Abstract
Recent work has revealed that autophagy plays a significant role in the process of white adipocyte differentiation. In both in vitro and in vivo model systems, autophagy inactivation by targeted deletion of essential autophagy genes results in alterations in white adipocyte structure. In both models, postdifferentiation cells exhibit atypical morphology, with many small lipid droplets and large numbers of mitochondria, rather than the single large lipid droplet and relatively few mitochondria observed in normal white adipocytes. The role of autophagy as the primary means of the degradation of mitochondria has long been studied, and it is likely that a deficiency in the degradation of mitochondria contributes to the unusual phenotypes observed in mice with autophagy-deficient adipose tissue, including reduced adiposity, resistance to diet-induced obesity, and increased insulin sensitivity. What is not yet known is whether the process of mitochondria-specific autophagy, often referred to as “mitophagy,” is specifically induced during adipogenesis or if a general increase in the nonspecific autophagic degradation of mitochondria plays a role in normal adipose differentiation. Despite remaining questions, these findings not only establish the critical role of autophagy in white adipose tissue development, but also suggest that the manipulation of autophagy in adipose tissue may provide novel therapeutic opportunities for metabolic diseases. Antioxid. Redox Signal. 14, 1971–1978.
Introduction
In mammals, white adipose tissue (WAT) is traditionally considered as a metabolically active storage depot for lipids. In recent years, studies have unequivocally demonstrated that WAT is an endocrine organ that contributes to energy homeostasis not only through the storage and release of lipids, but also by secreting adipokines that exercise effects on other tissues (12, 36). With the growing pandemic of human obesity and its associated diseases, improving our understanding of the development, function, and manipulation of WAT has increased considerably in importance (5, 11, 21). The differentiation of white adipocytes is accompanied by an initial increase in mitochondria biogenesis (51), followed by a vast reduction in the number of mitochondria in the cell. This review provides an overview of our understanding of the role mitochondria play in adipogenesis and how autophagic degradation of mitochondria contributes to the normal differentiation of WAT. We also explore the possibility of targeting autophagy in adipose tissue as a novel intervention for the prevention and treatment of metabolic diseases.
White Adipocyte Function and Structure
WAT was originally identified as a repository for excess lipids within the body. While a wide variety of alternate tissues can store varying amounts of lipids under the right circumstances, the bulk of lipid storage and release in adult mammals is handled by WAT. The process by which this occurs has been thoroughly explored and is well established in the literature. In the simplest terms, an enzyme present on endothelial cells known as lipoprotein lipase breaks down triglycerides from blood-borne lipoproteins into free fatty acids and glycerol. Adipocytes take up free fatty acids and convert them back into triglycerides for long-term storage. These triglycerides can be broken back down into fatty acids and glycerol via lipolysis for release into the blood during periods of energetic need. The processes of lipogenesis and lipolysis are primarily regulated by the hormone insulin, although a number of other factors may exercise influence on the balance between fatty acid storage and release. The coordinate regulation of lipolysis/lipogenesis contributes to energy homeostasis within the organism in an environment of unstable nutrition.
Work in recent decades has revealed a more complex role for WAT as an active endocrine tissue. Adipocytes assist in the regulation of whole-body metabolism through the expression of hormones like leptin and adiponectin (12, 17), as well assert influence at the local level through the expression of cytokines, such as inflammation-associated tumor necrosis factor alpha and interlukin-6 (36). Collectively referred to as adipokines, these active molecules regulate neurological activities such as appetite and behavior as well as metabolic activities of peripheral tissues. This represents yet another way in which WAT regulates whole-organism energy homeostasis. There is a growing body of evidence suggesting that the pathological aspects of obesity are due in large part to the altered adipokine secretion profile exhibited by hyperplastic WAT (18).
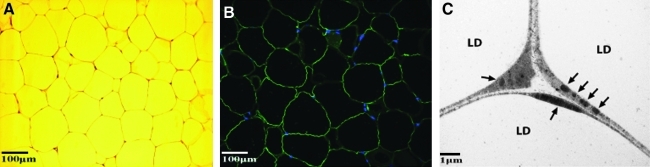
Consistent with their function, white adipocytes possess a unique and highly differentiated cellular structure. A typical mature white adipocyte is often described as having an engagement ring profile. When seen under a light microscope, the cell is almost completely occupied by a single, large lipid droplet, with the nucleus sandwiched between the droplet and the cell membrane representing the diamond on the ring (Fig. 1A, B). Electron microscopy reveals the presence of a relatively small number of mitochondria distributed thinly along the periphery of the cell, sandwiched like the nucleus between the lipid droplet and the cell membrane (Fig. 1C). This stands in stark contrast to brown adipocytes, in which the ample cytoplasmic content is heavily interspersed with mitochondria, other organelles, and a large number of small lipid droplets.
