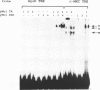
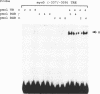
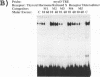
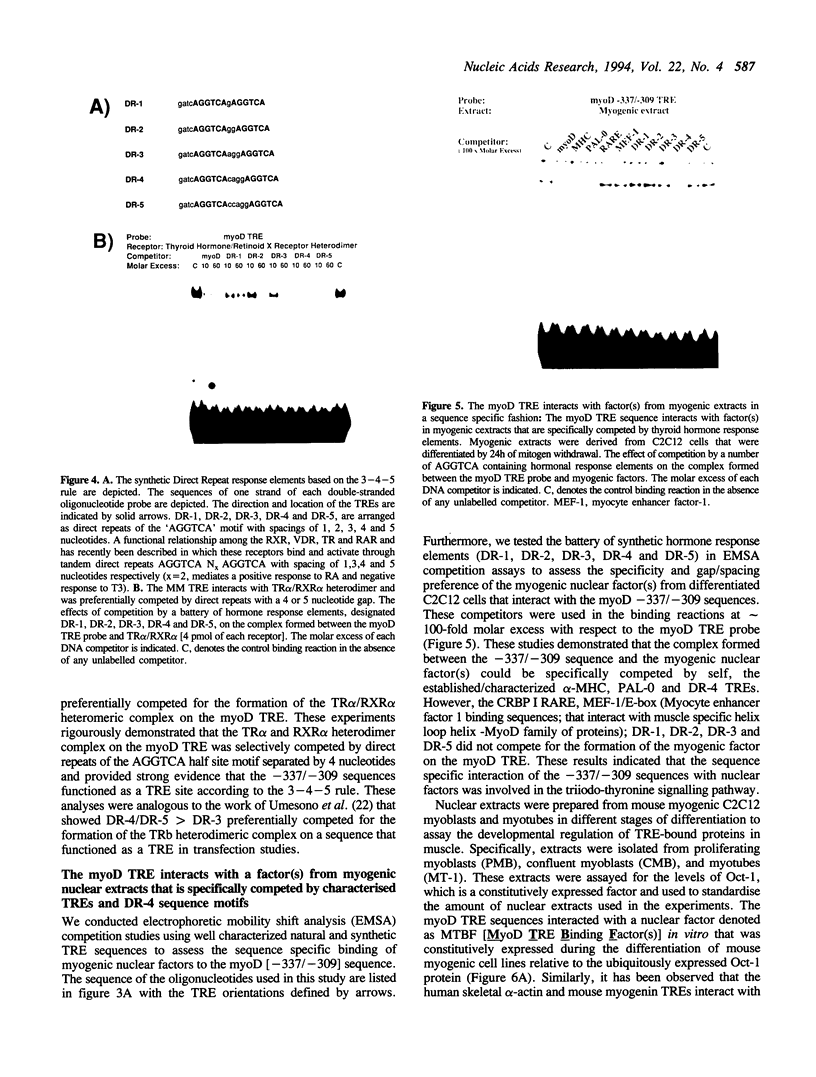
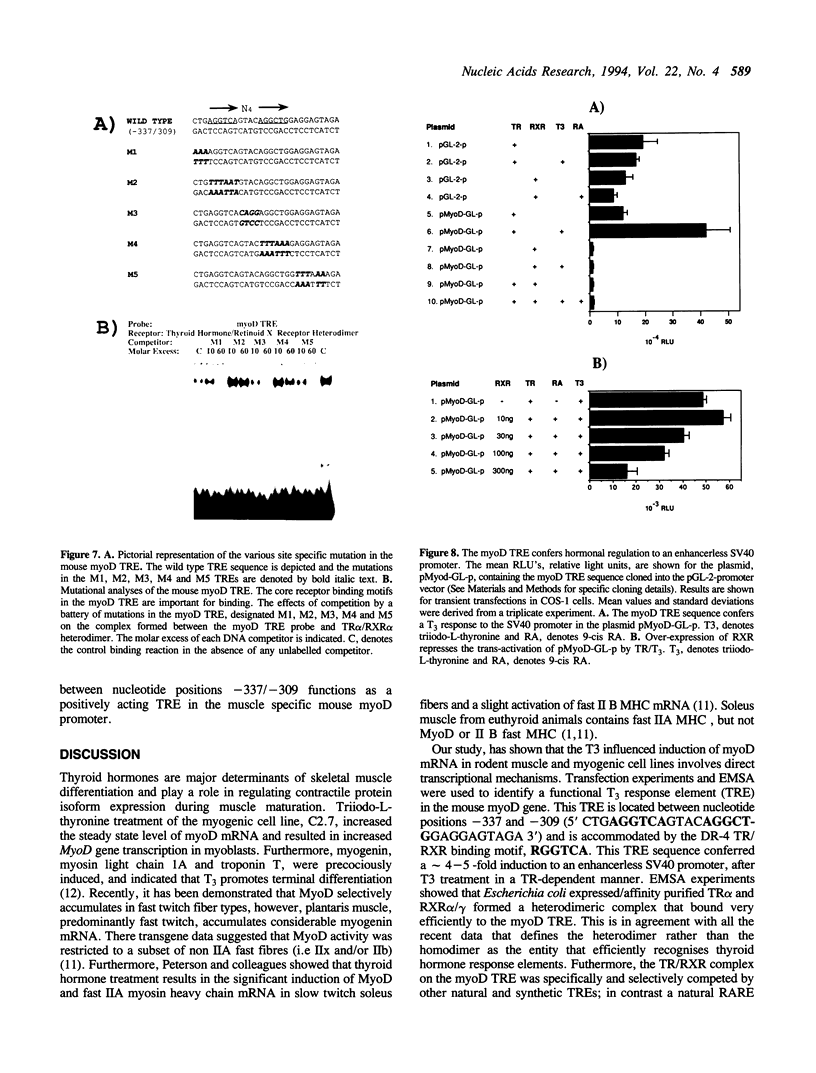
Abstract
Thyroid hormones are major determinants of skeletal muscle differentiation in vivo. Triiodo-L-thyronine treatment promotes terminal muscle differentiation and results in increased MyoD gene transcription in myogenic cell lines; furthermore myoD and fast myosin heavy chain gene expression are activated in rodent slow twitch muscle fibers (Molecular Endocrinology 6: 1185-1194, 1992; Development 118: 1137-1147, 1993). We have identified a T3 response element (TRE) in the mouse MyoD promoter between nucleotide positions -337 and -309 (5' CTGAGGTCAGTACAGGCTGGAGGAGTAGA 3'). This sequence conferred an appropriate T3 response to an enhancerless SV40 promoter. In vitro binding studies showed that the thyroid hormone receptor alpha (TR alpha) formed a heterodimeric complex, with either the retinoid X receptor alpha or gamma 1 isoforms (RXR alpha, RXR gamm), on the MyoD TRE that was specifically competed by other well characterised TREs and not by other response elements. Analyses of this heterodimer with a battery of steroid hormone response elements indicated that the complex was efficiently competed by a direct repeat of the AGGTCA motif separated by 4 nucleotides as predicted by the 3-4-5 rule. EMSA experiments demonstrated that the nuclear factor(s) present in muscle cells that bound to the myoD TRE were constitutively expressed during myogenesis; this complex was competed by the myosin heavy chain, DR-4 and PAL-0 TREs in a sequence specific fashion. Western blot analysis indicated that TR alpha 1 was constitutively expressed during C2C12 differentiation. Mutagenesis of the myoD TRE indicated that the sequence of the direct repeats (AGGTCA) and the 4 nucleotide gap were necessary for efficient binding to the TR alpha/RXR alpha heterodimeric complex. In conclusion our data suggest that the TRE in the helix loop helix gene, myoD, is a target for the direct heterodimeric binding of TR alpha and RXR alpha/gamma. These results provide a molecular mechanism/model for the effects of triiodo-L-thyronine on in vitro myogenesis; the activation of myoD gene expression in the slow twitch fibres and the cascade of myogenic events regulated by thyroid hormone.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler-Browne G. S., Herlicoviez D., Whalen R. G. Effects of hypothyroidism on myosin isozyme transitions in developing rat muscle. FEBS Lett. 1984 Jan 23;166(1):71–75. doi: 10.1016/0014-5793(84)80047-2. [DOI] [PubMed] [Google Scholar]
