Abstract
The significance of the hypoxia component of stroke injury is highlighted by hypermetabolic brain tissue enriched with arachidonic acid (AA), a 22:6n-3 polyunsaturated fatty acid. In an ischemic stroke environment in which cerebral blood flow is arrested, oxygen-starved brain tissue initiates the rapid cleavage of AA from the membrane phospholipid bilayer. Once free, AA undergoes both enzyme-independent and enzyme-mediated oxidative metabolism, resulting in the formation of number of biologically active metabolites which themselves contribute to pathological stroke outcomes. This review is intended to examine two divergent roles of molecular dioxygen in brain tissue as (1) a substrate for life-sustaining homeostatic metabolism of glucose and (2) a substrate for pathogenic metabolism of AA under conditions of stroke. Recent developments in research concerning supplemental oxygen therapy as an intervention to correct the hypoxic component of stroke injury are discussed. Antioxid. Redox Signal. 14, 1889–1903.
Brain Oxygen Consumption and Regulation
The human brain is one of the most metabolically active organs in the body, despite not performing mechanical work like skeletal muscle or the heart. The normal human brain consumes 3.5 ml of O2 per 100 g of brain tissue per minute, a value which remains constant throughout periods of wakefulness and sleep. This relatively high rate of oxygen consumption is appreciable when compared to the metabolic rate of the entire body. The average man weighs 70 kg and consumes ∼250 ml of O2 per minute in the resting state. With the average human brain weighing 1400 g (∼2% of total body weight), it therefore consumes ∼49 ml O2 per minute, or 20% of total body oxygen consumed while at rest (21a, 46, 82) (Table 1). Within the brain, oxygen consumption is highly dynamic and region specific. Gray matter consumes more than twice as much oxygen as white matter, with highest consumption occurring in the medial occipital lobe (125). Oxygen transport from blood to tissue is driven by the oxygen concentration gradient between blood and tissue, moving from the source to the sink. While literature supports capillaries as the principal suppliers of blood to brain tissue, a growing body of evidence suggests that precapillary arterioles also play an active role in oxygen transport (169). Indeed, the major oxygen concentration gradient has been found at the blood–tissue interface of arterioles, not capillaries (38).
Table 1.
Oxygen Consumption by Tissue
| Tissue | O2 consumption (ml O2/min/100g) | % of total body O2 consumption* |
|---|---|---|
| Skin | 0.2 | 5 |
| Resting muscle | 1 | 20 |
| Liver | 2 | 20 |
| Brain | 3.5 | 20 |
| Kidney | 5 | 7.2 |
| Resting heart | 8 | 10–12 |
| Contracting muscle | 50 | NA |
At the cellular level, oxygen-sensing systems have evolved to ensure tight regulation of cerebral blood flow and oxygen homeostasis in the brain to avoid metabolic compromise. The average rate of blood flow in the human brain as a whole is 800 ml/min, or ∼15% of the total basal cardiac output. By adjusting vascular tone, brain perfusion is tightly controlled over a wide range of blood pressures, a process termed cerebral autoregulation (2). This powerful autonomic response permits a dynamic flux of cerebral blood flow on the basis of changes in neural activity and metabolic demand within brain regions. This phenomenon is the foundation of functional magnetic resonance imaging (fMRI) which uses blood oxygenation level-dependent (BOLD) contrast imaging to identify changes in the hemodynamic response related to neural activity (73).
When cerebral autoregulation is impaired, or in pathological conditions such as stroke in which cerebral blood flow is disrupted, neuron function is both directly and indirectly regulated in response to brain hypoxia. Direct regulation of neuronal excitation occurs in a hypoxic state by O2-sensing ion channels that are reversibly inhibited by hypoxia (70). Hypoxia-induced modulation of these channels lead to increased excitability, including “leak” K+ currents from TWIK-related acid sensitive (TASK) channels (114). Hypoxia-induced K+ leak in TASK channels is an instantaneous open-rectifier current that influences both neuronal resting membrane potential and the duration of action potential (19). In contrast, hypoxia-inducible factor (HIF) transcriptional complex is an indirect regulator of neuronal activity and excitability under hypoxic conditions. Widely conserved among mammalian species and invertebrates alike, the HIF complex exists as a heterodimer composed of constitutively expressed HIF-β and O2-sensitive HIF-α subunits. Oxygen-dependent enzymatic hydroxylation of proline residues within HIF-α subunits governs protein stability, and transcriptional activity of the HIF complex. More than 70 putative HIF-target genes have been identified to date, expression of which governs an array of vital cellular processes including energy metabolism, angiogenic response, and cell survival (145).
Stroke-Induced Disruption of Cerebral Oxygen Delivery
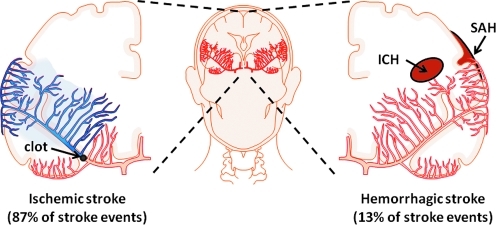
Today, stroke is the leading cause of long-term disability and the third leading cause of death in the United States, with more than 795,000 Americans afflicted by a new or recurring stroke each year (98). Broadly defined by a cerebrovascular disruption of blood supply to the brain, stroke etiology is classified as either ischemic or hemorrhagic in origin (Fig. 1). Given the hypermetabolic activity of brain tissue and the relatively high oxygen consumption rate, the brain is dependent upon an uninterrupted blood borne supply of substrate for oxidative metabolism. Oxygen is utilized in the brain almost entirely for the oxidation of carbohydrate (21a). Glucose is the preferred metabolic substrate for brain, and without glucose and oxygen stores available to maintain oxidative metabolism, homeostatic brain function is quickly lost. In the case of global cerebral blood flow arrest, consciousness is lost within 10 seconds. The critical level of brain tissue pO2 beneath which consciousness and normal EEG pattern are lost is 20 mmHg (127). The high oxidative metabolic demands of brain tissue therefore exacerbate the pathological consequences of interrupted circulation of blood to brain tissue as compared to other organs. Irreversible pathological injury to brain tissue occurs within minutes of cerebral blood flow interruption. The adage “time is brain” is commonly expressed to emphasize that human nervous tissue is rapidly and irretrievably lost as the duration of cerebral blood flow disruption progresses. A quantitative assessment of “time is brain” in supratentorial acute ischemic stroke estimated that 1.9 million neurons, and 14 billion synapses are lost per minute due to ischemic cerebrovascular disruption (141).
FIG. 1.