FIG. 1.
The structure of normal white adipocytes. (A) Hematoxylin and eosin staining of mouse white adipose tissue. (B) A section of normal white adipose tissue subjected to immunofluorescent staining with antibody against perilipin to observe lipid droplets and DAPI staining to observe nuclei. (C) An electron microscopy picture of portions of white adipocytes. LD, lipid droplet; arrows, mitochondria.
The hallmark structure of the white adipocyte is the single, large lipid droplet that serves as the reservoir for triglyceride storage. This specialized structure contains a number of unique membrane-associated structural proteins such as the perilipins (4, 7, 19). These proteins assist in the genesis of the lipid droplet and regulation of lipid transport in and out of the lipid droplet. For example, the perilipin family proteins play a key role in regulating the stability of the lipid droplet and the control of basal and hormonally stimulated lipolysis (4).
White Adipocyte Differentiation
The precise cellular lineage of white adipocytes remains uncertain at this time, but they are most likely derived from mesenchymal stem cells (MSCs) (32, 37). It is generally believed that adipocyte differentiation occurs as a two-step process. In the first step, pluripotent MSCs undergo a process known as determination. This process, which is not yet thoroughly understood, results in fibroblast-like cells that appear morphologically identical to pluripotent MSCs but are now capable of differentiating into only adipocytes. As a result, postdetermination MSCs are thereafter referred to as either preadipocytes or adipoblasts.
The second stage of differentiation is the process of the formation of the structurally distinct mature adipocyte from the fibroblast-like preadipocyte and is commonly referred to as adipogenesis. Extensive research using the preadipocytic cell line 3T3-L1 has demonstrated that the process of adipogenesis is coordinately regulated by a network of transcription factors in which peroxisome proliferator-activated receptor gamma (PPAR-γ) and CCAAT-enhancer binding proteins (C/EBPs) play a central role (9, 14, 32, 35, 37, 38). PPAR-γ is of particular importance and is considered the master regulator of adipogenesis as studies have shown that upregulation of PPAR-γ expression is both necessary and sufficient to induce adipogenesis. The increased expression of PPAR-γ in adipoblasts sets in motion a complex cascade of temporally controlled transcriptional events that result in the morphological and biochemical changes that impart the abilities necessary for a white adipocyte to function both as a lipid storage vessel and as an endocrine organ. Importantly, PPAR-γ is required not only for the initiation of adipogenesis, but also for the maintenance of the mature adipocyte (1, 15).
The Role of Mitochondria in WAT Differentiation and WAT Function
Classic ultrastructural study of 3T3-L1 adipogenesis by electron microscopy has elegantly recorded the changes in mitochondrial morphology as well as their relative abundance (29). Recent molecular studies have demonstrated the dramatic changes in the components of mitochondria present in adipocytes during the process of adipogenesis. Data obtained from the two-dimensional polyacrylamide gel electrophoresis analysis of mitochondrial protein expression shows a 20- to 30-fold increase in abundance of these proteins in the early stages of differentiation (51). Electron microscopy studies of preadipocytes undergoing differentiation confirm the massive increase in the numbers of mitochondria present in these cells beginning in early adipogenesis (51). This upregulation of mitochondria biogenesis appears to be coordinated with the initiation of adipogenesis, as PPAR-γ, as well as other transcription factors closely associated with adipogenesis, like cAMP responsive element-binding protein (CREB), C/EBP-α, estrogen-related receptor alpha (ERR-α), and the gene expression coactivator PGC-1α, are involved in the stimulation of both processes (36, 37).