- Carnac G., Albagli-Curiel O., Vandromme M., Pinset C., Montarras D., Laudet V., Bonnieu A. 3,5,3'-Triiodothyronine positively regulates both MyoD1 gene transcription and terminal differentiation in C2 myoblasts. Mol Endocrinol. 1992 Aug;6(8):1185–1194. doi: 10.1210/mend.6.8.1406697. [DOI] [PubMed] [Google Scholar]
- Collie E. S., Muscat G. E. The human skeletal alpha-actin promoter is regulated by thyroid hormone: identification of a thyroid hormone response element. Cell Growth Differ. 1992 Jan;3(1):31–42. [PubMed] [Google Scholar]
- Darling D. S., Beebe J. S., Burnside J., Winslow E. R., Chin W. W. 3,5,3'-triiodothyronine (T3) receptor-auxiliary protein (TRAP) binds DNA and forms heterodimers with the T3 receptor. Mol Endocrinol. 1991 Jan;5(1):73–84. doi: 10.1210/mend-5-1-73. [DOI] [PubMed] [Google Scholar]
- Downes M., Griggs R., Atkins A., Olson E. N., Muscat G. E. Identification of a thyroid hormone response element in the mouse myogenin gene: characterization of the thyroid hormone and retinoid X receptor heterodimeric binding site. Cell Growth Differ. 1993 Nov;4(11):901–909. [PubMed] [Google Scholar]
- Forman B. M., Samuels H. H. Interactions among a subfamily of nuclear hormone receptors: the regulatory zipper model. Mol Endocrinol. 1990 Sep;4(9):1293–1301. doi: 10.1210/mend-4-9-1293. [DOI] [PubMed] [Google Scholar]
- Gambke B., Lyons G. E., Haselgrove J., Kelly A. M., Rubinstein N. A. Thyroidal and neural control of myosin transitions during development of rat fast and slow muscles. FEBS Lett. 1983 Jun 13;156(2):335–339. doi: 10.1016/0014-5793(83)80524-9. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Devary O. V., Rosenfeld M. G. Multiple cell type-specific proteins differentially regulate target sequence recognition by the alpha retinoic acid receptor. Cell. 1990 Nov 16;63(4):729–738. doi: 10.1016/0092-8674(90)90139-6. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M. Regulation of gene expression by the thyroid hormone receptor. Biochim Biophys Acta. 1990 Dec 11;1032(2-3):157–176. doi: 10.1016/0304-419x(90)90002-i. [DOI] [PubMed] [Google Scholar]
- Gossett L. A., Kelvin D. J., Sternberg E. A., Olson E. N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989 Nov;9(11):5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. Nuclear hormone receptors. Promiscuous liaisons. Nature. 1993 Feb 18;361(6413):590–591. doi: 10.1038/361590a0. [DOI] [PubMed] [Google Scholar]
- Hermann T., Hoffmann B., Zhang X. K., Tran P., Pfahl M. Heterodimeric receptor complexes determine 3,5,3'-triiodothyronine and retinoid signaling specificities. Mol Endocrinol. 1992 Jul;6(7):1153–1162. doi: 10.1210/mend.6.7.1324421. [DOI] [PubMed] [Google Scholar]