Stroke etiology. Stroke is a general term used to define pathological conditions in which cerebral blood flow is disrupted. The cause of stroke can be classified as one of two etiological origins: ischemic (left) and hemorrhagic (right). Ischemic stroke describes the arrest of cerebral blood flow by narrowing and blockage of a cerebral artery (thrombosis) or by a peripheral artery clot (emboli) traveling to and blocking a cerebral artery. Hemorrhagic stroke describes the rupture of a cerebral artery in intracerebral space (ICH) or subarachnoid space (SAH). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Ischemic Stroke
The word ischemia originates from the Greek words ischaimos which means “to restrain” and haima meaning “blood”. In simple terms, ischemic stroke occurs when an artery of the brain is blocked. The brain depends on continuous blood flow to deliver oxygen, nutrients (i.e., glucose, vitamins), and to remove carbon dioxide and cellular waste. When cerebral blood flow is obstructed and brain tissue is no longer able to sustain homeostatic function, the terminal result is energetic failure and cell death. Ischemic stroke has several etiological origins. The most common is due to the narrowing of the arteries in the neck or head. This is most often caused by atherosclerosis—a chronic disease process directly influenced by diet. High fat diets leading to elevated LDL cholesterol and triglyceride levels are significant risk factors for atherogenesis. If cerebrovascular arteries become too narrow, blood cells collect and form blood clots. These blood clots can block the artery where they are formed (thrombosis), or can dislodge and become trapped in smaller or more distant arteries of the brain (embolism).
A thrombotic stroke occurs when diseased or damaged cerebral arteries become blocked by the formation of a blood clot within the brain. Clinically referred to as focal cerebral thrombosis or cerebral infarction, this classification of stroke is responsible for 50% of all clinically presented stroke cases. Cerebral thrombosis can also be divided into an additional two categories that correlate to the location of the blockage within the brain: large vessel thrombosis and small vessel thrombosis. Large vessel thrombosis is the term used when the blockage is in one of the brain's larger blood-supplying arteries such as the carotid or middle cerebral artery, while small vessel thrombosis involves one (or more) of the brain's smaller, yet deeper penetrating arteries (Φ:0.2–15 mm). This latter type of stroke is also referred to as a “lacunar” stroke event.
An embolic stroke is also caused by cerebrovascular occlusion, but in this case the clot (or emboli) is formed in peripheral arteries outside of the cerebrovascular system. Often from the heart, these emboli will travel the bloodstream until they become lodged in the brain and cannot travel any further. This naturally restricts the flow of blood to the brain and results in almost immediate physical and neurological deficits. While thrombotic and embolic events are the most common cause of ischemic stroke, there are many other pathological events, including traumatic injury to the blood vessels of the neck, or disorders of blood clotting, which also induce ischemic stroke of the brain.
Focal Ischemic Stroke and the Ischemic Penumbra
Following focal cerebral ischemia, arrest of blood flow at the site of occlusion produces an ischemic infarct core in which local tissue pO2 approaches anoxic levels. Liu et al. reported that in a rodent model of middle cerebral artery occlusion (MCAO) focal cerebral ischemia rapidly decreased interstitial brain tissue pO2 to ∼4% of baseline at the infarct core as measured by electron paramagnetic resonance (EPR) oximetry (96). Moving away from the ischemic core, cerebral blood perfusion and tissue oxygenation gradually improves (Fig. 2) (96, 133). Still hypoperfused as compared to baseline, neurons in this region of tissue, termed the ischemic penumbra, are still able to preserve ion homeostasis and transmembrane electrical potentials (12, 165). While stringent delineation of the ischemic penumbra is difficult, the most clinically relevant and straightforward definition characterizes it as the ischemic region which, without intervention, would evolve into ischemic infarction over time (175a). This definition, while vague, can be readily applied to characterize the penumbra in a preclinical setting where reproducible stroke-induced infarct is compared across control and stroke intervention groups (133). Additional criteria used to define the ischemic penumbra include site specific cerebral blood flow measurement, documenting diminished protein synthesis in approaching the ischemic core, and spatially resolved mRNA expression analysis of heat-shock protein 70 (hsp70) that is reported to be selectively expressed in the penumbra 3h after permanent MCAO in mice (58, 59, 63).
FIG. 2.
Characterization of the ischemic penumbra in focal ischemic stroke. In a focal ischemic stroke event, brain tissue oxygenation increases toward baseline (normoxia, green) with distance from the ischemic core (red). Ischemic penumbra and core are delineated by quantitative molecular and physiological differences. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
While rapid restoration of cerebral blood flow at the ischemic site via thrombolytic therapy or mechanical intervention reduces infarct lesion size by salvage of the ischemic penumbra, reperfusion itself also paradoxically contributes to ischemic stroke brain injury. Specifically, reperfusion of cerebral blood flow following ischemia rapidly increases cerebral pO2 in tissue that is metabolically compromised and highly susceptible to oxidative stress (133). In this state, the sudden rise in tissue oxygenation contributes to oxidative damage of brain tissue. Reperfusion also enables peripheral leukocyte recruitment to stroke-affected tissue (124). Leukocytes contribute to stroke pathology by obstructing the microcirculation, disrupting the blood–brain barrier, and infiltrating brain tissue where they release cytokines and propagate inflammation. Furthermore, leukocytes contribute to platelet aggregation in the stroke affected microcirculation causing vasogenic brain edema and hemorrhagic transformation following stroke (135).
Molecular Mechanisms of Ischemic Stroke Pathology
Ischemic stroke-induced hypoxia causes energetic failure (ATP deficiency) due to the lack of oxygen required to maintain cellular respiration. Within 15 minutes of ischemic stroke onset, ATP levels of stroke-affected brain tissue decrease by one-third. As a result, ATP-dependent ion pumps (i.e., Na+/K+ ATPase) fail to maintain transmembrane ion gradients, resulting in Ca2+ influx through voltage-sensitive Ca2+ channels. Ischemia-induced energetic failure in brain tissue also results in excessive extracellular glutamate release. Consequently, glutamate-mediated overstimulation of ionotropic NMDA receptors continues to drive intracellular Ca2+ uptake. The subsequent rapid accumulation of intracellular Ca2+ results in activation of lipases (i.e., phospholipase A2) that contribute to membrane degradation and lipid peroxidation in stroke-affected brain tissue enriched with polyunsaturated fatty acids (PUFAs). Moreover, the intracellular Ca2+ influx also induces Ca2+ and calmodulin co-dependent activation of neuronal and endothelial nitric oxide synthase (NOS). NOS generates nitric oxide (NO) gas, a signaling molecule with well-characterized vasodilatory effects. The potential for NO to improve ischemic stroke-affected cerebral blood flow via vasodilation is clear, however increasing evidence suggests that NO also contributes to oxidative damage during ischemic stroke. With one unpaired electron, NO reacts with most free radicals at near diffusion-limited rates (178). Reaction of NO with superoxide radical (O2-) leads to the production of highly reactive peroxynitrite (ONOO-). As much as 2% of oxygen consumed by mitochondria is converted to superoxide anion under normal respiratory conditions (18, 170). In a pathological setting of ischemic stroke, generation of superoxide is greatly enhanced and in combination with elevated NO levels contributes to a massive increase in peroxynitrite formation (154, 155). In addition to evidence supporting peroxynitrite-mediated lipid peroxidation, peroxynitrite also exerts cytotoxic effects directly on mitochondria by inhibiting respiration at complexes I, II, III and V (97, 174).
