Abstract
Tumor angiogenesis appears to be achieved by the expression of vascular endothelial growth factor (VEGF) within solid tumors that stimulate host vascular endothelial cell mitogenesis and possibly chemotaxis. VEGF's angiogenic actions are mediated through its high-affinity binding to 2 endothelium-specific receptor tyrosine kinase, Flt-1 (VEGFR1), and Flk-1/KDR (VEGFR2). RNA interference-mediated knockdown of protein expression at the messenger RNA level provides a new therapeutic strategy to overcome various diseases. To achieve high efficacy in RNA interference-mediated therapy, it is critical to develop an efficient delivering system to deliver small interference RNA (siRNA) into tissues or cells site-specifically. We previously reported an angiogenic endothelial cell-targeted polymeric gene carrier, PEI-g-PEG-RGD. This targeted carrier was developed by the conjugation of the ανβ3/ανβ5 integrin-binding RGD peptide (ACDCRGDCFC) to the cationic polymer, branched polyethylenimine, with a hydrophilic polyethylene glycol (PEG) spacer. In this study, we used PEI-g-PEG-RGD to deliver siRNA against VEGFR1 into tumor site. The physicochemical properties of PEI-g-PEG-RGD/siRNA complexes was evaluated. Further, tumor growth profile was also investigated after systemic administration of PEI-g-PEG-RGD/siRNA complexes.
Introduction
Vascular endothelial growth factor (VEGF), a member of the platelet-derived growth factor family, is considered as a major inducer of angiogenesis and vessel permeability. Tumor angiogenesis can be achieved by the expression of VEGF within solid tumors that stimulate host vascular endothelial cell mitogenesis and possibly chemotaxis (GILES, 2001; MARGOLIN, 2002). In addition, expression of the VEGF is upregulated by hypoxia (Shweiki et al., 1992; Aiello et al., 1995) and inflammatory mediators (Cheng et al., 1998). VEGF's angiogenic actions are mediated through its high-affinity binding to 2 endothelium-specific receptor tyrosine kinase, Flt-1 (fms-like tyrosine kinase or VEGFR1), and Flk-1/KDR (fetal live kinase or VEGFR2). Flt-1 shows at least 10-fold higher affinity for VEGF relative to Flk-1/KDR (Terman et al., 1992; Millauer et al., 1993; Quinn et al., 1993). During developmental angiogenesis, VEGFR1 acts as a negative regulator of VEGF activity, but in adult mice with Lewis lung carcinoma, tumor angiogenesis was stimulated by VEGFR1 (Hiratsuka et al., 2001). In mice with ischemic retinopathy, intravenous injection of small interference RNA (siRNA) targeting VEGFR1 reduced retinal neovascularization by 32% compared to control siRNA (Shen et al., 2006). Also, a VEGFRs tyrosine kinase inhibitor, GW654652, significantly decreased cell proliferation and induced apoptosis in human umbilical vein endothelial cells and M6 mammary tumor cells (Huh et al., 2005). Thus, silencing of VEGF and VEGFR expression or inhibition of VEGF-VEGFR interaction with inhibitors has a therapeutic potential for suppression of tumor angiogenesis.
Numerous studies have been conducted via suppression of VEGF expression using antisense oligonucleotides (Robinson et al., 1996), soluble VEGF receptor (sFlt-1) (Kendall and Thomas, 1993; Goldman et al., 1998), VEGF receptor chimeric proteins (Aiello et al., 1995), and through interference with intracellular signal transduction pathways (Ozaki et al., 2000; Takahashi et al., 2003). More recently, siRNA has been used to silence the expression of VEGF (Reich et al., 2003; Kim et al., 2006a).
RNA interference (RNAi)-mediated knockdown of protein expression at the messenger RNA (mRNA) level provides a new therapeutic strategy to overcome various diseases. RNAi has been successfully utilized in various therapeutic applications such as combat against viral pathogenesis, cancer, and inflammation by siRNA-mediated silencing of the responsible genes (Song et al., 2003; Flynn et al., 2004; Yano et al., 2004). To achieve high efficacy in RNAi-mediated therapy, it is crucial to develop an efficient delivering system to deliver siRNA into tissues or cells site-specifically (Sorensen et al., 2003).
We previously reported an angiogenic endothelial cell-targeted polymeric gene carrier, PEI-g-PEG-RGD. This targeted carrier was developed by the conjugation of the ανβ3/ανβ5 integrin-binding RGD peptide (ACDCRGDCFC) to the cationic polymer, namely, branched polyethylenimine (BPEI) with a hydrophilic polyethylene glycol (PEG) spacer (Suh et al., 2002; Kim et al., 2005b, 2006b). In vitro transfection showed that PEI-g-PEG-RGD efficiently transferred therapeutic gene to angiogenic endothelial cells, but not to the nonangiogenic cells (Kim et al., 2005b). In addition, the PEI-g-PEG-RGD gene carrier delivered genes to tumors more efficiently than PEI-g-PEG after systemic administration (Kim et al., 2006b).
