Abstract
Introduction:
Vestibular schwannomas (VS) are benign tumors originating from Schwann cells in vestibulocochlear nerve. This study aimed at evaluating outcomes of microsurgical resections of VS based on tumor sizes in a South Asian country.
Methods:
The chart notes of 71 patients who underwent microsurgical resections of VS at a single academic center over a 20-year period (1990–2009) were reviewed, and relevant information was extracted. For analyzing outcomes, patients were divided into two groups based on tumor size at initial presentation: (1) Group A (tumor size ≤ 4 cm) and (2) Group B (tumor size > 4 cm). Pearson's chi-square and Fisher's exact tests were used for comparison of proportions; the independent sample t-test was used for comparison of means.
Results:
The average tumor diameter was 4.1 ± 1.5 (range, 1–6.6) cm. Complete resection was achieved more frequently in patients in Group A (P < 0.001). Duration of hospital stay and cost of treatment were significantly higher in Group B patients (P < 0.003 and P < 0.04, respectively). The severity of postoperative facial nerve injury, assessed by House–Brackmann grading system, was significantly higher in Group B (P < 0.01). Cerebrospinal fluid (CSF) leak and lower cranial nerve deficits also occurred more frequently after resection in Group B (P = 0.031 and P = 0.003, respectively).
Conclusion:
We conclude that advanced stage tumors suggestive of delayed presentation are fairly common in Pakistan, and limit curative resection in the majority of patients. Postoperative morbidity is significantly higher in patients with tumor size > 4 cm.
Keywords: Acoustic neuroma, vestibular schwannoma, microsurgical resection, neurotological outcome, South Asia, tumor size
INTRODUCTION
Vestibular schwannomas (VS) are benign, slow growing tumors which originate from Schwann cells at the oligodendrocyte–Schwann cell junction (i.e., the Obersteiner–Redlich zone), in the peripheral portion of superior and inferior vestibular as well as cochlear nerves. These tumors are frequently referred to as acoustic neuromas, but the preferred term to be used is VS because they are composed of Schwann cells, and more frequently originate in the vestibular portion of the eighth cranial nerve (CN).[4] These tumors comprise about 10% of primary intracranial tumors, 85% of tumors at the cerebellopontine angle, and 90% of intracranial schwannomas.[12,14]
Early in the twentieth century, the goal of treatment for VS was to prolong life with minimal operative mortality. However, by the end of the century, with improvement in neuroimaging modalities and with advent of microsurgical techniques and intraoperative monitoring tools, there was a paradigm shift toward excision of tumor with preservation of CN function.[16]
In developed countries, most VS are diagnosed early when they are still small in size but in developing countries such as Pakistan, where healthcare system is less refined, large VS are fairly common. Large tumors may be associated with compression of CNs, brainstem, and/or obstruction of cerebrospinal fluid (CSF) pathways causing life-threatening hydrocephalus, thus requiring urgent surgical treatment.[18,27] For large tumors, hearing preservation is generally not a consideration as most of the patients have poor or no hearing preoperatively.[27] In developing countries, most of the centers lack the facilities to perform intraoperative monitoring of facial nerves thus making its preservation even more difficult.
In this study, we have analyzed outcomes of microsurgical resections of VS in a South Asian country based on preoperative tumor sizes. The results are interpreted in the context of data from Western centers.
MATERIALS AND METHODS
Study design
This was a retrospective descriptive study based on chart note review of all patients who underwent microsurgical resection of VS at the Aga Khan University Hospital (AKUH), Karachi, Pakistan over a 20-year period (1990–2009).
Case identification
Surgically treated cases of VS were identified by searching institution's medical record system based on ICD-9-CM (International Classification of Diseases, 9th revision with clinical modification) coding. The chart notes of all identified patients were retrieved and reviewed. All data were collected on a pro forma specifically designed for the purpose. Diagnosis of VS was based on clinical presentation, radiological imaging, and postoperative histopathology reports in all patients.
Inclusion criteria
All cases of VS who underwent microsurgical excision, and whose chart notes were considered complete with regards the required baseline information, were included in the study.
Exclusion criteria
Conservatively managed cases and patients with incomplete chart notes were excluded from the study.