Hemorrhagic Stroke
Intracerebral hemorrhage (ICH) occurs when a vulnerable blood vessel within the brain bursts, allowing blood to leak inside the brain. The sudden increase in pressure within the brain can cause damage to the brain cells surrounding the blood. If the amount of blood increases rapidly, the sudden buildup in pressure can lead to unconsciousness or death. ICH usually occurs in selective parts of the brain, including the basal ganglia, cerebellum, brainstem, or cortex. The most common cause of ICH is high blood pressure (hypertension). Since high blood pressure itself is asymptomatic, many people at risk for ICH are not aware that they have high blood pressure, or that it needs to be treated. Less common causes of ICH include trauma, infection, tumors, blood clotting deficiencies, and abnormalities in blood vessels.
Subarachnoid hemorrhage (SAH) occurs when a blood vessel just outside the brain ruptures and the area between the arachnoid membrane and the pia mater rapidly fills with blood. A patient with SAH may have a sudden, intense headache (referred to as a thunderclap headache), neck pain, and nausea or vomiting. SAH may arise spontaneously or due to trauma with the sudden buildup of pressure outside the brain, causing rapid loss of consciousness or death. SAH is most often caused by cerebral aneurysms—small areas of rounded or irregular swellings in the arteries. Where the swelling is most severe, the blood vessel wall becomes weak and prone to rupture.
In addition to a disruption of cerebral perfusion which by itself imposes similar patterns of oxidative stress as compared to ischemic stroke, hemorrhagic stroke also incorporates patterns of oxidative stress associated with iron-mediated free radical injury. Specifically, the rupture of cerebral blood vessels and subsequent red blood cell lysis results in release of heme in hemorrhagic stroke-affected tissue. Heme is metabolized by heme oxygenase (HO) in the brain into iron, carbon monoxide, and biliverdin (64). Following heme degradation by HO, free iron concentration can reach as high as 10 mmol/L, which induces significant brain edema and directly contributes to free radical formation and lipid peroxidation (65). Free iron greatly enhances hydroxyl radical production and subsequent lipid peroxidation via the Fenton reaction (137, 138). Furthermore, as iron deposition in brain tissue increases with age, iron-mediated free radical formation, lipid peroxidation, and neurotoxicity become increasingly relevant in hemorrhagic stroke pathology (158). This is particularly noteworthy given the elevated risk of a cerebral stroke event with age, and the risk for intracranial bleeding in ischemic stroke cases—a phenomenon termed “hemorrhagic transformation” that occurs in as many as 70% of all ischemic stroke patients (13, 90). Finally, carbon monoxide produced by HO-mediated heme metabolism also amplifies generation of reactive oxygen species in brain mitochondria (182), thereby further contributing to elevated oxidative stress under hemorrhagic stroke conditions.
Stroke-Mediated Oxidative Stress in PUFA-Rich Brain
Regardless of stroke mechanism, brain tissue is highly susceptible to oxidative stress as it: a) consumes an inordinate amount of oxygen to meet high metabolic demands, b) contains high concentrations of polyunsaturated fatty acids (PUFAs) that are vulnerable to lipid peroxidation, and c) has lower antioxidant capacity as compared to other organ systems. Neurons are particularly vulnerable to oxidative damage in stroke-affected brain tissue due to lower levels of endogenous antioxidant, glutathione, as compared to resident glial cells (35). The consequence of increased generation of free radical species in stroke-affected brain tissue enriched with PUFAs is an accumulation of lipid peroxidation products that have proven to be culpable neuromodulators of the cell death cascade. Brain tissue is highly enriched with the n-6 PUFA arachidonic acid (AA, 20:4n-6) and the n-3 PUFA docosahexaenoic acid (DHA, 22:6n-3), which are major components of phospholipid membranes. Together, AA and DHA account for ∼20% of all fatty acids in the mammalian brain (34). Both AA and DHA are nutritionally essential to brain function and structure during early development (26–28, 116, 132, 171), and influence membrane fluidity, signal transduction, and gene transcription throughout life (39, 72, 132, 177). Neither AA nor DHA are synthesized de novo, but are obtained from the diet and circulated to the brain directly in the plasma (132), or elongated from n-6 and n-3 PUFA linoleic (18:2n-6) and α-linolenic acid (18:3n-3) precursors in the liver (156). A number of pathological conditions in the human brain are associated with disturbed PUFA metabolism of AA (42, 131), including stroke.
Arachidonic Acid Metabolism in Brain
In resting cells, AA is stored within phospholipid membranes, esterified to glycerol. A receptor-dependent event, requiring a transducing G-protein coupled receptor, initiates phospholipid hydrolysis and releases AA into the intracellular space. Three enzymes mediate this deacylation reaction: phospholipase A2 (PLA2), phospholipase C (PLC), and phospholipase D (PLD), each with different sites of attack on the phospholipid backbone. PLA2 catalyzes the hydrolysis of phospholipids at the sn-2 position, releasing AA in a single-step reaction. By contrast, PLC and PLD do not release free AA directly. Rather, they generate lipid products containing arachidonate (diacylglycerol and phosphatidic acid, respectively), which can be released subsequently by diacylglycerol- and monoacylglycerol-lipases.
Phospholipase A2
Phospholipase A2 (PLA2) isozymes encompass a diverse family of at least 15 different isozyme groups (20) classified into five distinct categories: (a) secreted small molecular weight sPLA2, (b) larger cytosolic calcium-dependent cPLA2, (c) calcium-independent iPLA2, (d) platelet-activating factor acetylhydrolases (PAFA), and (e) lysosomal PLA2 isozymes. The entire PLA2 family is characterized by a common function; the enzymatic hydrolysis of the sn-2 ester bond of glycerophospholipids, thereby producing a free fatty acid (i.e., AA) and lysophospholipid (i.e., lysophosphatidylcholine, LPC). Currently, only sPLA2 and cPLA2 have well-defined roles in stroke-mediated AA metabolism and are therefore the focus of discussion here.
Under conditions of stroke in which reactive oxygen and nitrogen species are abundant, there occurs a rapid accumulation of free fatty acids, due to increases in intracellular Ca2+ and activation of PLA2s (3, 32, 61, 91, 94, 123, 134, 139, 159, 172). The sPLA2s are characterized by the requirement of histidine in their active site, Ca2+ for catalysis and the presence of six conserved disulfide bonds (20). Under ischemic stroke conditions, sPLA2 mRNA and protein expression is significantly upregulated (5, 94) and activity is induced by inflammatory cytokine tumor necrosis factor-alpha (TNF-α) (10). The cPLA2s are the only PLA2 which demonstrate a preference for AA in the sn-2 position of phospholipids (21). Localized predominantly in gray matter (160), they lack the disulfide bonding network of sPLA2s and function through the action of a serine/aspartic acid dyad (20). Following ischemic stroke, cPLA2 subunit mRNA and protein expression is elevated (123, 159). Recently, a hypoxia-sensitive domain in the human cPLA2 promoter region has been characterized, suggesting oxygen-specific transcriptional regulation as well (6). Stroke-induced intracellular Ca2+ accumulation mediates cPLA2 subunit translocation to the membrane phospholipid bilayer (176) and activity is induced by phosphorylation of serine residue 505 by mitogen-activated protein kinase (93, 118, 175). In addition to AA, glycerophospholipid metabolism by PLA2s following stroke also elevate LPC levels in blood, cerebrospinal fluid, and brain tissue following ischemic and hemorrhagic stroke (61, 62, 79, 164). LPCs act as potent mediators of inflammation in the brain following stroke via stimulated release of interleukin 1β (162) and subsequent activation of microglia (122, 143).
