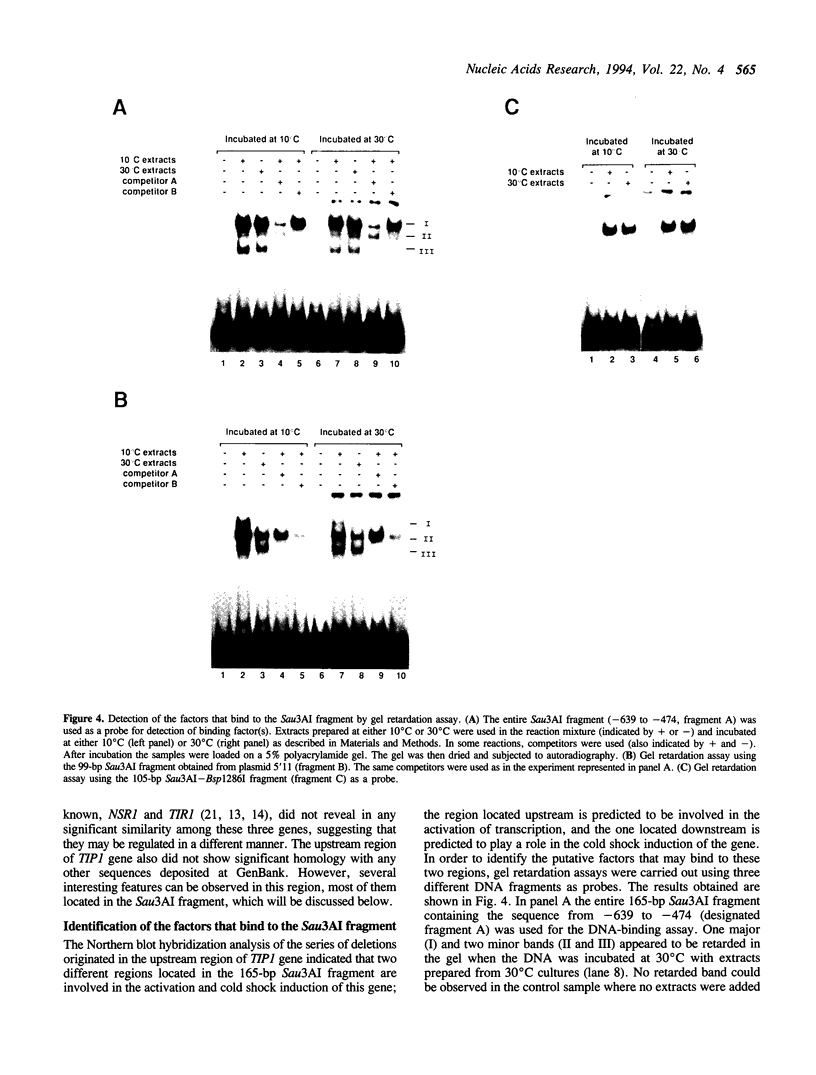
Northern blot hybridization analysis of a series of 5' end, 3' end and internal deletions has revealed that at least four different regions are involved in the regulation of the expression of TIP1, a cold shock-inducible gene of Saccharomyces cerevisiae. One of these four regions has negative effect on the expression of the TIP1 gene, while the others are responsible for the activation and cold shock-induction of the gene. A fragment involved in the cold-shock induction of TIP1 was used as a probe in gel retardation assays to identify the cold shock-factor. The cold shock-factor could be detected in cells grown at 30 degrees C as well as 10 degrees C, but both the amount of the factor and its affinity to DNA were found to increase 2-3-fold after cold shock. In addition, another factor was found to bind just upstream of the cold shock element, in a region where a transcriptional activator was predicted to function by Northern blot hybridization analysis. The amount of this activating factor and its affinity for DNA was not affected by temperature. Implications of our data on possible mechanisms of transcriptional regulation of the TIP1 gene by cold shock are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fantino E., Marguet D., Lauquin G. J. Downstream activating sequence within the coding region of a yeast gene: specific binding in vitro of RAP1 protein. Mol Gen Genet. 1992 Dec;236(1):65–75. doi: 10.1007/BF00279644. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. G., Krah R., Tafuri S. R., Wolffe A. P. DNA gyrase, CS7.4, and the cold shock response in Escherichia coli. J Bacteriol. 1992 Sep;174(18):5798–5802. doi: 10.1128/jb.174.18.5798-5802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. G., VanBogelen R. A., Neidhardt F. C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987 May;169(5):2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Inouye M. TIP 1, a cold shock-inducible gene of Saccharomyces cerevisiae. J Biol Chem. 1991 Sep 15;266(26):17537–17544. [PubMed] [Google Scholar]
- Kondo K., Inouye M. Yeast NSR1 protein that has structural similarity to mammalian nucleolin is involved in pre-rRNA processing. J Biol Chem. 1992 Aug 15;267(23):16252–16258. [PubMed] [Google Scholar]
- Kondo K., Kowalski L. R., Inouye M. Cold shock induction of yeast NSR1 protein and its role in pre-rRNA processing. J Biol Chem. 1992 Aug 15;267(23):16259–16265. [PubMed] [Google Scholar]
- La Teana A., Brandi A., Falconi M., Spurio R., Pon C. L., Gualerzi C. O. Identification of a cold shock transcriptional enhancer of the Escherichia coli gene encoding nucleoid protein H-NS. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10907–10911. doi: 10.1073/pnas.88.23.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. C., Xue Z. X., Mélèse T. The NSR1 gene encodes a protein that specifically binds nuclear localization sequences and has two RNA recognition motifs. J Cell Biol. 1991 Apr;113(1):1–12. doi: 10.1083/jcb.113.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Marguet D., Guo X. J., Lauquin G. J. Yeast gene SRP1 (serine-rich protein). Intragenic repeat structure and identification of a family of SRP1-related DNA sequences. J Mol Biol. 1988 Aug 5;202(3):455–470. doi: 10.1016/0022-2836(88)90278-1. [DOI] [PubMed] [Google Scholar]
- Marguet D., Lauquin G. J. The yeast SRP gene: positive modulation by glucose of its transcriptional expression. Biochem Biophys Res Commun. 1986 Jul 16;138(1):297–303. doi: 10.1016/0006-291x(86)90279-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin H., Marahiel M. A., Heinemann U. Universal nucleic acid-binding domain revealed by crystal structure of the B. subtilis major cold-shock protein. Nature. 1993 Jul 8;364(6433):164–168. doi: 10.1038/364164a0. [DOI] [PubMed] [Google Scholar]
- Schnuchel A., Wiltscheck R., Czisch M., Herrler M., Willimsky G., Graumann P., Marahiel M. A., Holak T. A. Structure in solution of the major cold-shock protein from Bacillus subtilis. Nature. 1993 Jul 8;364(6433):169–171. doi: 10.1038/364169a0. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Tanabe H., Goldstein J., Yang M., Inouye M. Identification of the promoter region of the Escherichia coli major cold shock gene, cspA. J Bacteriol. 1992 Jun;174(12):3867–3873. doi: 10.1128/jb.174.12.3867-3873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willimsky G., Bang H., Fischer G., Marahiel M. A. Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting cell viability at low temperatures. J Bacteriol. 1992 Oct;174(20):6326–6335. doi: 10.1128/jb.174.20.6326-6335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Tafuri S., Ranjan M., Familari M. The Y-box factors: a family of nucleic acid binding proteins conserved from Escherichia coli to man. New Biol. 1992 Apr;4(4):290–298. [PubMed] [Google Scholar]