The coordinated instigation of both adipogenesis and the biogenesis of mitochondria suggest that mitochondria play an important role in the differentiation and maturation of adipocytes. This is due not only to the increased energetic needs of the cell during differentiation, but also because only mitochondria can provide key substrates necessary to support the massive lipogenesis during adipogenesis. Energetically, adipocytic mitochondria are critical for adipogenesis. The differentiating cells must generate enough ATP for one of the most energy-consuming processes in the cell, the sustained synthesis of fatty acids, in addition to producing ATP in support of normal cellular activity. A second critical contribution from the mitochondria for lipogenesis is providing acetyl-CoA from glucose metabolism, the substrate for the fatty acid synthesis. The conversion of the glucose metabolite pyruvate to acetyl-CoA takes places exclusively in the mitochondrial matrix, catalyzed by a mitochondrial enzyme complex known as the pyruvate decarboxylase complex, or PDC. Finally, glycerol 3-phosphate, which is the substrate for fatty acid esterification, is produced exclusively by the mitochondria and is needed for the packaging of lipids in the form of triglycerides in the lipid droplet (10).
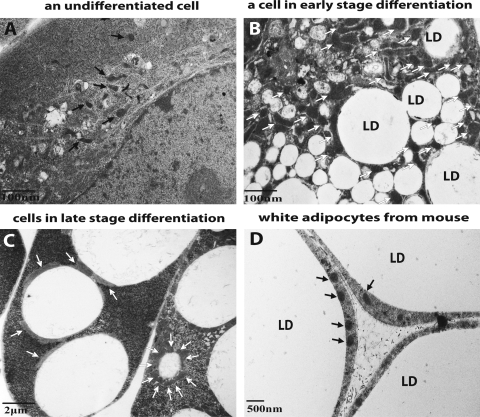
The number of mitochondria in mature white adipocytes is significantly lower than that observed during the process of differentiation. Figure 2A–C illustrates the changes in mitochondria number experienced by primary mouse embryonic fibroblasts (MEFs) undergoing adipogenesis. Before the induction of differentiation, the cells contain sparsely distributed mitochondria (Fig. 2A). As adipogenesis progresses, there is a dramatic increase in mitochondria biogenesis. As a result, mitochondria are heavily populated in almost every region within the cytoplasm (Fig. 2B). As adipogenesis proceeds and the lipid droplets consolidate, the cytoplasmic area containing mitochondria recede. The majority of mitochondria are then clustered around the very few lipid droplets within the cells and the number of mitochondria decreases (Fig. 2C). Finally, as shown in Figure 2D, an electron microscopic picture of WAT from mouse, mature white adipocytes contain very few mitochondria compared to those cells still undergoing the differentiation process (Fig. 2B). This decrease in mitochondria content likely reflects the decreased need for lipogenesis in the mature white adipocyte.
FIG. 2.
Dynamic alterations in the number of mitochondria observed in cells undergoing adipogenesis. Electron microscopy images of primary mouse embryonic fibroblasts undergoing white adipogenesis. (A) A cell before differentiation, (B) a differentiating cell in an early stage of adipogenesis, and (C) a cell in a late stage of adipogenesis. (D) An electron microscopy picture of mouse white adipose tissue highlighting mitochondria and lipid droplets. Mitochondria are denoted by arrows; LD, lipid droplet.
With the abundance of free fatty acids in adipocytes, it is not surprising that one feature of WAT mitochondria is high levels of fatty acid β-oxidation (13, 23), which provides an important source of ATP to the mature adipocytes. In addition, the mitochondrial oxidation of free fatty acids by adipocytes likely plays an important role in whole-body lipid homeostasis control. Indeed, treatment with thiazolidinediones (a.k.a. glitazones), the drugs for treating dyslipidemia and type II diabetes, are associated with increase of mitochondria number in adipocytes, which is believed to cause the salutary effects of these drugs (51, 52).
The Autophagic Degradation of Mitochondria in WAT Differentiation
Similar to the differentiation of many other tissues in the body, the WAT differentiation process involves the substantial remodeling of progenitor cells to result in a tissue highly optimized to serve its specific function. A unique aspect of WAT differentiation, however, is the removal of the bulk of the cytoplasmic contents, particularly the removal of excess mitochondria, and their predominant substitution with a single, large lipid droplet that occupies nearly the entire cytoplasmic space. This process of massive cellular remodeling has not been well investigated.