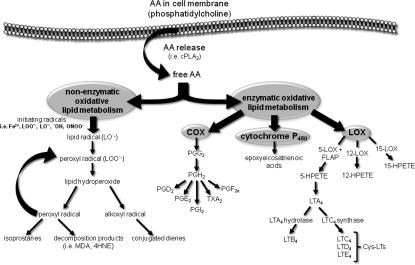
Once released, free AA has three potential fates: reincorporation into phospholipids, diffusion outside the cell, and metabolism. In a pathological setting, such as ischemic and hemorrhagic stroke, free AA accumulates and undergoes uncontrolled oxidative metabolism by both enzymatic and nonenzymatic processes (Fig. 3). This uncontrolled metabolism, referred to as the “arachidonic acid cascade”, includes the formation of prostaglandins, leukotrienes, thromboxanes, isoprostanes, and nonenzymatic lipid peroxidation products (129). The arachidonic cascade amplifies the overall production of free radicals, both reactive oxygen and nitrogen species, and subsequently oxidative damage to lipids, proteins, and nucleic acids. Taken together, the release of AA by PLA2 and downstream metabolism plays a significant role in oxidative tissue injury following stroke-induced hypoxia.
FIG. 3.
Stroke-induced hypoxia mediates oxidative metabolism of arachidonic acid. Following release from the lipid membrane bi-layer by oxygen-sensitive phospholipase A2 (PLA2), arachidonic acid (AA) undergoes oxidative metabolism under enzymatic and nonenzymatic mechanisms. FLAP, 5-LOX activating protein; 4HNE, 4-hydroxynonenal; 5-HPETE, 5-hydroperoxyeicosatetraenoic acid; 12-HPETE, 12-hydroperoxyeicosatetraenoic acid; 15-HPETE, 15-hydroperoxyeicosatetraenoic acid; 5-LOX, 5-lipoxygenase; 12-LOX, 12-lipoxygenase; 15-LOX, 15-lipoxygenase; LTA4, leukotriene A4; LTB4, leukotriene B4; LTC4, leukotriene C4; LTD4, leukotriene D4; MDA, malondialdehyde; PGD2, prostaglandin D2; PGF2a, prostaglandin F2a; PGG2, prostaglandin G2; PGH2, prostaglandin H2; PGI2, prostaglandin I2; TXA2, thromboxane A2.
Nonenzymatic Oxidative Lipid Metabolism
Beyond the initial damage to lipid membranes, reaction of free radical species with double bonds of PUFAs produce alkyl radicals, which in turn react with molecular oxygen to form a peroxyl radical (ROO•). Peroxyl radical can abstract hydrogen from adjacent PUFAs to produce a lipid hydroperoxide (ROOH) and a second alkyl radical, thereby propagating a chain reaction of lipid oxidation (38a). Lipid peroxides degrade and give rise to α,β-unsaturated aldehydes that include 4-hydroxynonenal (4HNE), malondialdehyde (MDA), and acrolein (4, 40, 76, 126). These aldehydes covalently bind to proteins through reaction with thiol groups and alter their function. They also react with amino groups to form cyclic adducts.
The most well-characterized of the lipid peroxide aldehydes is 4HNE; a nine-carbon α,β-unsaturated aldehyde that at low concentration modulates cellular signaling in brain tissue. Under pathological conditions such as stroke, 4HNE-mediated protein carbonylation induces a number of deleterious effects in cells, including inhibition of nucleic acid synthesis, elevation of intracellular calcium, and inhibition of mitochondrial respiration (43). The localized concentration of 4HNE may increase to as high as 4.5 mM within the phospholipid bilayer, causing protein cross-linking of essential membrane transporters (i.e., glucose and glutamate transporters, sodium/potassium ATPases) (77, 101, 107). The modification of adenine nucleotide translocation by 4HNE also suppresses ADT and ATP transport through the inner mitochondrial phospholipid membrane. Taken together, these actions significantly attenuate the energy-producing capacity of mitochondria in tissue already devastated by stroke-induced energetic failure. The C-3 position of 4HNE is highly reactive: undergoing Michael addition reaction with cellular thiol. This leads to the formation of adducts with endogenous antioxidant glutathione, and ultimately efflux from the cell via glutathione conjugate transporter RLIP76 (51). 4HNE-mediated inactivation of thioredoxin and thioredoxin reductase through modification of cysteine and selenocysteine residues at the active site has also been linked to dysregulation of cellular redox status (41).
Reactive species attack on lipid hydroperoxides also result in the formation of isoprostanes via β-cleavage of the peroxyl acid and subsequent molecular rearrangement. Isoprostanes have D-, E-, and F-ringed structures similar to cyclooxygenase-generated prostaglandins, except that their hydrocarbon chains are in the cis position in relation to the pentane ring as opposed to the trans position observed in prostaglandins. While measurement of isoprostanes is employed as a “gold standard” to quantify cumulative oxidative stress from neurological stress, their bioactivity remains poorly characterized (109, 179). One particular isoprostane metabolite, 15-A2t-IsoP, has been shown to induce neurodegeneration in cultured neurons via mitochondrial ROS production, glutathione depletion, 12-lipoxygenase activation, and caspase cleavage (109). Furthermore, 15-A2t-IsoP has recently been shown to potentiate hypoxia-induced neuronal cell death, suggesting a greater role in stroke pathology (179). Finally, the F2-isoprostane acts as a potent vasoconstrictor of brain capillaries by inducing COX-mediated synthesis of thromboxane in endothelial cells (88). In a stroke setting, vasoconstriction and platelet aggregation by thromboxane is counterproductive to resolving arteriothrombosis.
Enzymatic Oxidative Lipid Metabolism
Cyclooxygenase, lipoxygenase, and epoxygenase enzymes are pivotal players in the generation of oxygenated derivatives of AA in the pathological setting of stroke. Cyclooxygenases convert AA to prostaglandins and thromboxanes, lipoxygenases catalyze the metabolism of AA into leukotrienes and lipoxins, and epoxygenase activity produces epoxyeicosatrienoic acids. The functional role of these enzymes and downstream lipid-derived products is largely dependent upon environment and cellular localization. In a stroke setting, several products of these pathways act within neurons to modulate the activities of ion channels, protein kinases, ion pumps, and neurotransmitter uptake systems. The newly formed eicosanoids may also exit the cell of origin and act at a distance, by binding to G-protein-coupled receptors present on nearby neurons or glial cells. Finally, the actions of the eicosanoids may be terminated by diffusion, uptake into phospholipids, or enzymatic degradation.