In the present study, we introduced synthetic siRNA targeting VEGFR for inhibiting tumor growth. To deliver siRNA into tumor site, we used the targeted polymeric gene carrier, PEI-g-PEG-RGD, which was previously developed in our group. The physicochemical properties of PEI-g-PEG-RGD/siRNA complexes were evaluated. Further, tumor growth profile was also investigated after systemic administration of PEI-g-PEG-RGD/siRNA complexes.
Materials and Methods
Materials
BPEI (average molecular weight 25 kDa; average degree of polymerization 580) and Rosewell Park Memorial Institute (RPMI 1640) medium were purchased from Sigma-Aldrich (Milwaukee, WI). Dulbecco's modified Eagle's medium, penicillin–streptomycin, trypsin-like enzyme (TrypLE Express), and Dulbecco's phosphate buffered saline were purchased from Gibco BRL (Carlsbad, CA). N-hydroxysuccinimide-vinyl sulfone PEG (molecular weight 3400) was purchased from NEKTAR (Huntsville, AL). RGD peptide, ACDCRGDCFC, was purchased from the Genemed Synthesis, Inc. (San Franscisco, CA). After synthesis, peptides were purified via reverse-phase high-performance liquid chromatography and then analyzed by mass spectrometry performed using matrix-assisted laser desorption/ionization time of flight mass spectrometer. Fetal bovine serum (FBS) was purchased from HyClone (Logan, UT). siRNAs were purchased from IDT Tech., Inc. (Coralville, IA) and sequences of siRNA are shown in Fig. 1B. All siRNAs were 21-nucleotide-long double-stranded RNA oligos with a 2 nucleotide overhang (TT) at the 3′ end. The siRNA and scRNA stand for mouse siRNA against VEGF receptor 1 and scrambled siRNA, respectively. We selected siRNA sequences as reported by Kim et al. (2004). SVR cell line and CT-26 colon adenocarcinoma cell lines were purchased from American Type Culture Collection (Manassas, VA).
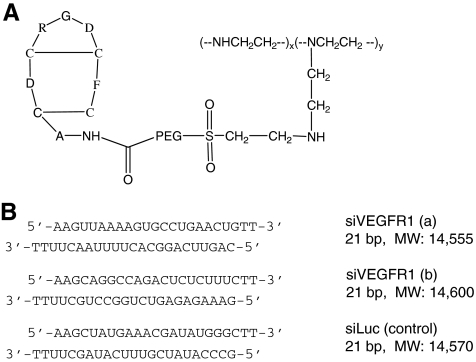
FIG. 1.
Structure of PEI-g-PEG-RGD polymer (A) and sequences of siRNAs targeting VEGFR1 and unrelated siRNAs (B). VEGFR, vascular endothelial growth factor receptor; siRNA, small interference RNA.
Synthesis of PEI-g-PEG-RGD
The PEI-g-PEG-RGD conjugate (Fig. 1A) was synthesized and purified as reported earlier (Suh et al., 2002; Kim et al., 2005b). Briefly, in the first step, RGD peptide was conjugated to N-hydroxysuccinimide-vinyl sulfone PEG in anhydrous N,N-dimethylformamide (DMF) in presence of 4 molar excess of triethylamine (TEA). In the subsequent step, 2 molar excess of RGD-PEG-VS conjugates were mixed with BPEI solution in pH 9.0 sodium carbonate buffer and incubated at room temperature overnight. The final product, PEI-g-PEG-RGD, was purified by dialysis and lyophilized. The composition of PEI-g-PEG-RGD conjugates were analyzed by 1H-nuclear magnetic resonance (1H-NMR).1H-NMR spectra were obtained on a Varian Inova 400 MHz NMR spectrometer (Varian, Palo Alto, CA) using standard proton parameters. Chemical shifts were referenced to the residual HDO resonance at ∼4.7 ppm. The molar ratios of RGD to PEG and RGD to PEI of conjugates were 1 and 1.3, respectively as determined by NMR spectrum analysis (Kim et al., 2005b).
Polyacrylamide gel electrophoresis studies
Various amounts of PEI-g-PEG-RGD, ranging from 0 to 115 ng, were added to 7 ng of siRNA at various N/P ratios (nitrogen of PEI-g-PEG-RGD/phosphate of siRNA) ranging from 0 to 100 in 5% glucose solution and incubated for 30 minutes at room temperature. After incubation, each sample was electrophoresed on a 13% polyacrylamide gel (w/v) for 1 hour at 100 V. TBE (89 mM Tris-borate, 2 mM EDTA) buffer was used as electrophoresis buffer. After ethidium bromide (0.1 μg/mL) staining, the gel was illuminated with a UV illuminator to determine the location of uncomplexed siRNA.
Stability of siRNA in serum
Stability assay of siRNA in serum was performed as described in previous a report (Kim et al., 2007). Briefly, PEI-g-PEG-RGD/siRNA complexes (N/P = 10) or free siRNA (63 ng) were incubated at 37°C in the 5% glucose solution containing 20% FBS. At 0, 0.5, 1, 3, and 6 hour postincubation, 10 μL of samples was taken into Eppendorf tubes, and stored at −70°C. The samples were thawed and mixed with 2 μL of 2% sodium dodecyl sulfate and analyzed by electrophoresis using a 13% polyacrylamide gel. After electrophoresis, gels were stained with ethidium bromide and observed on a UV-transilluminator. The gel images were obtained using a GelDoc system (BioRad, Hercules, CA).