Macroautophagy, commonly referred to simply as autophagy, is a process by which cells degrade macromolecular intracellular material via sequestration in a double-membrane structure, known as an autophagosome, which then delivers the enclosed material to a lysosome for degradation (20). The molecular machinery of autophagy is highly conserved evolutionarily from yeast to humans (25, 30). Key roles for autophagy in a variety of pathophysiological conditions have been identified, including immunity (27, 31), tumorigenesis (20, 34, 53), programmed cell death (49), the selective degradation of organelles (41, 56), aging (6), neurodegenerative diseases (39), and the differentiation of erythrocytes and lens (22, 40, 44). Autophagy has also been implicated in hepatocyte lipid droplet formation (45), and global lipid metabolism (46).
Early morphological study using electron microscopy has clearly documented the massive autophagy activation as well as the engulfment of mitochondria by autophagosomes during 3T3-L1 adipogenesis (29). Recent studies from our laboratory (2, 55) and from the Czaja laboratory (47) have shown that autophagy also plays an important functional role in the process of adipogenesis. Several in vitro cell culture models have now shown that when autophagic activity is inactivated or reduced in adipocyte progenitor cells, they lose their ability to differentiate into normal white adipocytes. One model that illustrates this finding consists of primary MEFs obtained from mice with the homozygous deletion of an essential autophagy gene, atg5 (2). The Atg5 protein forms a conjugate with the ubiquitin-like autophagy protein Atg12, and together they allow the normal formation of the autophagosome (26). When stimulated to differentiate into white adipocytes, the atg5−/− MEFs used in this study failed to complete the differentiation process, with the majority of cells developing multiple small lipid droplets and then dying (2). The wild-type cells, however, were capable of developing into mature white adipocytes bearing a large unilocular lipid droplet or large oligolocular lipid droplets without difficulty. The findings in the atg5−/− knockout model were recapitulated in primary MEFs obtained from mice bearing the homozygous deletion of a second critical autophagy gene, atg7 (55). When atg7−/− MEFs were stimulated to undergo adipogenesis, the results were identical to those observed in atg5−/− MEFs.
Another study showed that when autophagy was eliminated in preadipocytic 3T3-L1 cells either via a pharmacological agent or via stable transfection with shRNA directed against either atg7 or atg5, respectively, the autophagy-compromised cells failed to accumulate as much triglyceride as their wild-type counterparts (47). Further, measurements of 3T3-L1 proteins indicative of normal adipocytic differentiation were reduced in the autophagy-compromised cells, indicating that differentiation in these cells had been effectively blocked (47).
The inactivation of autophagy in adipose tissue in mice also results in significant phenotypic change at both the tissue and cellular levels (47, 55). Similar mouse models in which the atg7 gene is conditionally knocked-out in adipose tissue in mice were independently generated and characterized by both the Jin and Czaja laboratories (47, 55). This was accomplished through the crossing of mice bearing a floxed atg7 gene with mice bearing the cre gene driven by the adipose-specific ap2 (a.k.a. fatty acid binding protein 4) promoter. Both groups reported that in these mutant mice, WAT mass was significantly reduced when compared to wild-type littermates. Histological examination revealed that the white adipocytes in atg7 conditional knockout mice were generally smaller in size than wild-type white adipocytes, and also contained multiple small lipid droplets, a significant volume of cytoplasm, and a much higher number of mitochondria than was observed in normal WAT cells.
Additional metabolic changes were also observed in these mutant mice. First, adipose-specific atg7 conditional knockout mice display an increased sensitivity to insulin. Second, adipose-specific atg7 conditional knockout mice are highly resistant to high fat diet-induced obesity. Further, there are decreased levels of blood triglycerides, cholesterol, and the key metabolic adipokine leptin.
Among the structural alterations, the most significant changes observed in the white adipocytes of adipose-specific atg7 conditional knockout mice are the significantly increased number of mitochondria present compared to the relatively few mitochondria observed in normal WAT cells, and the presence of multiple small lipid droplets rather than a single large droplet. Mitochondria have been identified in autophagosomes almost since the discovery of the autophagic process, and since then the functional role of autophagy in degrading mitochondria has been firmly established. Morphological analysis and gene expression profiling data show that autophagy-deficient adipogenesis initially follows a normal path in which the biogenesis of mitochondria is upregulated to facilitate the differentiation process (51), but the relatively few mitochondria present in normal mature white adipocytes implies that considerable degradation of mitochondria takes place later in adipogenesis. The observed massive accumulation of mitochondria in autophagy-deficient cells thus provides direct experimental evidence supporting the critical role of autophagy in mitochondria degradation during adipogenesis as a means of regulating the total number of mitochondria in mature adipocytes. However, it is not clear whether the selective and specific degradation of mitochondria via autophagy (mitophagy) or the nonselective process of general autophagy is responsible for the clearance of excess mitochondria during the process of white adipogenesis. Recent literature supports a specific autophagy/mitophagy pathway requiring that NIX is involved in the mitochondria clearance during reticulocyte maturation (40, 44). It will be interesting to test the involvement of this pathway or a similar selective mitophagy pathway in adipogenesis.