Cyclooxygenase
The cyclooxygenase (COX) family of isozymes is composed of heme-containing bifunctional enzymes with two catalytic sites. The first active site adds two oxygen molecules to AA to form the hydroxy endoperoxide prostaglandin G2 (PGG2). Subsequently, PGG2 translocates to a second active site for peroxidative reduction to prostaglandin H2 (PGH2). As there is no channel within the COX monomer to shuttle PGG2 to the active reducing site, PGG2 may be reduced on the same or a neighboring enzyme (150). Importantly, the peroxidase activity of COX converts PGG2 to PGH2 by removal of oxygen, which may thereby serve as a source of oxygen radicals (86, 153). It has recently been demonstrated that COX-dependent neuronal death is linked to superoxide anion generation (68).
Three cyclooxygenase isoforms (COX-1, COX-2, and COX-3) exist in brain tissue (67). COX-1 and COX-2 are both constitutively expressed in neural tissue (30, 50). COX-1 is traditionally characterized as a “housekeeping” enzyme in homeostatic production of prostaglandins for activities that include maintenance of glycerophospholipid levels and membrane remodeling (37, 103). Unlike COX-1, however, COX-2 expression and activity is induced in response to pathogenic stimuli including ischemic and hemorrhagic stroke (66, 106, 110, 120, 121). Suggestive evidence supports COX-2 isozyme coupling with upstream phospholipase and downstream synthase activity for the production of lipid signaling eicosanoids. Both cPLA2 and COX-2 knockout mice share phenotypic similarities and decreased susceptibility to ischemia/reperfusion brain injury (140, 166, 167), supporting a functional coupling between cPLA2 and COX-2 enzymes (111, 144, 167). While COX-1 and COX-2 are homodimers, sharing 60% homology in cDNA and amino acids, subtle yet significant differences in substrate binding and catalytic sites confer unique properties. COX-1 contains valine residues at positions 434 and 523, while COX-2 possesses isoleucine. This small difference results in a larger and more flexible active site in COX-2 as compared to COX-1; thus accounting for greater eicosanoid production by COX-2 at low AA concentration (87, 129, 152). COX-3 is a recently identified acetaminophen-sensitive COX isoform, highly abundant in astrocytes, endothelial cells, and pericytes, though absent in neurons (29, 81). First described in canines in 2002, COX-3 mRNA is identical to that of COX-1 with the exception that intron 1 is retained. The existence of a COX-1 splice variant that includes intron 1 has also been reported in humans, however, whether this putative human COX-3 encodes a functional COX protein remains questionable and the source of controversy (80).
The COX-mediated metabolism of AA to PGH2 is a precursor to five primary bioactive prostanoids (PGD2, PGE2, PGF2α), thromboxane (TXA2), and prostacyclin (PGI2). The highest levels of extracellular protanoid release in an ischemic stroke setting occurs during reperfusion (161). The prostanoids bind to G protein-coupled receptors that differ in their effects on cAMP and/or phosphoinositol turnover, and intracellular Ca2+ mobilization (92). For example, PGE2 has been shown to elicit both neuroprotective and neurotoxic effects following cerebral ischemia that are dependent on receptor class binding. PGE2 activation of the E-prostanoid 1 receptor (EP1) disrupts neuronal calcium homeostasis by impairing sodium/calcium exchange, further agonizing Ca2+ activation of phospholipase and the arachidonic acid cascade. Pharmacological and gene inhibition of EP1 receptor has been demonstrated to reduce stroke-induced brain injury, oxygen-glucose deprivation, and neurotoxicity (75). Conversely, PGE2 signaling via EP2 receptor is neuroprotective in cerebral ischemia and dependent on activation of the cAMP–PKA pathway (102). Such paradoxical outcomes suggest an additional level of complexity in the prostanoid response to ischemic injury that warrants further investigation.
Lipoxygenases
The first human lipoxygenase (LOX) activity was described in platelets via the transformation of AA to a prostaglandin-like endoperoxide (54). Today, the LOX enzyme family includes four members that are classified on the basis of the carbon position in which they oxidize arachidonic acid: 5-, 8-, 12-, and 15-LOX. Despite variances in amino acid sequences and tissue distribution amongst family members, the active site of each isozyme is highly conserved (11). The structure of LOX at the active site is composed of an N-terminal beta-barrel domain and a C-terminal domain containing a hydrophobic substrate-binding site (49). A non-heme iron atom is coordinated by three histidine residues and the carboxy-terminal isoleucine. The oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+) activates the enzyme that is then capable of excising a hydrogen atom from a hydrocarbon at one of the four double bonds. This abstraction generates a radical metabolite of AA that rapidly reorganizes its double bonds to take on a more stable conformation. Next, insertion of molecular oxygen generates a hydroperoxide radical which is reduced to the hydroperoxide anion by the simultaneous oxidation of iron to the ferric state (49). A proton is then accepted to form a highly reactive fatty acid hydroperoxide; hydroperoxyeicosatetraenoic acid (HPETE). These hydroperoxide derivatives of AA are short-lived, and readily metabolized into more stable compounds, including hydroxyeicosatetraenoic acids (HETEs) and leukotrienes.
While each LOX isoform carries out the same general reaction, namely hydrogen abstraction from arachidonic acid, each has a unique gene structure, amino acid sequence, and tissue distribution profile. Only three forms of LOX are present in brain tissue, 5-, 12-, and 15-LOX (129). Of these, 12-LOX is the most abundant isoform found in the brain (55), with significant mRNA expression in rat cortical neurons, astrocytes, and oligodendrocytes (16). 5-LOX activity requires a small 18 kDa protein known as FLAP (5-LOX activating protein) for leukotriene synthesis (22). Evidence suggests that FLAP binds AA for presentation to 5-LOX (1, 100). The LOX-mediated metabolites of AA serve as second messengers following stroke by modulating inflammation, apoptosis, and synaptic activity.
Hydroperoxy- and Hydroxy- Eicosatetraenoic Acids
While short-lived, HPETEs are potent neurotoxins. Highly reactive oxygen radicals are produced during the conversion of HPETEs to HETEs (74), contributing to the overall burden of oxidative stress following stroke. Under conditions of glutathione (GSH) depletion, as in acute focal stroke, 12-LOX derived 12-HPETE triggers nitric oxide (NO) induced neural cell death (24). Meanwhile, inhibitors of cPLA2 and 12-LOX have been demonstrated to prevent neurotoxicity in vitro (84). Recent evidence supports that the hydroxy- (HETE) derivatives of LOX-mediated metabolism also possess potent biological activity as well. 12-HETE has been shown to increase mitochondrial NO production, to induce cytochrome C release, and subsequently cause mitochondrial dysfunction (112). 5-, 12-, and 15-HETE are potent mediators of increased vascular permeability (57). Elevated levels of HETEs in a setting of ischemic and hemorrhagic stroke may therefore contribute to the documented phenomenon of blood brain barrier breakdown and stroke-induced edema (71).