Zeta potential and size distribution measurement
Surface charge and particle size distribution assay of polymer/siRNA complexes were performed as described previously (Kim et al., 2007). Briefly, PEI-g-PEG-RGD/siRNA complexes were prepared at various N/P ratios ranging from 0 to 30 by adding various amounts of PEI-g-PEG-RGD into the same volume of siRNA (1 μg in 5% glucose solution). The mixture was then incubated for 30 minutes at room temperature. Zeta potential and size distribution of each sample were determined by 3 serial measurements using a Zetasizer 3000HSA (MALVERN Instruments, Worcestershire, United Kingdom).
Cell culture
SVR cells (CRL-2280, ATCC) and CT-26 colon adenocarcinoma cell line were grown and maintained in Dulbecco's modified Eagle's medium and RPMI 1640 medium, respectively; supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin; and maintained at 37°C under humidified atmosphere.
Reverse transcription–polymerase chain reaction
SVR cells were grown to 80% confluency in 6-well plates and transfected with 8.73 μg of PEI-g-PEG-RGD complexed with either siLuc or siVEGFR. After 24-hour incubation, the cells were trypsinized from the plate and total RNA was isolated from the transfected cells using the Qiagen RNeasy kit according to the manufacturer's DNase protocol (Qiagen, Valencia, CA) to eliminate DNA contamination. The concentration of RNA was estimated by measuring the absorbance at 260 nm. Equal amount of cDNAs was synthesized in 20 μL of reaction mixtures by reverse transcription using SuperScript™ III Reverse Transcriptase (Invitrogen, Carlsbad, CA). To this mixture, we added 100 ng of total RNA. The reaction was allowed to proceed at 65°C for 5 minutes and at 4°C for 5 minutes, and subsequently heated for 50 minutes at 50°C and at 85°C for 5 minutes. Specific oligonucleotide primers were the forward primer 5′-CAG GAC GAT GAA TCT GAG CTG-3′ and backward primer 5′-CAC TGC TCC TTC CTG TCC AG -3′. The polymerase chain reaction (PCR) reaction involved heating at 95°C for 5 minutes, 30 cycles at 94°C for 15 seconds, 58.5°C for 30 seconds, and 72°C for 35 seconds, followed by an extension of 5 minutes at 72°C. The PCR products were separated by electrophoresis in 1% agarose gels.
Mice
Five-week-old female BALB/c mice were purchased from Simonsen Laboratories (Gilroy, CA) and housed in the Animal Care Facility, Biomedical Polymers Research Building, University of Utah. Mice were maintained on ad libitum rodent feed and water at room temperature and 40% humidity. All mice were acclimated for at least 1 week before tumor implantation. All studies were performed in accordance with the approved animal protocol.
Tumor implantation and treatment
To generate tumors, 5-week-old female BALB/c mice were injected subcutaneously in the middle of the right flank with 100 μL of a single-cell suspension containing 1 × 106 CT-26 cells. Tumor size was measured using a digital vernier caliper across its longest (a) and shortest diameters (b) and its volume was calculated using the formula V = 0.5ab2. Treatment of the tumors was started after 10–15 days when the tumor size attained a volume of ∼65–70 mm3.
Tumor growth inhibition studies
Mice received intravenous injections of siVEGFR or siLuc (7 μg) complexed with polymers in a 200 μL solution of 5% glucose at N/P ratio of 10 (n = 5). All tumor-bearing mice were administrated with injections at an interval of twice a week (day 1, 4, 8, and 11). In all cases, tumors were measured every 3 days and mice were examined for monitoring the appearance and growth of necrosis as well as decreased physical activity. Tumor progression was reported in terms of tumor volume over a period of 11 days.
Statistical analysis
Results were reported as the mean ± SEM. The statistical analysis between groups was determined using a nonpaired t-test. P < 0.05 was considered to be significant.
Results
Polyelectrolyte complex formation of PEI-g-PEG-RGD with siRNA
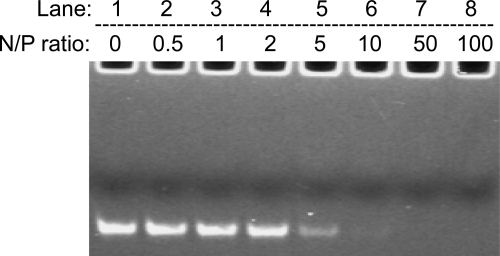
To deliver siRNA into cells, polymeric carrier should form stable polyplex with siRNA. To ascertain the formation of PEI-g-PEG-RGD/siRNA complexes, the gel retardation assay was performed at various N/P ratios and observed on a UV-transilluminator. The electrophoretic mobility of the siRNA in the gel was retarded as the amount of the PEI-g-PEG-RGD was increased, indicating the charge neutralization due to the effective binding between PEI-g-PEG-RGD and siRNA. As shown in Fig. 2, when the value of N/P ratio of PEI-g-PEG-RGD/siRNA reached 10, free siRNA could not be detected on polyacrylamide gel electrophoresis, indicating that complete retardation of siRNA occurred at N/P ratio 5–10.