Chart review
Information on following variables was extracted from hospital, laboratory, and radiology records: patient age and gender; signs and symptoms of VS; audiometric tests; laboratory and radiological investigations; tumor size at initial presentation; surgical approach and extent of resection; duration of stay and cost of treatment; and postoperative complications and outcome. The duration of follow-up (from surgery to last clinic visit) was also recorded.
The tumor size was measured (on computed tomography (CT) and/or magnetic resonance imaging (MRI) scan) in three axes, i.e. diameter parallel to the petrous ridge, diameter perpendicular to the petrous ridge, and the vertical diameter in the coronal slices. The size of VS was taken as the largest diameter in any one of the three axes. For analyzing outcomes, patients were divided into two groups based on tumor size at initial presentation: (1) Group A (tumor size ≤ 4 cm) and (2) Group B (tumor size >4 cm). Both pre- and postoperative tumor sizes were recorded. Tumor extension was described using the Hannover grading system:[15] : Class T1, purely intrameatal; Class T2, intra- and extrameatal; Class T3a, filling the cerebellopontine cistern; Class T3b, reaching the brain stem; Class T4a, compressing the brain stem; Class T4b, severely dislocating the brain stem and compressing the fourth ventricle.
Facial nerve (FN) function was assessed using the House-Brackmann grade[8] (HB grade) and was classified as excellent (HB grade 1–2), intermediate (HB grade 3–4), and poor (HB grade 5–6). HB grade at last follow-up was used to analyze FN outcome.
Data analysis
We used Statistical Package for Social Sciences (SPSS) version 16.0 (© SPSS Inc., 1989-2007) for data entry and analysis. Continuous variables are reported as means (± standard deviation), with median (and range) also shown in the case of a skewed distribution. Pearson's chi-square and Fisher's exact tests were used for comparison of proportions; the independent sample t-test was used for comparison of means. For all comparisons, a P-value of <0.05 was considered statistically significant.
RESULTS
General patient data
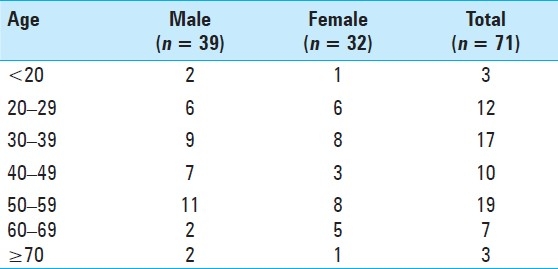
Between 1990 and 2009, seventy-eight consecutive patients with VS underwent microsurgical resection at authors′ institution. Among these, 71 patients in whom the clinical and operative records were available were entered into the study. The average age of the patients was 43 ± 15 (range, 12–75) years. Thirty-nine (55%) patients were male and 32 (45%) were female. The most prevalent age was in the sixth decade [Table 1]. The average age of female patients was higher than that of male patients (43.25 versus 42 years), although this was not statistically significant. The average length of follow-up was 24.2 ± 35.8 months (range, 9 days to 225 months).
Table 1.
Patients’ age by decade and gender
Sixty-nine (97.2%) patients had unilateral tumors; 35 right-sided tumors and 34 left-sided tumors. Two patients (2.8%) had bilateral tumors (neurofibromatosis type-2). In one of these patients, bilateral surgery was performed and complete excision of the tumor was achieved on the side with larger tumor and a partial resection was done on the contralateral side after a period of time. This patient had a co-existing medullary astrocytoma which was also surgically excised. The other patient with bilateral VS underwent surgical resection for the left-sided tumor; the contralateral tumor was conservatively managed. This patient had co-existing trigeminal and olfactory nerve schwannomas and multiple dural meningiomas.
Previous surgery had been performed elsewhere in four patients. These patients underwent subtotal resection 9 months to 3 years before initial presentation at authors’ institution. Three out of these four patients had lost hearing and FN in previous surgery and presented with facial paralysis.
Based on 58 available CT and/or MRI scans (discussed later), there were 32 (55.2%) patients in Group A (tumor size ≤ 4cm) and 26 (44.8%) patients in Group B (tumor size > 4 cm).