Leukotrienes
The 5-LOX catalyzed dehydration reaction generates an epoxide intermediate, leukotriene A4 (LTA4) (129). LTA4 is a highly unstable intermediate that is readily hydrolyzed to LTB4 or conjugated with glutathione by cysteinyl leukotriene C4 synthase to produce leukotriene C4 (LTC4), leukotriene D4 (LTD4), and leukotriene E4 (LTE4) (147). Together, LTC4, LTD4, and LTE4 are collectively referred to as cysteinyl-leukotrienes (Cys–LTs). Total leukotriene levels accumulate in the mammalian brain and cerebral spinal fluid during and after cerebral ischemia (130, 173). LTB4 is characterized as a powerful chemotactic agent that induces adhesion of pro-inflammatory leukocytes to the endothelium; stimulates phagocytosis, and activates neutrophils and other leukocytes in a paracrine manner (25, 33, 44, 146). The Cys-LTs are potent vasoconstrictors of both venous and arterial smooth muscle (105, 142), and are also reported to produce vascular leak and vasogenic edema (36). Furthermore, pharmacological inhibition of Cys–LT receptors attenuates pathological outcomes in a rodent model of focal cerebral ischemia, suggesting roles for Cys–LTs in mediating ischemic injury (31, 44). Like LTB4, Cys–LTs also operate as potent activators and chemoattractants for leukocytes, particularly eosinophils and monocytes, thereby indirectly propagating inflammation and oxidative stress (44).
Cytochrome P450
Cytochrome P450 epoxygenases (cP450) are heme-containing enzymes that use molecular oxygen to oxidize PUFAs by transfer of one atom of oxygen to their substrate and the other to water. Brain cP450 epoxygenases metabolize AA by inserting a single oxygen atom into an unactivated hydrocarbon at one of the four double bonds; thereby producing four regioisomers: 5,6-, 8,9-, 11,12-, and 14,15-epoxyeicosatrienoic acids (EETs). Compared to other organs, expression of cP450 in brain tissue is quite low, accounting for only 1%–10% of levels observed in liver (60, 163). Relative expression of cP450 in brain tissue is found to be highest in astrocytes localized near blood vessels, as well as the adjacent vascular endothelium (128). The activity of cP450 epoxygenases localized in and around the cerebrovasculature has been shown to modulate angiogenesis via capillary endothelial migration and tube formation (104, 108). In a pathological setting, such as ischemic stroke, EETs also serve as potent vasodilators of cerebral arterioles by increasing K+ currents in cerebral arteriole smooth muscle cells (47). Elevated extracellular glutamate, as released by neurons and astrocytes under conditions of acute ischemic stroke, increases the formation of EETs in cultured astrocytes (8). Importantly, glutamate alone has no effect on isolated cerebral blood vessels (56, 168). However, when injected into rat brain tissue via cranial window, glutamate induces significant dilation of pial arterioles (8). A cP450 inhibitor, miconazole, abrogates this effect (7, 8), suggesting that glutamate-stimulated activity of cP450 enhances EET formation and vasodilation.
In addition to their vasodilatory effects, beneficial roles of EETs have been demonstrated within the vasculature that are independent of smooth muscle cell relaxation (85). Specifically, it has been shown that EETs possess potent anti-inflammatory effects by inhibiting cytokine-induced endothelial cell adhesion molecule expression and preventing leukocyte adhesion to the vascular wall (23, 119, 181). More recently, EETs have been shown to protect neurons (83) and astrocytes (95) against ischemic cell death induced in vitro by oxygen–glucose deprivation. Like other eicosanoids, the biological activity of EETs is expected to occur via cell surface receptors. To date, however, the identification of putative EET receptors and mechanisms of protection remain to be adequately characterized (85).
Once metabolized by cP450, AA-derived EETs are subject to several metabolic fates, including incorporation back into membrane phospholipids, and hydration by soluble epoxide hydrolase (sEH) (180) to di-hydroxyeicosatrienoic acids (DHET). No essential function of DHETs has yet to be identified (157). Strategies to inhibit sEH activity in order to prolong the neuroprotective action of EETs are currently of therapeutic interest. Inhibitors of sEH have historically been developed as anti-hypertensive agents, but recent data indicate they also decrease vascular smooth muscle proliferation, prevent cardiac hypertrophy, and improve renal hemodynamics (157).
Supplemental Oxygen Therapy in Focal Ischemic Stroke
In 1662, an English physician and clergyman named Henshaw was the first to use compressed air controlled by large organ bellows to manipulate the atmospheric pressure in a sealed room. He suggested that “in times of good health this domicilium is proposed as a good expedient to help digestion, to promote insensible respiration, and consequently, of excellent use for the prevention of most affections at the lungs” (31a). Two centuries later, a French physician, Junod, was the first to commission the construction of a hyperbaric chamber dedicated for medical use (151). While the purported benefits of early hyperbaric chambers were anecdotal, they laid the foundation for rigorous scientific development. In 1960, Dutch surgeon and engineer Ite Boerema published groundbreaking research demonstrating the capacity for hyperbaric oxygen (HBO)-induced plasma-dissolved oxygen to sustain life in exsanguinated pigs (17). Five years after Boerema's pivotal discovery, Swedish physicians Ingvar and Lassen reported improved outcomes in four stroke patients treated with HBO—reversing neurological deficits and electroencephalography abnormalities (69).
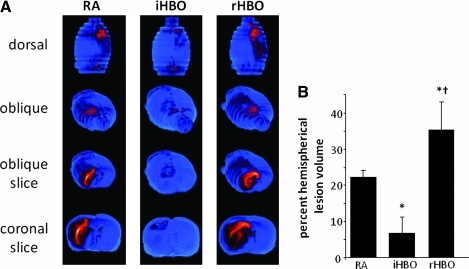
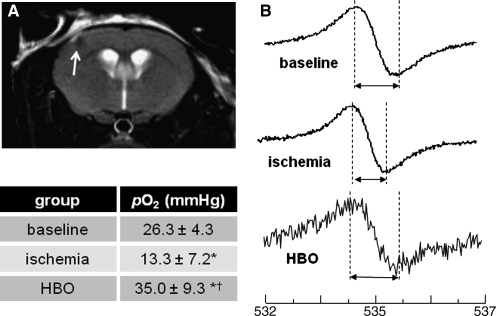
Since then, a number of small animal and clinical case reports have demonstrated both efficacious and deleterious outcomes for HBO and normobaric oxygen (100% O2, 1 ATA, NBO) in the treatment of AIS. Three clinical pilot studies to probe the efficacy of HBO to treat acute ischemic stroke reported mixed and potentially harmful outcomes with HBO treatments that overlapped thrombolytic therapy or were applied as late as 2 weeks after the onset of stroke (9, 117, 136). Indeed, preclinical investigation by our laboratory has uncovered specific application phases during ischemia and following reperfusion in which HBO mediates both protective and harmful outcomes (Fig. 4) (133). HBO applied during MCAO-induced ischemia was found to correct the hypoxia component of stroke injury sufficienty. Mice were selectively implanted with oxygen-sensitive EPR probe in cortical tissue of the ischemic penumbra (Fig. 5). This region was identified as ischemic penumbra as it was spared from stroke-induced lesion when HBO was applied during ischemia. In addition to reduction of stroke lesion volume, rodents receiving HBO during ischemia had significantly attenuated markers of oxidative stress (i.e., 4HNE) in the penumbra region (133). Conversely, HBO at the onset of ischemic stroke reperfusion, when ROS are known to be elevated (78), exacerbated oxidative stress while worsening stroke outcomes. Others have reported a limited window between 3 h and 6 h acute ischemic stroke reperfusion in which HBO is beneficial, but harmful at 12 h and beyond (14, 99). Given the distinct and limited window of HBO therapy, the challenge now comes in translating these findings to an efficient and effective clinical response. Improved diagnostic processes to rapidly and repeatedly monitor cerebral perfusion of acute ischemic stroke patients would benefit the clinical prescription of HBO therapy.