Despite the clear-cut differences between the wild-type and atg7−/− adipose tissues, it is important to point out that a large percentage (around 50%) (55) of white adipocytes in the atg7−/− adipose tissue appear normal morphologically. With the exception of a drastic reduction in cell size, they are indistinguishable from a wild-type mature white adipocyte. These cells contain only a single large lipid droplet and their cytoplasm occupies minimal space. One trivial explanation for this observation is the incompletion of atg7 deletion, which is dependent on Cre expression controlled by the aP2 promoter, in white adipocytes. Alternatively, the observation argues the involvement of a complementary atg7-independent degradation process. It remains to be determined if the recently discovered atg5- and atg7-independent autophagy pathway (28), which is implicated in reticulocyte maturation (54), is a backup process for the atg7- and atg5-dependent autophagy in white adipocyte differentiation.
One difference in observations between the adipose-specific atg7 conditional knockout mouse studies is that while Czaja's group interpreted the altered phenotype of mutant WAT to represent transdifferentiation from white adipocytes to brown adipocytes as a result of atg7 deletion (47), our group did not find that the mutant adipose tissue expressed increased levels of the mitochondrial uncoupling protein known as UCP1, which is the hallmark of brown adipose tissue (55). Further work will be required to settle the issue of whether or not autophagy deficiency leads to transdifferentiation. Despite this minor difference, these independent studies validate each other and confirm the belief that autophagy plays a key role in the adipogenic process, particularly through the control of mitochondria number and function in mature white adipocytes.
Adipose-Specific Autophagy Inactivation Improves Whole-Organism Metabolism: Implications in Obesity and Type II Diabetes Treatment
As previously noted, whole-body metabolism changes were observed in atg7 conditional knockout mice that may have profound implications for interventions in obesity and insulin resistance. First, adipose-specific atg7 conditional knockout mice are highly resistant to high fat diet-induced obesity. After several weeks of access exclusively to high-fat food, wild-type mice increase their overall body mass and their WAT mass significantly compared to those fed with a normal, balanced diet. In contrast, both total body mass and WAT mass of the mutant mice remain virtually identical to those fed a normal diet (55). Second, adipose-specific atg7 conditional knockout mice display an increased sensitivity to insulin, and there is no evidence of increased lipid accumulation in nonadipose tissues throughout the body (55). This stands in contrast to other models of reduced adipose tissue in mice, in which abnormal fat accumulates in other tissues such as muscles, liver, and heart, leading to decreased sensitivity to insulin (42). Further, the mutant mice maintained decreased levels of blood triglycerides and cholesterol. Finally, adipose-specific atg7 conditional knockout mice were observed to have a higher basal rate of physical activity than their wild-type counterparts. When placed in an open-field test, the mutant mice spent 300% more time in motion and traveled ∼300% further than their wild-type littermates (55).