Clinical presentation
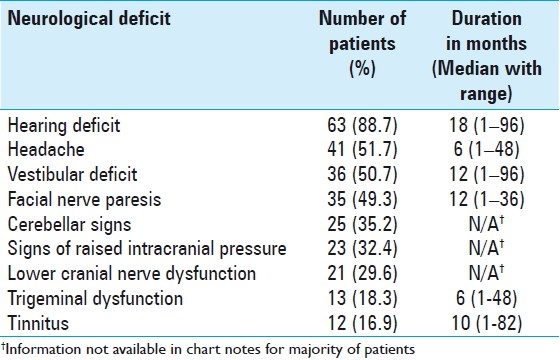
Varying degrees of hearing loss were found in 63 (88.7%) patients (28 in Group A and 24 in Group B, P = 0.293). Twenty-six (41.3%) out of these 63 patients had a completely deaf ear whereas 37 (58.7%) had variable hearing deficits. Hearing deficits were unilateral in 61 (96.8%) patients and bilateral in 2 (3.2%). Among the 46 patients in whom the character of hearing loss was recorded, 36 (78.3%) had a history of progressive hearing loss and 10 patients (21.7%) had an acute attack with total deafness.
Forty-one patients (51.7%) had headaches (18 in Group A and 19 in Group B, P = 0.589). Tinnitus was present in 12 (16.9%) patients (4 in Group A and 5 in Group B, P = 0.98). Vestibular deficits were noted in 36 (50.7%) (13 in Group A and 15 in Group B, P = 0.291). Varying degrees of facial paresis were observed in 35 (49.3%) (12 in Group A and 17 in Group B, P = 0.129). Three out of these 35 patients had facial paralysis after previous surgery for VS outside authors’ institution. Trigeminal nerve deficits were present in 13 (18.3%) patients (4 in Group A and 6 in Group B, P = 0.540); altered sensation of face was identified in 10 (77%), diminished or absent corneal reflex in 9 (69.2%), weakness of muscles of mastication in 4 (30.8%), and neuralgia in 1 (7.7%) patient. Other CN deficits involved optic nerve in 7 (9.8%), oculomotor nerve in 1 (1.4%), glossopharyngeal nerve in 12 (16.9%), and vagus nerve in 9 (12.7%).
Cerebellar signs were present in 25 (35.2%) patients (8 in Group A and 14 in Group B, P=0.193). Twenty-two (88%) had gait disturbances, 7 (28%) had nystagmus, and 5 (20%) had ataxia. Clinically, raised intracranial pressure (ICP) was suspected in 23 (32.4%) patients (5 in Group A and 16 in Group B, P < 0.01). Clinical features suggesting raised ICP were headaches in all, nausea/vomiting in 16 (69.6%) patients, drowsiness in 4 (17.4%), and papilledema in 3 (13%) cases.
Table 2 depicts the documented major clinical features along with their duration in 71 patients with VS.
Table 2.
Preoperative cranial nerve and other neurological deficits in 71 patients with vestibular schwannomas
Preoperative diagnosis
Audiometric studies were performed in 17 (23.9%) patients. On pure tone audiometry (PTA), all had a “dead ear”. Brainstem evoked response audiometry was done in 4 (5.6%) patients. Latency of response waves and interauricular latency of greater than 0.2 ms was identified in all four cases.
Diagnostic neuroradiologic imaging was available in 58 patients, which included CT scan only in 8 (13.8%), MRI in 40 (69%), and both CT and MRI in 10 (17.2%) patients.
Tumor size and extension
The average preoperative tumor size was 4.1 ± 1.5 (range, 1–6.6) cm which was measured in the largest diameter on 58 available CT and/or MRI scans. Thirty-two (55.2%) patients had tumor size ≤4 cm (Group A) whereas remaining 26 (44.8%) patients had tumor size >4 cm (Group B). Tumor extension based on the Hannover grading system is demonstrated in Table 3. Twenty-nine (50%) patients had a T4 tumor. Imaging reports documented hydrocephalus in 18 (31%) out of these 58 patients. Fourteen (53.8%) patients in Group B had hydrocephalus whereas only 4 (12.5%) in Group A developed hydrocephalus (P = 0.001). Preoperative extraventricular drains and ventriculoperitoneal shunts were placed in 10 (14.08%) and 12 (16.9%) patients, respectively.
Table 3.