FIG. 4.
Hyperbaric oxygen therapy reduces during, exacerbates at reperfusion, acute ischemic stroke infarct volume. (A) Representative T2-weighted MR images were acquired 48 h after MCAO in rodents kept in room air during ischemia (RA, control), or receiving hyperbaric oxygen (100% O2 at 2 ATA) during ischemia (iHBO) or immediately following reperfusion (rHBO). Color look up table applied—shift from blue to red denotes edema and stroke-induced infarct. Three-dimensional reconstruction of coronal slices permits visualization of the brain from dorsal and oblique positions. HBO during MCAO significantly decreased stroke-induced lesion volume in iHBO animals, while in rHBO animals is increased lesion volume. (B) Percent hemispherical lesion volume corrected for edema >5 *p < 0.05 vs RA, †p < 0.05 vs iHBO. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 5.
HBO corrects stroke-induced hypoxia during middle cerebral artery occlusion in rodents. (A) Oxygen-sensitive EPR oximetry probe was implanted in the ischemic penumbra of rodents in the week prior to MCAO using stereotaxic coordinates −0.1 mm bregma, +2.0 mm lateral, −1.0 mm dorsal. Placement of the probe was verified using MRI. (B) EPR spectra were acquired prior to MCAO to characterize normal cortical pO2 (baseline). After MCAO-induced focal ischemia, brain pO2 in the ischemic penumbra significantly dropped to ∼ 50% of baseline level. Correction of stroke-induced hypoxia was achieved with HBO during ischemia in the penumbra zone. Increased noise in EPR signal was encountered in the HBO group due to greater distance between probe and resonator as a result of the hyperbaric chamber. Representative EPR spectra shown. N ≥ 3, *p < 0.05 vs baseline, †p < 0.05 vs ischemia.
Conclusions
When one considers the unique physiological and biochemical properties of hypermetabolic brain tissue, with a voracious appetite for oxygen in an environment enriched with oxidation-prone PUFAs such as arachidonic acid, the dire physiological consequences of stroke-induced hypoxia is readily apparent. Preclinical models of stroke have revealed substantive evidence of elevated oxidants as early as 20 minutes following the onset of stroke (89, 113), and uncontrolled oxidative metabolism of AA as early as 1 hour following hemorrhagic and ischemic stroke (45, 148). In the case of ischemic stroke, a significant increase in reactive oxygen species and AA metabolism accompany reoxygenation of brain tissue following reperfusion of stroke-affected tissue (52). The rapid and overwhelming imbalance of pro-oxidants over antioxidants in stroke injury leads to a pathological setting that is highly resistant to late phase (>6 h after stroke onset) therapeutic intervention. Potential neuroprotective agents administered after the onset of stroke that aim to salvage ischemic tissue, limit infarct size, and resolve stroke-mediated oxidative stress and inflammation have failed in clinical studies. Of 114 neuroprotective agents tested during the 20th century, none were proven successful in clinical trials (48). As clinicians and scientists recognize the significant contribution of the hypoxia component of stroke injury to the limited therapeutic window for intervention, research emphasis is shifting from late phase treatment modalities toward acute phase (<6 hours) and prophylactic treatment regimens.
Abbreviations Used
- 4HNE
4-hydroxynonenal
- AA
arachidonic acid
- AIS
acute ischemic stroke
- BOLD
blood oxygenation level-dependent
- COX
cyclooxygenase
- cP450
cytochrome P450
- cPLA2
cytosolic calcium dependent phospholipase A2
- Cys-LTs
cysteinyl-leukotrienes
- DHA
docosahexaenoic acid
- DHET
dihydroxyeicosatrienoic acid
- EETs
epoxyeicosatrienoic acids
- EP1
E-prostanoid 1 receptor
- EPR
electron paramagnetic resonance
- Fe2+
ferrous iron
- Fe3+
ferric iron
- FLAP
5-LOX activating protein
- fMRI
functional magnetic resonance imaging
- HBO
hyperbaric oxygen
- HETE
hydroxyeicosatetraenoic acid
- HIF
hypoxia-inducible factor
- HO
heme oxygenase
- HPETE
hydroperoxyeicosatetraenoic acid
- ICH
intracerebral hemorrhage
- iHBO
hyperbaric oxygen during ischemia
- iPLA2
calcium independent phospholipase A2
- LDL
low density lipoprotein
- LOX
lipoxygenase
- LPC
lysophosphatidylcholine
- LTA4
leukotriene A4
- LTB4
leukotriene B4
- LTC4
leukotriene C4
- LTD4
leukotriene D4
- LTE4
leukotriene E4
- MCAO
middle cerebral artery occlusion
- MDA
malondialdehyde
- NO
nitric oxide
- NOS
nitric oxide synthase
- O2-
superoxide radical
- ONOO-
peroxynitrite
- PAFA
platelet-activating factor acetylhydrolase
- PGF2a
prostaglandin F2a
- PGG2
hydroxy endoperoxide prostaglandin G2
- PGH2
prostaglandin H2
- PGI2
prostacyclin I
- PLA2
phospholipase A2
- PLC
phospholipase C
- PLD
phospholipase D
- PUFA
polyunsaturated fatty acid
- rHBO
hyperbaric oxygen after reperfusion
- ROO-
peroxyl radical
- ROOH
lipid hydroperoxide
- ROS
reactive oxygen species
- SAH
subarachnoid hemorrhage
- sHE
soluble epoxide hydrolase
- sPLA2
secreted small molecular weight phospholipase A2
- TASK
TWIK-related acid sensitive channel
- TNF-α
tumor necrosis factor alpha
- TXA2
thromboxane A2
Acknowledgment
These studies are supported in part by NIH Grant NS42617.