FIG. 2.
Electrophoretic mobility assay of siRNA complexes with PEI-g-PEG-RGD. Lane 1: siRNA only; lanes 2–8, the N/P ratio of PEI-g-PEG-RGD/siRNA = 0.5, 1, 2, 5, 10, 50, and 100, respectively.
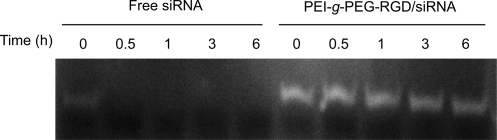
Stability of siRNA in serum
siRNA becomes subjected to facile degradation by various serumic enzymes such as nucleases and RNases when injected into blood stream. This enzymatic degradation of siRNA can be averted by the formation of compact polyelectrolyte complex between siRNA and cationic polymer so that the physical shield of cationic polymer may prevent the approach of serumic enzymes toward the siRNA. To evaluate the protection of siRNA by PEI-g-PEG-RGD from serum enzymes, we performed a serum stability assay. The PEI-g-PEG-RGD/siRNA polyplex was formed by incubation of siRNA with PEI-g-PEG-RGD for 30 minutes, and then, the polyplexes were incubated at 37°C in the presence of 20% FBS. When incubated with 20% FBS, free siRNAs were completely degraded due to RNase in the FBS within 30 minutes of incubation. However, the condensed siRNAs by PEI-g-PEG-RGD were efficiently protected at N/P ratio 10 (Fig. 3). As shown in Fig. 3, siRNA remained intact at lease for 6 hour even in the presence of 20% FBS, which might ensure prolonged contact between the polyplex and the target cells during circulation when injected into the blood stream.
FIG. 3.
siRNA protection assay in serum. Degradation of siRNA exposed to serum was measured for PEI-g-PEG-RGD/siRNA complexes and compared with free siRNA. The PEI-g-PEG-RGD/siRNA complexes, or free siRNA, was incubated in 20% serum from 0 to 6 hour. Undegraded, intact siRNA is detected after polyacrylamide gel electrophoresis and ethidium bromide staining.
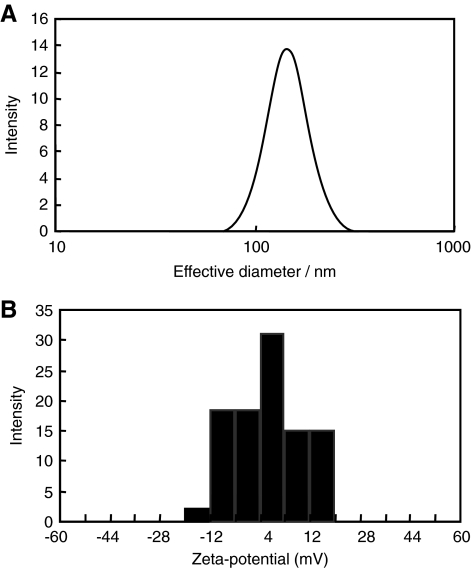
Physicochemical properties of PEI-g-PEG-RGD/siRNA complex
To achieve high efficiency for siRNA delivery, it is essential to optimize the physicochemical properties of polyplexes such as size and surface charge. Therefore, surface charge and effective particle size of PEI-g-PEG-RGD/siRNA were studied. Different N/P ratios of PEI-g-PEG-RGD to siRNA were investigated at constant siRNA concentration. As shown in Table 1, the sizes of all the complexes were lying between 114 and 180 nm at various N/P ratios ranging from 5 to 30. Initially, the size of polyplexes decreased abruptly to 114 nm at N/P ratio 5, and then it attained constant size of 134 nm at N/P ratio 30. This result is in good agreement with other reports (Shim and Kwon, 2009; Mok et al., 2010). In addition, the relative homogenous size distributions of complexes are unimodal as shown in Fig. 4A. The surface charges of complexes were also studied at various N/P ratios (Table 1). The zeta potential for all complexes remained in the range of 0.8–5.8 mV with the exception of N/P ratio 1, where the stable polyplex could not be formed completely. The relative surface charge also showed unimodal distribution of complexes as shown in Fig. 4B. For enhanced cellular uptake of complexes, positive surface charge of complexes is necessary for binding to anionic cell surfaces. We believed that the nano-sized PEI-g-PEG-RGD/siRNA complexes charged positively had a potential as an efficient siRNA delivery carrier.
Table 1.
Particle Characterization of PEI-g-PEG-RGD/Small Interference RNA Complex
| N/P | 1 | 5 | 10 | 20 | 30 |
|---|---|---|---|---|---|
| Mean particle size (nm) | >1000 | 114.3 ± 8.5 | 160.9 ± 5.5 | 180.0 ± 6.1 | 134.0 ± 5.7 |
| Zeta-potential (mV) | −2.9 ± 5.5 | 0.8 ± 2.1 | 4.2 ± 1.6 | 5.8 ± 0.8 | 5.8 ± 1.7 |
FIG. 4.