Tumor extension using the Hannover grading system determined from 58 available CT and/or MRI scans
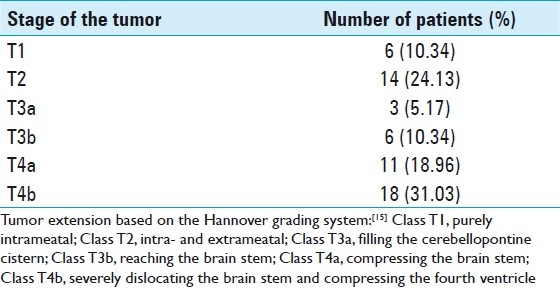
Figure 1 depicts the MRI scans of a VS patient with 4.2 cm and stage T4b tumor.
Figure 1.
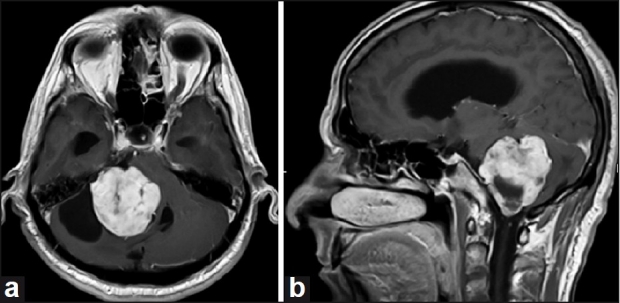
(a and b) Axial and sagittal, T1 weighted, post-contrast magnetic resonance imaging (MRI) brain scans of a patient with a 4.2 cm vestibular schwannoma (stage T4b)
Surgical procedure and extent of resection
Microsurgical resection was done using posterior fossa (suboccipital/retrosigmoid) approach in the majority (n = 67, 94.4%) of patients. Translabyrinthine excision of VS was performed in 3 (4.2%) cases; one (1.4%) resection was done using the middle cranial fossa approach. Surgical resections were performed by seven different neurosurgeons during a period of 20 years. An otolaryngeal surgeon was involved in three cases. Intraoperative facial nerve monitoring was done in eight patients. Neuronavigation-guided craniotomy was done in one patient. Intraoperatively, tumor was found to be vascular in 32 (45%), adherent to adjacent tissues in 31 (43.6%), encapsulated in 8 (11.3%), cystic in 6 (8.45%), and hemorrhagic in 4 (5.6%) patients. All tumors on histopathology received a diagnosis of benign schwannomas and none of the samples revealed any malignant change.
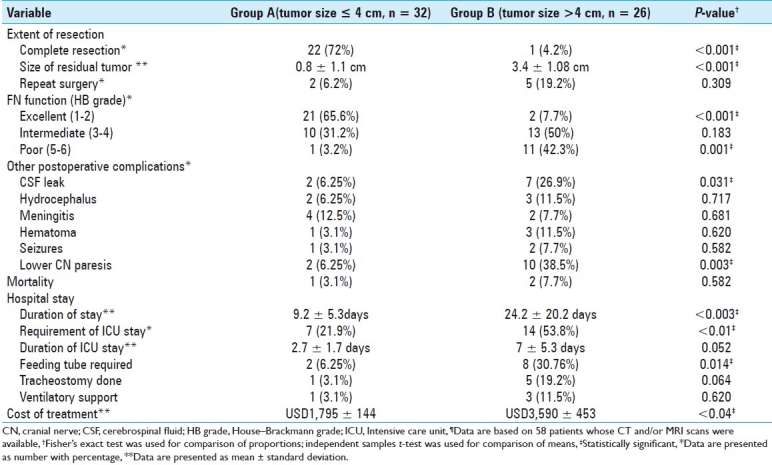
The extent of tumor resection as determined by the operative records and postoperative CT and/or MRI was as follows: complete resection in 34 (47.9%) and partial/subtotal resection in 37 (52.1%). Complete resection was done in 22 (71%) patients in Group A and in 1 (4.2%) patient in Group B (P < 0.001). The mean size of postoperative residual tumor calculated from 34 available scans was 2.1 ± 1.7 (range, 0–5) cm. The residual tumor size was significantly higher in Group B (3.4 ± 1.08 cm) as compared to Group A (0.8 ± 1.1cm) (P < 0.001). In cases where the partial/subtotal resection was done, some portion of tumor adherent to the facial nerve or brain stem was left behind. A redo surgery was done after a period of time in 9 (12.67%) patients, 2 in Group A and 5 in Group B (P = 0.309).