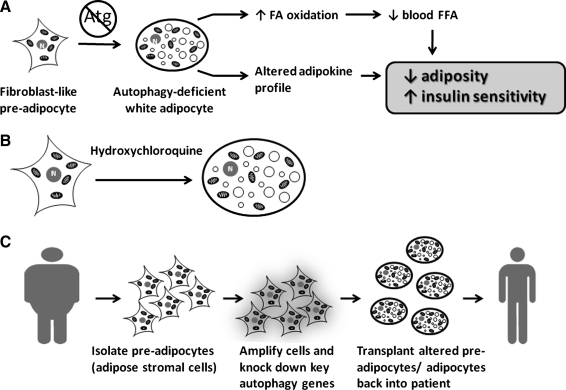
How the inactivation of autophagy in adipose tissue directly or indirectly improves whole-organism metabolism is not yet fully understood. The current information supports the working model illustrated in Figure 3A. First, the mutant adipose tissue may influence peripheral tissue metabolism through enhanced free fatty acid metabolism. Consistent with the increased levels of mitochondria, the autophagy-deficient white adipocytes exhibit increased fatty acid β-oxidation rates (47, 55), which may be responsible for the observed decreased fed plasma levels of free fatty acids in these mice (55). Elevated plasma free fatty acid levels decrease insulin sensitivity (3); therefore, the reduction of plasma free fatty acids may be responsible for sensitizing peripheral tissues to insulin in these mutant mice. Second, the mutant adipose tissue may influence peripheral tissue metabolism through the secretion of an altered profile of adipokines. In the adipose-specific atg7 conditional knockout mice we observed circulating leptin levels significantly lower than levels found in wild-type mice, whereas adiponectin levels remained largely unchanged, indicating the profile of adipokine secretion between the wild-type and mutant cells is different. It is possible that the net result of changes to the adipokine profile may lead to these beneficial metabolic outcomes.
FIG. 3.
A working model and potential implications in treating metabolic diseases. (A) A working model on how inhibition of autophagic degradation of mitochondria affects adipose tissue and improves whole-body metabolism. (B) The small molecule-based approach to treating metabolic diseases with lipophilic autophagy inhibitors like hydroxychloroquine, which will be highly enriched in adipose tissue. (C) The ex vivo, or cell-based, approach to treating metabolic diseases relies upon the removal of preadipocytes from the host, amplification and autophagy gene knockdown, induction for terminal white adipocyte differentiation, and then autologously transplanting the altered cells back into the host.
Regardless of the exact mechanism by which it occurs, all of the findings described thus far demonstrate that the inhibition of autophagy can have an impact on adipose tissue development and can significantly improve whole-body metabolism. For this to be clinically significant, however, we must be able to manipulate autophagy in adult human adipose tissue while exposing the patient to minimal risk. Potential therapeutic interventions are possible through the use of lipophilic pharmacological inhibitors of autophagy [e.g., hydroxychloroquine (Fig. 3B), a drug approved by FDA for chronic use, which has a large volume-of-distribution and is highly enriched 200- to 700- fold in lipophilic compartments versus plasma (16)]. Interestingly, a prospective, multicenter, observational, clinical study suggests that hydroxychloroquine has effect in preventing the development of type II diabetes (50). Currently, a clinical trial is ongoing to test the effect of hydroxychloroquine in preventing and treating metabolic syndrome by Clay Semenkovich group at Washington University School of Medicine on the ground that hydroxychloroquine inhibits ATM (43). The outcomes of this trial will be very exciting, and apparently the contribution of autophagy inhibition by hydroxychloroquine to the outcomes should also be examined.
Another potential therapeutic approach to achieve adipose tissue-specific inactivation of autophagy is through ex vivo manipulation of adipose stromal cells (adipose stem cells) (Fig. 3C). Adipose tissue contains a pool of stromal cells that exhibit preadipocyte characteristics (24). They can be easily isolated from the adipose tissue of adult subjects, propagated and genetically manipulated in vitro (ex vivo), and induced to differentiate into adipocytes. The resulting cells, which are terminally differentiated and genetically altered adipocytes, can then be autologously transplanted back to the same individual. The safety of the autologous adipose tissue transplantation, which is routinely performed in plastic surgery, is well established (8). In theory, by ex vivo manipulation of adipose stromal cells followed by induction of adipocyte differentiation and autologous transplantation, we can safely create autophagy-compromised adipose tissue in obesity and diabetic patients. This makes the cell-based therapeutic strategy appealing. Future research in these areas is warranted.