Physicochemical properties of PEI-g-PEG-RGD/siRNA complexes. Distribution of particle size (A) and zeta potential (B) of PEI-g-PEG-RGD/siRNA complexes at N/P ratio of 10.
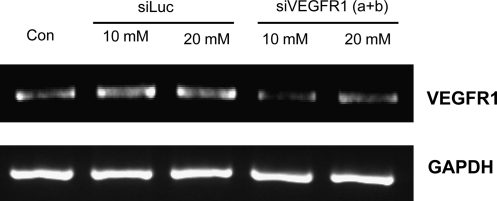
In vitro knockdown of VEGFR1 mRNA by PEI-g-PEG-RGD/siRNA polyplexes
The successful suppression of VEGFR expression could be induced through the inhibition of mRNA of VEGFR by efficient delivery of siRNA into cells. This reduced expression of VEGFR on the surface of endothelial cells could inhibit angiogenesis that is induced by VEGF-VEGFR interaction, and thereby could arrest the tumor growth and metastasis. To investigate whether polyplex can suppress the amount of mRNA of VEGFR or not, the siVEGFR was mixed with PEI-g-PEG-RGD polymer and then transfected into SVR cells that endogenously express the VEGFR1. As a control, unrelated siRNA (siLuc) was also transfected separately. siVEGFR against VEGFR1 suppressed the mRNA level as shown in Fig. 5, whereas control siLuc did not show any inhibitory effect even at higher amount of siRNA. These results indicated that the designed siVEGFR against VEGFR1 in SVR cells was able to inhibit the production of VEGFR1 mRNA in vitro and had a potential to inhibit tumor angiogenesis in vivo.
FIG. 5.
In vitro VEGFR silencing by PEI-g-PEG-RGD/siRNA complexes. Reverse transcriptase–polymerase chain reaction measurement of VEGFR1 after transfection of siVEGFR1 or siLuc complexed with PEI-g-PEG-RGD into SVR cells.
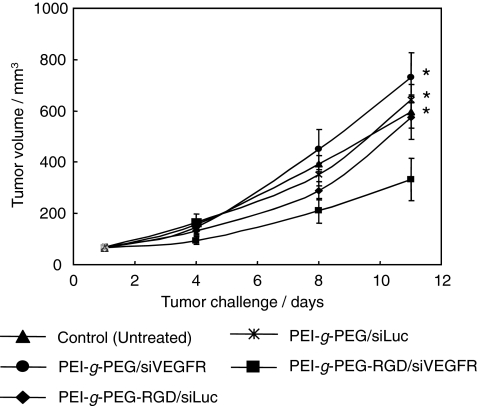
In vivo inhibition of tumor growth with PEI-g-PEG-RGD/siRNA polyplex
Previously, we have demonstrated that the systemic transfection of the soluble VEGFR1 (sFlt-1) by PEI-g-PEG-RGD polymer inhibited the tumor growth in subcutaneous mouse tumor model (Kim et al., 2006b). Therefore, we used this model to test whether the systemic application of PEI-g-PEG-RGD/siVEGFR complexes results in the growth inhibition of established subcutaneous (s.c.) tumors or not. PEI-g-PEG-RGD/siRNA complexes were administrated to tumor-bearing mice intravenously through the tail vein. PEI-g-PEG-RGD-complexed siRNA (PEI-g-PEG-RGD/siVEGFR) or PEI-g-PEG-complexed siRNA (PEI-g-PEG/siVEGFR) or unrelated control siRNA complexed with PEI-g-PEG-RGD (PEI-g-PEG-RGD/siLuc) as a negative control was injected into mice every 3 days. As shown in Fig. 6, tumors in mice treated with PEI-g-PEG/siVEGFR or PEI-g-PEG-RGD/siLuc grew very rapidly reaching a mean size of over 570 mm3 after 11 days. The treatment with the PEI-g-PEG-RGD/siVEGFR, however, resulted in a significantly suppressed tumor growth (the mean tumor volume is <330 mm3 after 11 days), suggesting that the RGD moiety of the carrier and therapeutic effect of the siRNA were responsible for tumor suppression (Fig. 6).
FIG. 6.
Antitumor effect of siRNAs in CT-26 tumor-bearing mice. PEI-g-PEG-RGD/siVEGFR, PEI-g-PEG/siVEGFR, PEI-g-PEG-RGD/siLuc, and PEI-g-PEG/siLuc complexes were injected into mice intravenously twice a week. Day 1 corresponds to 2 weeks after inoculation of CT-26 cells when the tumor volume was ∼65–70 mm3. Tumor diameters were measured at a regular interval for up to 11 days with calipers. Results represent the means standard deviation (n = 5). *P < 0.05 compared to PEI-g-PEG-RGD/siVEGFR group.