Duration of stay and cost of treatment
The mean duration of hospital stay was 16.2 ± 17.8 (range, 3–102) days. The mean duration of stay in Group B (24.2 ± 20.2) was significantly higher than Group A (9.2 ± 5.3) (P<0.003). Postoperatively, intensive care unit (ICU) stay was required in 27 (38%) patients. Seven out of 32 (21.9%) patients in Group A stayed in ICU whereas 14 out of 26 (53.8%) patients in Group B required ICU stay (P < 0.01). The average duration of ICU stay was 5.4 ± 4.6 (range, 1–10) days. The difference in mean duration of ICU stay between Groups A and B (2.7 ± 1.7 versus 7 ± 5.3) was approaching statistical significance (P = 0.052).
The average cost of treatment (including surgery and in-hospital stay) was Pakistan rupee (PKR) 205,755.54 ± 26,059.86 [United States dollars (USD) 2404.81 ± 304.58, based on the current exchange rate, 1USD = 85.56 PKR]. The mean cost of treatment in Group B [PKR 307,165.52 ± 38,825.42 (USD 3,590.06 ± 453.78)] was significantly higher as compared to Group A [PKR 153,647.79 ± 12,380.53 (USD 1,795.79 ± 144.70)](P < 0.04).
Facial nerve and hearing preservation
The frequency of new-onset FN injury was 38%. The HB grades of FN injury were significantly higher in Group B as compared to Group A (P < 0.001). Ten patients with facial weakness received gold weight lid implants, and four patients underwent facial-hypoglossal nerve anastomosis.
Hearing function was impaired in the majority (88.7%) of patients preoperatively. New-onset hearing loss was documented in two patients postoperatively (both in Group B). Overall, postoperative hearing was normal in nine (12.7%) patients only. None of the patients in Group B had normal hearing at last follow-up. However, it is difficult to comment about hearing preservation due to lack of objective evaluation (like audiometric tests) in the majority of patients.
Other neurological outcomes and surgical complications
Overall, CSF leak occurred in 13 (18.3%) patients, managed with lumbar drain in 12 (92.3%) patients. CSF leak was observed more frequently in Group B as compared to Group A (P = 0.031). Associated meningitis occurred in seven (9.9%) patients. Postoperative hydrocephalus developed in nine (12.7%) patients. CN IX, X, and XII deficits occurred in 10 (14.08%), 9 (12.67%), and 1 (1.4%) patients, respectively. Overall frequency of postoperative lower CN lesions was significantly higher in Group B than Group A (P = 0.003). The feeding tube was required in 11 (15.5%) patients including the nasogastric tube in 8 and PEG in 3 patients. The feeding tube was required more frequently in Group B as compared to Group A (P = 0.014). Tracheostomy was done in 8 (11.26%) patients and ventilatory support was required in 5 (7%) patients. The lower CN paresis improved significantly in the majority of patients on 6–8 weeks follow-ups. Varying degrees of postoperative hemorrhage occurred in 6 (8.5%) patients. Postoperative seizures were observed in 3 (4.2%) patients.
Mortality occurred in 3 (4.2%) patients, 1 in Group A and 2 in Group B (P = 0.582). First patient had aspiration pneumonia and later developed a lung abscess with Enterobacter species in the tracheal aspirate. This patient received ventilatory support and died on ninth postoperative day. Respiratory failure accounted for mortality in the second patient who died 1 month after the surgical excision of tumor. Aspiration pneumonia accompanied by the left-sided thoracic empyema was the cause of death in the third patient who died on 10th postoperative day.
Table 4 depicts the comparison of extent of resection, FN outcome and postoperative morbidity, and hospital stay and cost of treatment between groups A and B.
Table 4.
Comparison of extent of resection, facial nerve outcome and other postoperative neurological deficits and surgical complications, hospital stay and cost of treatment based on the tumor size¶
DISCUSSION
The actual incidence and natural history of VS in Pakistani population is not known. Very little data are available on the topic.[1] Our study has brought forth an important aspect of VS in Pakistan, i.e. delayed presentation with advanced stage tumors. Up to the best of our knowledge, no previous surgical series has reported such a large mean tumor size for VS patients. Inadequate screening and referral systems account for delayed presentation resulting in poor prognosis. Previous studies on other brain tumors in Pakistan have shown a similar trend.[19] The fact that only 78 patients had VS surgery during a 20-year period at AKUH, one of the largest tertiary care hospitals in the country with a wide catchment both geographically and across social strata reflects the inaccessibility to expeditious health care provision to the majority of our population.