- Hughes S. M., Taylor J. M., Tapscott S. J., Gurley C. M., Carter W. J., Peterson C. A. Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development. 1993 Aug;118(4):1137–1147. doi: 10.1242/dev.118.4.1137. [DOI] [PubMed] [Google Scholar]
- Ianuzzo D., Patel P., Chen V., O'Brien P., Williams C. Thyroidal trophic influence on skeletal muscle myosin. Nature. 1977 Nov 3;270(5632):74–76. doi: 10.1038/270074a0. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa R., Yu V. C., När A., Kyakumoto S., Han Z., Silverman S., Rosenfeld M. G., Glass C. K. Differential orientations of the DNA-binding domain and carboxy-terminal dimerization interface regulate binding site selection by nuclear receptor heterodimers. Genes Dev. 1993 Jul;7(7B):1423–1435. doi: 10.1101/gad.7.7b.1423. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Zhang X. K., Graupner G., Lee M. O., Hermann T., Hoffmann B., Pfahl M. Formation of retinoid X receptor homodimers leads to repression of T3 response: hormonal cross talk by ligand-induced squelching. Mol Cell Biol. 1993 Dec;13(12):7698–7707. doi: 10.1128/mcb.13.12.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Liu Q., Linney E. The mouse retinoid-X receptor-gamma gene: genomic organization and evidence for functional isoforms. Mol Endocrinol. 1993 May;7(5):651–658. doi: 10.1210/mend.7.5.8391126. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Mercadier J. J., Schwartz K. Changes in gene expression during cardiac growth. Int Rev Cytol. 1991;124:137–186. doi: 10.1016/s0074-7696(08)61526-0. [DOI] [PubMed] [Google Scholar]
- Mader S., Leroy P., Chen J. Y., Chambon P. Multiple parameters control the selectivity of nuclear receptors for their response elements. Selectivity and promiscuity in response element recognition by retinoic acid receptors and retinoid X receptors. J Biol Chem. 1993 Jan 5;268(1):591–600. [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Murray M. B., Towle H. C. Identification of nuclear factors that enhance binding of the thyroid hormone receptor to a thyroid hormone response element. Mol Endocrinol. 1989 Sep;3(9):1434–1442. doi: 10.1210/mend-3-9-1434. [DOI] [PubMed] [Google Scholar]
- Muscat G. E., Emery J., Collie E. S. Tissue-specific expression of the skeletal alpha-actin gene involves sequences that can function independently of MyoD and Id. Gene Expr. 1992;2(3):241–257. [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Gobius K., Emery J. Proliferin, a prolactin/growth hormone-like peptide represses myogenic-specific transcription by the suppression of an essential serum response factor-like DNA-binding activity. Mol Endocrinol. 1991 Jun;5(6):802–814. doi: 10.1210/mend-5-6-802. [DOI] [PubMed] [Google Scholar]