Discussion
In our previous reports with in vitro and in vivo experiments, we have demonstrated that the employment of tumor-targeting ligand, RGD, into nonviral polymeric gene delivery system improved the transgene expression in tumors compared with nontargeted nonviral gene delivery system (Kim et al., 2005b, 2006b). In those studies, we have shown that the tumor-targeted gene delivery system reduced the amount of transgene delivered to other organs; in other words, relatively high amount of accumulations in tumor site was attained by targeted polymeric gene delivery system as observed in biodistribution study. Thus, targeted system has numerous advantages compared with nontargeted system. Through the use of the targeted polymeric vectors, vector wastage can be reduced, thereby enhancing the efficiency of gene transfer in specific site and minimizing the risk of gene transfer into nontargeted sites that can reduce cytotoxicity and side effects. There have been numerous studies on generating nonviral targeted gene delivery system using a variety of cell-specific ligands, some more successful than others. These systems include glucosylated vehicle (Zanta et al., 1997; Choi et al., 1998), folate (Kim et al., 2005a), transferrin (Ogris et al., 2003), antibodies (O'Neill et al., 2001; Suh et al., 2001), and growth factors (Sosnowski et al., 1996; Blessing et al., 2001). With developing efficient delivery vectors, it is also necessary to design and generate the powerful therapeutic agents based on nucleic acids for the higher therapeutic effect. Several modulating systems of gene function have been introduced as a therapeutic strategy. Antisense and ribozyme-based therapies provide the possibility of specific downregulation of the expression of particular genes predominantly by interaction with mRNA (Kim et al., 1998; Pichon et al., 2001; KASHANI-SABET, 2004). Other strategies such as knockout gene therapy, gene replacement, and suicide gene therapy have been performed successfully. Recently, a newly developing approach for targeting mRNA, RNAi, has been used successfully for gene silencing in various experimental systems, specially tumor therapy, where RNAi silences the specific mRNA and inhibits the tumor growth and metastasis.
In the present study, we introduced synthetic siRNA targeting VEGFR for inhibiting tumor growth. VEGF is a potent angiogenic factor that binds to VEGFR present on endothelial cells, evoking on intracellular signaling cascade leading to a number of physiological responses. Thus, silencing of VEGFR with siRNA or inhibition of VEGFR function with inhibitors has a therapeutic potential. To deliver siRNA into tumor site, we developed the targeted polymeric gene carrier. This PEI-g-PEG-RGD polymer formed polyelectrolyte complex with siRNA and protected siRNA from enzymatic degradation as shown in Figs. 2 and 3. Efficient complexation and protection of siRNA with polymeric gene carrier are required for safe delivery of siRNA to the target site. As measured by dynamic light scattering, the average hydrodynamic diameter of PEI-g-PEG-RGD/siRNA complex was estimated at about 150 nm. Also, as shown in gel retardation and zeta-potential measurements, the negative charges on siRNA at N/P ratio of 1 are neutralized and the charge of polyplexes became positive with the increase of N/P ratios. This is relevant for enhanced cellular uptake of complexes, increasing their absorption efficiency in the negatively charged cellular membranes. In addition to physicochemical characterization of complexes, this study also suggested that a viable strategy attacking VEGFR is knockdown the mRNA of VEGFR1 with siRNA. With the transfection of PEI-g-PEG-RGD/siRNA complex in vitro, there was considerable reduction of VEGFR mRNA level as confirmed by reverse transcriptase–PCR in Fig. 5. These data suggest that the intravenous injection of PEI-g-PEG-RGD/siRNA deserves further investigation as a potential treatment approach for inhibition of tumor growth. As shown in Fig. 6, intravenous delivery of siRNA with targeted polymeric gene carrier suppressed the tumor growth, whereas there is no considerable inhibition of tumor growth in other control groups, indicating that silencing of VEGFR1 with sequence-specific siRNA interfered with the interaction of VEGF-VEGFR and their further signal transduction. Taking all these results into account, we consider that this targeted polymeric siRNA delivery system is applicable to the clinical cancer gene therapy, whereas further improvement of this system is necessary.
Conclusion
The present study has demonstrated that siRNA-mediated reduction of endogenous VEGFR1 could be achieved in vitro via the targeted polymeric gene delivery of synthetic siRNAs, at least in terms of mRNA. This study advocates a potential avenue for tumor gene therapy with significantly suppressed tumor growth in vivo. Although the developed system can be considered as an efficient means to achieve successful tumor therapy, there remains considerable scope to improve the efficiency of delivery system to address the impediments associated with in vivo delivery of siRNA.