Most patients in our study sought treatment for varying degrees of hearing loss, which was found in 88.7% of patients at the initial examination. This is consistent with Western data where reported incidence of hearing loss in patients with VS ranges from 80% to 96%.[9,18,26] However, 16.9% of our patients reported tinnitus in contrast to 40–90% documented by others.[15,26] Also, many more patients in our series (51.7%) complained of headaches compared to 5–30% reported by others.[1] Both these findings can be attributed to late presentation and larger tumor size. An inverse association has been suggested between tinnitus and tumor extension; patients with smaller tumors have higher rates of tinnitus.[1,15] Our study further corroborates the evidence.
As tumor enlarges and extends to the cerebellopontine angle, other CNs and structures may become involved. The sensory division of the trigeminal nerve is usually the first CN affected other than the vestibulocochlear nerve.[1] FN shows resilience even under extreme pressure and stretching with sensory division getting involved first.[1] Other authors have reported deficits of CN V as 20% and CN VII as 6–33% which is in congruence with our data.[15,24,26]
In contrast to certain Western settings where signs of raised ICP and cerebellar dysfunction are rarely the presenting features,[10,11] many of our patients had clinical evidence of raised ICP (32.4%) and/or cerebellar deficits (35.2%) at the time of admission. It may also be related to delayed presentation in our series. These large tumors caused brain stem compression and obstructive hydrocephalus in many of our patients (Group B > Group A). Preoperative ventriculoperitoneal shunts were required in 16.9% patients in our series. The frequency of preoperative shunt has been reported as 8.3% in a recent study from India.[9]
Microsurgical resection of VS can be accomplished via three operative approaches: translabyrinthine approach, middle fossa or temporal approach, and posterior fossa (retrosigmoid/suboccipital) approach.[13,17] We used the posterior fossa (retrosigmoid/suboccipital) approach in the majority (94.4%) of our patients. In the literature on microsurgical experience, complete resection of tumors has been achieved in 96–98% patients and FN function has been preserved in 65–100% patients.[3,13,16] The rate of hearing preservation ranges from 13 to 82% with the posterior fossa approach.[3,13,16,20]
Complete resection could be achieved in less than half (47.9%) patients in our series which is lower than the Western reports.[3,13,16] However, the rate of complete resection was markedly higher (71%) for tumors ≤4 cm.
In our study, the facial nerve could only be preserved in 34.6% patients with tumor size >4 cm. Large VS are associated with a worse functional facial nerve outcome as reported by Tos et al.[23] and by Grey et al.[7] Ojemann[16] in his series of 410 patients reported that HB grade 1 or 2 FN function was achieved in 98% of small tumors, 96% of medium tumors (1.0–1.9 cm), 75% of medium-large tumors (2.0–2.9 cm), 56% of large tumors (3.0–4.0 cm), and 56% of giant tumors (>4.0 cm). Somers et al.[21] also reported that FN functional outcome is dependent on tumor size, with HB grade 1 or 2 function being the rule (92%) for small tumors (<1.0 cm), still attainable (82%) for medium tumors (1.1-2.5 cm), but less common (56%) for large tumors (>2.6 cm). However, most of these papers are reports of a single surgeon's experience whereas our series presents results of microsurgical resections performed by seven different neurosurgeons over a period of 20 years with many of them performing a maximum of 12 surgeries over a period of 5 years. Thus the concept of a learning curve for VS surgery[22] cannot be applied to our series. According to Whittaker and Luetje[25] a surgeon operating less than 12 cases per year cannot expect to get equal results in large series. The preoperative size of tumor is an essential factor determining the FN preservation rate. As reported by others, a reciprocal relationship between tumor size and FN preservation rate was observed in our study. Also, a lack of intraoperative facial nerve monitoring may have adversely affected FN preservation rate. Such monitoring is highly warranted especially for resections of large-size tumors.[27,28]
Several studies also suggest that hearing preservation is more likely with smaller tumors with good preoperative hearing.[5,9] Ebersold et al.[3] have reported no postoperative hearing in any of their patients with preoperative tumor size >4 cm. Our study further corroborates the evidence that hearing preservation is very poor for tumors >4 cm.