- Muscat G. E., Griggs R., Downes M., Emery J. Characterization of the thyroid hormone response element in the skeletal alpha-actin gene: negative regulation of T3 receptor binding by the retinoid X receptor. Cell Growth Differ. 1993 Apr;4(4):269–279. [PubMed] [Google Scholar]
- Muscat G. E., Perry S., Prentice H., Kedes L. The human skeletal alpha-actin gene is regulated by a muscle-specific enhancer that binds three nuclear factors. Gene Expr. 1992;2(2):111–126. [PMC free article] [PubMed] [Google Scholar]
- Nagpal S., Saunders M., Kastner P., Durand B., Nakshatri H., Chambon P. Promoter context- and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors. Cell. 1992 Sep 18;70(6):1007–1019. doi: 10.1016/0092-8674(92)90250-g. [DOI] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Olson E. N. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992 Dec;154(2):261–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Perlmann T., Rangarajan P. N., Umesono K., Evans R. M. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993 Jul;7(7B):1411–1422. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- Sugie H., Verity M. A. Postnatal histochemical fiber type differentiation in normal and hypothyroid rat soleus muscle. Muscle Nerve. 1985 Oct;8(8):654–660. doi: 10.1002/mus.880080805. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986 Jul;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Toutant M., Butler-Browne G. S., Watkins S. C. Hereditary pituitary dwarfism in mice affects skeletal and cardiac myosin isozyme transitions differently. J Cell Biol. 1985 Aug;101(2):603–609. doi: 10.1083/jcb.101.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zilz N. D., Murray M. B., Towle H. C. Identification of multiple thyroid hormone response elements located far upstream from the rat S14 promoter. J Biol Chem. 1990 May 15;265(14):8136–8143. [PubMed] [Google Scholar]
- Zingg J. M., Alva G. P., Jost J. P. Characterisation of a genomic clone covering the structural mouse MyoD1 gene and its promoter region. Nucleic Acids Res. 1991 Dec 11;19(23):6433–6439. doi: 10.1093/nar/19.23.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hardeveld C., Kassenaar A. A. Thyroid hormone uptake and T4 derived T3 formation in different skeletal muscle types of normal and hyperthyroid rats. Acta Endocrinol (Copenh) 1978 Jun;88(2):306–320. doi: 10.1530/acta.0.0880306. [DOI] [PubMed] [Google Scholar]