Acknowledgments
This work was supported by National Institute of Health Grant CA 107070. This research was also supported by the Basic Science Research Program (20100015472 and 20100016020), and the Pioneer Research Center Program (20100002175) through the National Research Foundation of Korea by the Ministry of Education, Science and Technology, Republic of Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- AIELLO L.P. PIERCE E.A. FOLEY E.D. TAKAGI H. CHEN H. RIDDLE L. FERRARA N. KING G.L. SMITH L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLESSING T. KURSA M. HOLZHAUSER R. KIRCHEIS R. WAGNER E. Different strategies for formation of pegylated EGF-conjugated PEI/DNA complexes for targeted gene delivery. Bioconjug. Chem. 2001;12:529–537. doi: 10.1021/bc0001488. [DOI] [PubMed] [Google Scholar]
- CHENG T. CAO W. WEN R. STEINBERG R.H. LAVAIL M.M. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest. Ophthalmol. Vis. Sci. 1998;39:581–591. [PubMed] [Google Scholar]
- CHOI Y.H. LIU F. PARK J.S. KIM S.W. Lactose-poly(ethylene glycol)-grafted poly-L-lysine as hepatoma cell-tapgeted gene carrier. Bioconjug. Chem. 1998;9:708–718. doi: 10.1021/bc980017v. [DOI] [PubMed] [Google Scholar]
- FLYNN M.A. CASEY D.G. TODRYK S.M. MAHON B.P. Efficient delivery of small interfering RNA for inhibition of IL-12p40 expression in vivo. J. Inflamm. (Lond) 2004;1:4. doi: 10.1186/1476-9255-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILES F.J. The vascular endothelial growth factor (VEGF) signaling pathway: a therapeutic target in patients with hematologic malignancies. Oncologist. 2001;6:32–39. doi: 10.1634/theoncologist.6-suppl_5-32. [DOI] [PubMed] [Google Scholar]
- GOLDMAN C.K. KENDALL R.L. CABRERA G. SOROCEANU L. HEIKE Y. GILLESPIE G.Y. SIEGAL G.P. MAO X. BETT A.J. HUCKLE W.R. THOMAS K.A. CURIEL D.T. Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis, and mortality rate. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8795–8800. doi: 10.1073/pnas.95.15.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRATSUKA S. MARU Y. OKADA A. SEIKI M. NODA T. SHIBUYA M. Involvement of Flt-1 tyrosine kinase (vascular endothelial growth factor receptor-1) in pathological angiogenesis. Cancer Res. 2001;61:1207–1213. [PubMed] [Google Scholar]
- HUH J.I. CALVO A. STAFFORD J. CHEUNG M. KUMAR R. PHILP D. KLEINMAN H.K. GREEN J.E. Inhibition of VEGF receptors significantly impairs mammary cancer growth in C3(1)/Tag transgenic mice through antiangiogenic and non-antiangiogenic mechanisms. Oncogene. 2005;24:790–800. doi: 10.1038/sj.onc.1208221. [DOI] [PubMed] [Google Scholar]
- KASHANI-SABET M. Non-viral delivery of ribozymes for cancer gene therapy. Expert Opin. Biol. Ther. 2004;4:1749–1755. doi: 10.1517/14712598.4.11.1749. [DOI] [PubMed] [Google Scholar]
- KENDALL R.L. THOMAS K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM B. TANG Q. BISWAS P.S. XU J. SCHIFFELERS R.M. XIE F.Y. ANSARI A.M. SCARIA P.V. WOODLE M.C. LU P. ROUSE B.T. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am. J. Pathol. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM J.S. KIM B.I. MARUYAMA A. AKAIKE T. KIM S.W. A new non-viral DNA delivery vector: the terplex system. J. Control. Release. 1998;53:175–182. doi: 10.1016/s0168-3659(97)00251-4. [DOI] [PubMed] [Google Scholar]
- KIM S.H. JEONG J.H. CHO K.C. KIM S.W. PARK T.G. Target-specific gene silencing by siRNA plasmid DNA complexed with folate-modified poly(ethylenimine) J. Control. Release. 2005a;104:223–232. doi: 10.1016/j.jconrel.2005.02.006. [DOI] [PubMed] [Google Scholar]
- KIM W.J. CHANG C.W. LEE M. KIM S.W. Efficient siRNA delivery using water soluble lipopolymer for anti-angiogenic gene therapy. J. Control. Release. 2007;118:357–363. doi: 10.1016/j.jconrel.2006.12.026. [DOI] [PubMed] [Google Scholar]
- KIM W.J. CHRISTENSEN L.V. JO S. YOCKMAN J.W. JEONG J.H. KIM Y.H. KIM S.W. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol. Ther. 2006a;14:343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- KIM W.J. YOCKMAN J.W. LEE M. JEONG J.H. KIM Y.H. KIM S.W. Soluble Flt-1 gene delivery using PEI-g-PEG-RGD conjugate for anti-angiogenesis. J. Control. Release. 2005b;106:224–234. doi: 10.1016/j.jconrel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- KIM W.J. YOCKMAN J.W. JEONG J.H. CHRISTENSEN L.V. LEE M. KIM Y.H. KIM S.W. Anti-angiogenic inhibition of tumor growth by systemic delivery of PEI-g-PEG-RGD/pCMV-sFlt-1 complexes in tumor-bearing mice. J. Control. Release. 2006b;114:381–388. doi: 10.1016/j.jconrel.2006.05.029. [DOI] [PubMed] [Google Scholar]