CSF leak remains an important issue in the surgical treatment of VS. The reported frequency ranges between 0 and 30%, with an approximate average rate of 12%, however, making comparisons between various published series are difficult because of methodological issues.[2,9] In this study, frequency of CSF leak was 18.3% (18/71 patients) with 12 patients requiring lumbar drain; the frequency is in congruence with the series published by Fishman et al.[6] Brennan et al.[2] have reported a significant effect of tumor size on the leakage rate after retrosigmoid resection of VS which is at par with our findings. The frequency of associated meningitis in our series (9.9%) was slightly higher as compared to some of the recent series (between 3.7% and 9.2%).[9,11]
Microsurgical resection of VS is associated with a concomitant risk of lower CN injuries in large size tumors which can complicate postoperative course and may lead to increased morbidity and mortality.[9] The 21% (15/71 patients) frequency of lower CN injuries in our series is higher than the 1.5–6.8% reported incidence in published reports.[12,15] Nonetheless, the majority of lower CN injuries in our study were noted in Group B (tumor size > 4 cm).
The higher frequency of postoperative complications in Group B led to a higher duration of hospital stay and thus increased cost of treatment in these patients. Early detection when the tumor is of small size can not only result in better outcome but may also lead to reduction in cost of treatment.
CONCLUSION
Advanced stage tumors suggestive of a delayed presentation are more common in Pakistan. Postoperative morbidity including FN injury, hearing loss, lower CN deficits, and CSF leak are significantly higher in patients with tumor size > 4 cm leading to higher cost of treatment in these patients. We infer from our results that reduction in postoperative morbidity in our part of the world can be achieved with early detection of tumor emphasizing the need of effective screening and referral system.
Contributor Information
Syed Faraz Kazim, Email: farazkazim@gmail.com.
Muhammad Shahzad Shamim, Email: shahzad.shamim@aku.edu.
Syed Ather Enam, Email: ather.enam@aku.edu.
Muhammad Ehsan Bari, Email: ehsan.bari@aku.edu.
REFERENCES
- 1.Awan MS, Qureshi HU, Sheikh AA, Ali MM. Vestibular schwannomas: Clinical presentation, management and outcome. J Pak Med Assoc. 2001;51:63–7. [PubMed] [Google Scholar]
- 2.Brennan JW, Rowed DW, Nedzelski JM, Chen JM. Cerebrospinal fluid leak after acoustic neuroma surgery: Influence of tumor size and surgical approach on incidence and response to treatment. J Neurosurg. 2001;94:217–23. doi: 10.3171/jns.2001.94.2.0217. [DOI] [PubMed] [Google Scholar]
- 3.Ebersold MJ, Harner SG, Beatty CW, Harper CM, Jr, Quast LM. Current results of the retrosigmoid approach to acoustic neurinoma. J Neurosurg. 1992;76:901–9. doi: 10.3171/jns.1992.76.6.0901. [DOI] [PubMed] [Google Scholar]
- 4.Eldridge R, Parry D. Summary: Vestibular Schwannoma (Acoustic Neuroma) Consensus Development Conference. Neurosurgery. 1992;30:962–4. [PubMed] [Google Scholar]
- 5.Fischer G, Fischer C, Rémond J. Hearing preservation in acoustic neurinoma surgery. J Neurosurg. 1992;76:910–7. doi: 10.3171/jns.1992.76.6.0910. [DOI] [PubMed] [Google Scholar]
- 6.Fishman AJ, Hoffman RA, Roland JT Jr, Lebowitz RA, Cohen NL. Cerebrospinal fluid drainage in the management of CSF leak following acoustic neuroma surgery. Laryngoscope. 1996;106:1004–6. doi: 10.1097/00005537-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Grey PL, Moffat DA, Palmer CR, Hardy DG, Baguley DM. Factors which influence the facial nerve outcome in vestibular schwannoma surgery. Clin Otolaryngol Allied Sci. 1996;21:409–13. doi: 10.1046/j.1365-2273.1996.00816.x. [DOI] [PubMed] [Google Scholar]
- 8.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1995;113:179–80. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 9.Jain VK, Mehrotra N, Sahu RN, Behari S, Banerji D, Chhabra DK. Surgery of vestibular schwannomas: An institutional experience. Neurol India. 2005;53:41–5. doi: 10.4103/0028-3886.15052. [DOI] [PubMed] [Google Scholar]