Future Directions
The discovery of the involvement of the autophagic degradation of mitochondria in WAT development could have wide-reaching implications for both basic and clinical research. On the basic research side, it would be of great interest to investigate how the PPAR-γ transcriptional network coordinately regulates autophagy activation. In addition, it would be interesting to determine whether and how mitochondria are selectively and specifically degraded through mitophagy during adipogenesis. From the clinical research side, these studies have discovered a novel way to manipulate white adipocyte structure, leading to an increase in the number of mitochondria and enhanced mitochondrial function in autophagy-compromised adipose tissue. In turn, altered adipose tissue improves metabolic features by reducing adiposity and increasing insulin sensitivity. It is clear that human adult adipose tissue undergoes significant turnover (33, 48). It is imperative for future studies to experimentally test whether or not the inactivation of autophagy in the adipose tissue of adult animals has salutary effects in preventing or treating obesity and insulin resistance. Moreover, elucidating the molecular mechanism by which autophagy-compromised adipose tissue improves whole-body metabolism will be an exciting exercise. By addressing these questions we expect to gain mechanistic insight into the interaction between autophagy and adipogenesis, hopefully providing us with the possibility of discovering a novel approach for preventing and treating the many metabolic diseases associated with WAT.
Abbreviations Used
- C/EBPs
CCAAT-enhancer binding proteins
- CREB
cAMP responsive element-binding protein
- ERR-α
estrogen-related receptor alpha
- MEFs
mouse embryonic fibroblasts
- MSCs
mesenchymal stem cells
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- WAT
white adipose tissue
References
- 1.Agarwal AK. Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87:408–411. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- 2.Baerga R. Zhang Y. Chen PH. Goldman S. Jin S. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy. 2009;5:1118–1130. doi: 10.4161/auto.5.8.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden G. Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 4.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Bray GA. Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 6.Cuervo AM. Bergamini E. Brunk UT. Droge W. Ffrench M. Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 7.Ducharme NA. Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 8.ELFadl D. Garimella V. Mahapatra TK. McManus PL. Drew PJ. Lipomodelling of the breast: a review. Breast. 2010;19:202–209. doi: 10.1016/j.breast.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franckhauser S. Munoz S. Pujol A. Casellas A. Riu E. Otaegui P. Su B. Bosch F. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes. 2002;51:624–630. doi: 10.2337/diabetes.51.3.624. [DOI] [PubMed] [Google Scholar]
- 11.Frayn KN. Tan GD. Karpe F. Adipose tissue: a key target for diabetes pathophysiology and treatment? Horm Metab Res. 2007;39:739–742. doi: 10.1055/s-2007-990270. [DOI] [PubMed] [Google Scholar]
- 12.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89:973S–979S. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granneman JG. Li P. Zhu Z. Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E608–E616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 14.Hansen JB. Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem J. 2006;398:153–168. doi: 10.1042/BJ20060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai T. Takakuwa R. Marchand S. Dentz E. Bornert JM. Messaddeq N. Wendling O. Mark M. Desvergne B. Wahli W. Chambon P. Metzger D. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci U S A. 2004;101:4543–4547. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.INCHEM. Chemical Safety Information from Intergovernmental Organizations. http//wwwinchemorg/documents/pims/pharm/chloroquhtm. 2010. http//wwwinchemorg/documents/pims/pharm/chloroquhtm
- 17.Kadowaki T. Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 18.Kahn SE. Hull RL. Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel AR. Brasaemle DL. McAndrews-Hill M. Sztalryd C. Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 21.Kos K. Wilding JP. Adipokines: emerging therapeutic targets. Curr Opin Investig Drugs. 2009;10:1061–1068. [PubMed] [Google Scholar]
- 22.Kundu M. Lindsten T. Yang CY. Wu J. Zhao F. Zhang J. Selak MA. Ney PA. Thompson CB. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood. 2008;112:1493–1502. doi: 10.1182/blood-2008-02-137398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P. Zhu Z. Lu Y. Granneman JG. Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-alpha. Am J Physiol Endocrinol Metab. 2005;289:E617–E626. doi: 10.1152/ajpendo.00010.2005. [DOI] [PubMed] [Google Scholar]