- MARGOLIN K. Inhibition of vascular endothelial growth factor in the treatment of solid tumors. Curr. Oncol. Rep. 2002;4:20–28. doi: 10.1007/s11912-002-0044-9. [DOI] [PubMed] [Google Scholar]
- MILLAUER B. WIZIGMANN-VOOS S. SCHNURCH H. MARTINEZ R. MOLLER N.P. RISAU W. ULLRICH A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- MOK H. LEE S.H. Park J. Park T.G. Multimeric small interfering ribonucleic acid for highly efficient sequence-specific gene silencing. Nat. Mater. 2010;9:272–278. doi: 10.1038/nmat2626. [DOI] [PubMed] [Google Scholar]
- OGRIS M. WALKER G. BLESSING T. KIRCHEIS R. WOLSCHEK M. WAGNER E. Tumor-targeted gene therapy: strategies for the preparation of ligand-polyethylene glycol-polyethylenimine/DNA complexes. J. Control. Release. 2003;91:173–181. doi: 10.1016/s0168-3659(03)00230-x. [DOI] [PubMed] [Google Scholar]
- O'NEILL M.M. KENNEDY C.A. BARTON R.W. TATAKE R.J. Receptor-mediated gene delivery to human peripheral blood mononuclear cells using anti-CD3 antibody coupled to polyethylenimine. Gene Ther. 2001;8:362–368. doi: 10.1038/sj.gt.3301407. [DOI] [PubMed] [Google Scholar]
- OZAKI H. SEO M.S. OZAKI K. YAMADA H. YAMADA E. OKAMOTO N. HOFMANN F. WOOD J.M. CAMPOCHIARO P.A. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am. J. Pathol. 2000;156:697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICHON C. GONCALVES C. MIDOUX P. Histidine-rich peptides and polymers for nucleic acids delivery. Adv. Drug. Deliv. Rev. 2001;53:75–94. doi: 10.1016/s0169-409x(01)00221-6. [DOI] [PubMed] [Google Scholar]
- QUINN T.P. PETERS K.G. DE VRIES C. FERRARA N. WILLIAMS L.T. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH S.J. FOSNOT J. KUROKI A. TANG W. YANG X. MAGUIRE A.M. BENNETT J. TOLENTINO M.J. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol. Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- ROBINSON G.S. PIERCE E.A. ROOK S.L. FOLEY E. WEBB R. SMITH L.E. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN J. SAMUL R. SILVA R.L. AKIYAMA H. LIU H. SAISHIN Y. HACKETT S.F. ZINNEN S. KOSSEN K. FOSNAUGH K. VARGEESE C. GOMEZ A. BOUHANA K. AITCHISON R. PAVCO P. CAMPOCHIARO P.A. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- SHIM M.S. KWON Y.J. Acid-responsive linear polyethylenimine for efficient, specific, and biocompatible siRNA delivery. Bioconjug. Chem. 2009;20:488–499. doi: 10.1021/bc800436v. [DOI] [PubMed] [Google Scholar]
- SHWEIKI D. ITIN A. SOFFER D. KESHET E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- SONG E. LEE S.K. DYKXHOORN D.M. NOVINA C. ZHANG D. CRAWFORD K. CERNY J. SHARP P.A. LIEBERMAN J. MANJUNATH N. SHANKAR P. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J. Virol. 2003;77:7174–7181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORENSEN D.R. LEIRDAL M. SIOUD M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J. Mol. Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- SOSNOWSKI B.A. GONZALEZ A.M. CHANDLER L.A. BUECHLER Y.J. PIERCE G.F. BAIRD A. Targeting DNA to cells with basic fibroblast growth factor (FGF2) J. Biol. Chem. 1996;271:33647–33653. doi: 10.1074/jbc.271.52.33647. [DOI] [PubMed] [Google Scholar]
- SUH W. CHUNG J.K. PARK S.H. KIM S.W. Anti-JL1 antibody-conjugated poly (L-lysine) for targeted gene delivery to leukemia T cells. J. Control. Release. 2001;72:171–178. doi: 10.1016/s0168-3659(01)00273-5. [DOI] [PubMed] [Google Scholar]
- SUH W. HAN S.O. YU L. KIM S.W. An angiogenic, endothelial-cell-targeted polymeric gene carrier. Mol. Ther. 2002;6:664–672. [PubMed] [Google Scholar]
- TAKAHASHI K. SAISHIN Y. SILVA R.L. OSHIMA Y. OSHIMA S. MELIA M. PASZKIET B. ZERBY D. KADAN M.J. LIAU G. KALEKO M. CONNELLY S. LUO T. CAMPOCHIARO P.A. Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB J. 2003;17:896–898. doi: 10.1096/fj.02-0824fje. [DOI] [PubMed] [Google Scholar]
- TERMAN B.I. DOUGHER-VERMAZEN M. CARRION M.E. DIMITROV D. ARMELLINO D.C. GOSPODAROWICZ D. BOHLEN P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- YANO J. HIRABAYASHI K. NAKAGAWA S. YAMAGUCHI T. NOGAWA M. KASHIMORI I. NAITO H. KITAGAWA H. ISHIYAMA K. OHGI T. IRIMURA T. Antitumor activity of small interfering RNA/cationic liposome complex in mouse models of cancer. Clin. Cancer Res. 2004;10:7721–7726. doi: 10.1158/1078-0432.CCR-04-1049. [DOI] [PubMed] [Google Scholar]
- ZANTA M.A. BOUSSIF O. ADIB A. BEHR J.P. In vitro gene delivery to hepatocytes with galactosylated polyethylenimine. Bioconjug. Chem. 1997;8:839–844. doi: 10.1021/bc970098f. [DOI] [PubMed] [Google Scholar]