- 10.Jung S, Kang SS, Kim TS, Kim HJ, Jeong SK, Kim SC, et al. Current surgical results of retrosigmoid approach in extralarge vestibular schwannomas. Surg Neurol. 2000;53:370–7. doi: 10.1016/s0090-3019(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 11.Lanman TH, Brackmann DE, Hitselberger WE, Subin B. Report of 190 consecutive cases of large acoustic tumors (vestibular schwannoma) removed via the translabyrinthine approach. J Neurosurg. 1999;19:617–23. doi: 10.3171/jns.1999.90.4.0617. [DOI] [PubMed] [Google Scholar]
- 12.Lanser MJ, Sussman SA, Frazer K. Epidemiology, pathogensis, and genetics of acoustic tumors. Otolaryngol Clin North Am. 1992;25:499–520. [PubMed] [Google Scholar]
- 13.Lee SH, Willcox TO, Buchheit WA. Current results of the surgical management of acoustic neuroma. Skull Base. 2002;12:189–95. doi: 10.1055/s-2002-35750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machinis TG, Fountas KN, Dimpoulos V, Robinson JS. History of acousticneurinoma surgery. Neurosurg Focus. 2005;18:1–4. doi: 10.3171/foc.2005.18.4.10. [DOI] [PubMed] [Google Scholar]
- 15.Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): Clinical presentation. Neurosurgery. 1997;40:1–10. doi: 10.1097/00006123-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Ojemann RG. Management of acoustic neuromas (vestibular schwannomas) (honored guest presentation) Clin Neurosurg. 1993;40:498–535. [PubMed] [Google Scholar]
- 17.Ramamurthi B. The continuing challenge of acoustic neurinomas (1949-1993) Br J Neurosurg. 1995;9:361–6. doi: 10.1080/02688699550041368. [DOI] [PubMed] [Google Scholar]
- 18.Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): Hearing function in 1000 tumor resections. Neurosurgery. 1997;40:248–60. doi: 10.1097/00006123-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Shamim MS, Bari ME, Khursheed F, Jooma R, Enam SA. Pituitary adenomas: Presentations and outcomes in a South Asian country. Can J Neurol Sci. 2008;35:198–203. doi: 10.1017/s0317167100008635. [DOI] [PubMed] [Google Scholar]
- 20.Snyder WE, Pritz MB, Smith RR. Suboccipital resection of a medial acoustic neuroma with hearing preservation. Surg Neurol. 1999;51:548–52. doi: 10.1016/s0090-3019(97)00528-4. [DOI] [PubMed] [Google Scholar]
- 21.Somers T, Offeciers FE, Schatteman I. Results of 100 vestibular schwannoma operations. Acta Otorhinolaryngol Belg. 2003;57:155–66. [PubMed] [Google Scholar]
- 22.Tonn JC, Schlake HP, Goldbrunner R, Milewski C, Helms J, Roosen K. Acoustic neuroma surgery as an interdisciplinary approach: A neurosurgical series of 508 patients. J Neurol Neurosurg Psychiatry. 2000;69:161–6. doi: 10.1136/jnnp.69.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tos M, Thomsen J, Harmsen A. Results of translabyrinthine removal of 300 acoustic neuromas related to tumour size. Acta Otolaryngol Suppl. 1988;452:38–51. doi: 10.3109/00016488809124993. [DOI] [PubMed] [Google Scholar]
- 24.van Leeuwen JP, Cremers CW, Thewissen NP, Harhangi BS, Meijer E. Acoustic neuroma: Correlation among tumor size, symptoms, and patient age. Laryngoscope. 1995;105:701–7. doi: 10.1288/00005537-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Whittaker CK, Luetje CM. Vestibular schwannomas. J Neurosurg. 1992;76:897–900. doi: 10.3171/jns.1992.76.6.0897. [DOI] [PubMed] [Google Scholar]
- 26.Wiegand DA, Ojemann RJ, Fickel V. Surgical treatment of acoustic neuroma (vestibular schwannoma) in the United States: Report from the Acoustic Neuroma Registry. Laryngoscope. 1996;106:58–66. doi: 10.1097/00005537-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Fei Z, Chen YJ, Fu LA, Zhang JN, Liu WP, et al. Facial nerve function after excision of large acoustic neuromas via the suboccipital retrosigmoid approach. J Clin Neurosci. 2005;12:405–8. doi: 10.1016/j.jocn.2004.03.042. [DOI] [PubMed] [Google Scholar]