- 24.Meliga E. Strem BM. Duckers HJ. Serruys PW. Adipose-derived cells. Cell Transplant. 2007;16:963–970. doi: 10.3727/096368907783338190. [DOI] [PubMed] [Google Scholar]
- 25.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima N. Yamamoto A. Hatano M. Kobayashi Y. Kabeya Y. Suzuki K. Tokuhisa T. Ohsumi Y. Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa I. Amano A. Mizushima N. Yamamoto A. Yamaguchi H. Kamimoto T. Nara A. Funao J. Nakata M. Tsuda K. Hamada S. Yoshimori T. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 28.Nishida Y. Arakawa S. Fujitani K. Yamaguchi H. Mizuta T. Kanaseki T. Komatsu M. Otsu K. Tsujimoto Y. Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 29.Novikoff AB. Novikoff PM. Rosen OM. Rubin CS. Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol. 1980;87:180–196. doi: 10.1083/jcb.87.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohsumi Y. Mizushima N. Two ubiquitin-like conjugation systems essential for autophagy. Semin Cell Dev Biol. 2004;15:231–236. doi: 10.1016/j.semcdb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Paludan C. Schmid D. Landthaler M. Vockerodt M. Kube D. Tuschl T. Munz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 32.Park KW. Halperin DS. Tontonoz P. Before they were fat: adipocyte progenitors. Cell Metab. 2008;8:454–457. doi: 10.1016/j.cmet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Pouteau E. Beysen C. Saad N. Turner S. Dynamics of adipose tissue development by 2H2O labeling. Methods Mol Biol. 2009;579:337–358. doi: 10.1007/978-1-60761-322-0_17. [DOI] [PubMed] [Google Scholar]
- 34.Qu X. Yu J. Bhagat G. Furuya N. Hibshoosh H. Troxel A. Rosen J. Eskelinen EL. Mizushima N. Ohsumi Y. Cattoretti G. Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen ED. MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 36.Rosen ED. Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen ED. Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 38.Rosen ED. Walkey CJ. Puigserver P. Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 39.Rubinsztein DC. DiFiglia M. Heintz N. Nixon RA. Qin ZH. Ravikumar B. Stefanis L. Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 40.Sandoval H. Thiagarajan P. Dasgupta SK. Schumacher A. Prchal JT. Chen M. Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santi SA. Lee H. Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PloS One. 2011;6:e14614. doi: 10.1371/journal.pone.0014614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savage DB. Mouse models of inherited lipodystrophy. Dis Model Mech. 2009;2:554–562. doi: 10.1242/dmm.002907. [DOI] [PubMed] [Google Scholar]
- 43.Schneider JG. Finck BN. Ren J. Standley KN. Takagi M. Maclean KH. Bernal-Mizrachi C. Muslin AJ. Kastan MB. Semenkovich CF. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Schweers RL. Zhang J. Randall MS. Loyd MR. Li W. Dorsey FC. Kundu M. Opferman JT. Cleveland JL. Miller JL. Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shibata M. Yoshimura K. Furuya N. Koike M. Ueno T. Komatsu M. Arai H. Tanaka K. Kominami E. Uchiyama Y. The MAP1-LC3 conjugation system is involved in lipid droplet formation. Biochem Biophys Res Commun. 2009;382:419–423. doi: 10.1016/j.bbrc.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 46.Singh R. Kaushik S. Wang Y. Xiang Y. Novak I. Komatsu M. Tanaka K. Cuervo AM. Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh R. Xiang Y. Wang Y. Baikati K. Cuervo AM. Luu YK. Tang Y. Pessin JE. Schwartz GJ. Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spalding KL. Arner E. Westermark PO. Bernard S. Buchholz BA. Bergmann O. Blomqvist L. Hoffstedt J. Naslund E. Britton T. Concha H. Hassan M. Ryden M. Frisen J. Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 49.Tsujimoto Y. Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(Suppl 2):1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 50.Wasko MC. Hubert HB. Lingala VB. Elliott JR. Luggen ME. Fries JF. Ward MM. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187–193. doi: 10.1001/jama.298.2.187. [DOI] [PubMed] [Google Scholar]
- 51.Wilson-Fritch L. Burkart A. Bell G. Mendelson K. Leszyk J. Nicoloro S. Czech M. Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson-Fritch L. Nicoloro S. Chouinard M. Lazar MA. Chui PC. Leszyk J. Straubhaar J. Czech MP. Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yue Z. Jin S. Yang C. Levine AJ. Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J. Randall MS. Loyd MR. Dorsey FC. Kundu M. Cleveland JL. Ney PA. Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood. 2009;114:157–164. doi: 10.1182/blood-2008-04-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y. Goldman S. Baerga R. Zhao Y. Komatsu M. Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci U S A. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y. Qi H. Taylor R. Xu W. Liu LF. Jin S. The role of autophagy in mitochondria maintenance: characterization of mitochondrial functions in autophagy-deficient S. cerevisiae strains. Autophagy. 2007;3:337–346. doi: 10.4161/auto.4127. [DOI] [PubMed] [Google Scholar]