References
- 1.Abramovitz M. Wong E. Cox ME. Richardson CD. Li C. Vickers PJ. 5-lipoxygenase-activating protein stimulates the utilization of arachidonic acid by 5-lipoxygenase. Eur J Biochem. 1993;215:105–111. doi: 10.1111/j.1432-1033.1993.tb18012.x. [DOI] [PubMed] [Google Scholar]
- 2.Acker T. Acker H. Cellular oxygen sensing need in CNS function: Physiological and pathological implications. J Exp Biol. 2004;207:3171–3188. doi: 10.1242/jeb.01075. [DOI] [PubMed] [Google Scholar]
- 3.Adibhatla RM. Hatcher JF. Dempsey RJ. Phospholipase A2, hydroxyl radicals, and lipid peroxidation in transient cerebral ischemia. Antioxid Redox Signal. 2003;5:647–654. doi: 10.1089/152308603770310329. [DOI] [PubMed] [Google Scholar]
- 4.Adibhatla RM. Hatcher JF. Dempsey RJ. Lipids and lipidomics in brain injury and diseases. AAPS J. 2006;8:E314–21. doi: 10.1007/BF02854902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adibhatla RM. Hatcher JF. Larsen EC. Chen X. Sun D. Tsao FH. CDP-choline significantly restores phosphatidylcholine levels by differentially affecting phospholipase A2 and CTP: Phosphocholine cytidylyltransferase after stroke. J Biol Chem. 2006;281:6718–6725. doi: 10.1074/jbc.M512112200. [DOI] [PubMed] [Google Scholar]
- 6.Alexandrov PN. Cui JG. Lukiw WJ. Hypoxia-sensitive domain in the human cytosolic phospholipase A2 promoter. Neuroreport. 2006;17:303–307. doi: 10.1097/01.wnr.0000201506.61373.99. [DOI] [PubMed] [Google Scholar]
- 7.Alkayed NJ. Birks EK. Hudetz AG. Roman RJ. Henderson L. Harder DR. Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol. 1996;271:H1541–1546. doi: 10.1152/ajpheart.1996.271.4.H1541. [DOI] [PubMed] [Google Scholar]
- 8.Alkayed NJ. Birks EK. Narayanan J. Petrie KA. Kohler–Cabot AE. Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DC. Bottini AG. Jagiella WM. Westphal B. Ford S. Rockswold GL. Loewenson RB. A pilot study of hyperbaric oxygen in the treatment of human stroke. Stroke. 1991;22:1137–1142. doi: 10.1161/01.str.22.9.1137. [DOI] [PubMed] [Google Scholar]
- 10.Anthonsen MW. Solhaug A. Johansen B. Functional coupling between secretory and cytosolic phospholipase A2 modulates tumor necrosis factor-alpha- and interleukin-1beta-induced NF-kappa B activation. J Biol Chem. 2001;276:30527–30536. doi: 10.1074/jbc.M008481200. [DOI] [PubMed] [Google Scholar]
- 11.Aparoy P. Reddy RN. Guruprasad L. Reddy MR. Reddanna P. Homology modeling of 5-lipoxygenase and hints for better inhibitor design. J Comput Aided Mol Des. 2008;22:611–619. doi: 10.1007/s10822-008-9180-0. [DOI] [PubMed] [Google Scholar]
- 12.Astrup J. Siesjo BK. Symon L. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 13.Aviv RI. d'Esterre CD. Murphy BD. Hopyan JJ. Buck B. Mallia G. Li V. Zhang L. Symons SP. Lee TY. Hemorrhagic transformation of ischemic stroke: Prediction with CT perfusion. Radiology. 2009;250:867–877. doi: 10.1148/radiol.2503080257. [DOI] [PubMed] [Google Scholar]
- 14.Badr AE. Yin W. Mychaskiw G. Zhang JH. Dual effect of HBO on cerebral infarction in MCAO rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R766–770. doi: 10.1152/ajpregu.2001.280.3.R766. [DOI] [PubMed] [Google Scholar]
- 15. This reference has been deleted.
- 16.Bendani MK. Palluy O. Cook–Moreau J. Beneytout JL. Rigaud M. Vallat JM. Localization of 12-lipoxygenase mRNA in cultured oligodendrocytes and astrocytes by in situ reverse transcriptase and polymerase chain reaction. Neurosci Lett. 1995;189:159–162. doi: 10.1016/0304-3940(95)11482-c. [DOI] [PubMed] [Google Scholar]
- 17.Boerema I. Meyne NG. Brummelkamp WH. Bouma S. Mensch MH. Kamermans F. Stern Hanf M. van A. [Life without blood.] Ned Tijdschr Geneeskd. 1960;104:949–954. [PubMed] [Google Scholar]
- 18.Boveris A. Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckler KJ. Williams BA. Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke JE. Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23:49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke JE. Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50:S237–242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Butterworth RF. Metabolic encephalopathies. In: Siegel G, editor; Albers RW, editor; Brady S, editor; Price D, editor. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Amsterdam; Boston: Elsevier; 2006. pp. 593–602. [Google Scholar]
- 22.Byrum RS. Goulet JL. Griffiths RJ. Koller BH. Role of the 5-lipoxygenase-activating protein (FLAP) in murine acute inflammatory responses. J Exp Med. 1997;185:1065–1075. doi: 10.1084/jem.185.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell WB. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol Sci. 2000;21:125–127. doi: 10.1016/s0165-6147(00)01472-3. [DOI] [PubMed] [Google Scholar]
- 24.Canals S. Casarejos MJ. de Bernardo S. Rodriguez–Martin E. Mena MA. Nitric oxide triggers the toxicity due to glutathione depletion in midbrain cultures through 12-lipoxygenase. J Biol Chem. 2003;278:21542–21549. doi: 10.1074/jbc.M213174200. [DOI] [PubMed] [Google Scholar]
- 25.Canetti C. Hu B. Curtis JL. Peters–Golden M. Syk activation is a leukotriene B4-regulated event involved in macrophage phagocytosis of IgG-coated targets but not apoptotic cells. Blood. 2003;102:1877–1883. doi: 10.1182/blood-2003-02-0534. [DOI] [PubMed] [Google Scholar]
- 26.Carlson SE. Werkman SH. Peeples JM. Cooke RJ. Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci USA. 1993;90:1073–1077. doi: 10.1073/pnas.90.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson SE. Werkman SH. Peeples JM. Wilson WM. Long-chain fatty acids and early visual and cognitive development of preterm infants. Eur J Clin Nutr. 1994;48:S27–30. [PubMed] [Google Scholar]
- 28.Carlson SE. Werkman SH. Peeples JM. Wilson WM., 3rd Growth and development of premature infants in relation to omega 3 and omega 6 fatty acid status. World Rev Nutr Diet. 1994;75:63–69. doi: 10.1159/000423552. [DOI] [PubMed] [Google Scholar]
- 29.Chandrasekharan NV. Dai H. Roos KL. Evanson NK. Tomsik J. Elton TS. Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopra B. Giblett S. Little JG. Donaldson LF. Tate S. Evans RJ. Grubb BD. Cyclooxygenase-1 is a marker for a subpopulation of putative nociceptive neurons in rat dorsal root ganglia. Eur J Neurosci. 2000;12:911–920. doi: 10.1046/j.1460-9568.2000.00979.x. [DOI] [PubMed] [Google Scholar]
- 31.Ciana P. Fumagalli M. Trincavelli ML. Verderio C. Rosa P. Lecca D. Ferrario S. Parravicini C. Capra V. Gelosa P. Guerrini U. Belcredito S. Cimino M. Sironi L. Tremoli E. Rovati GE. Martini C. Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Clarke D. History of hyperbaric therapy. In: Meloni D, editor. Physiology and Medicine of Hyperbaric Oxygen Therapy. Philadelphia: Saunders/Elsevier; 2008. pp. 3–24. [Google Scholar]
- 32.Clemens JA. Stephenson DT. Smalstig EB. Roberts EF. Johnstone EM. Sharp JD. Little SP. Kramer RM. Reactive glia express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–535. doi: 10.1161/01.str.27.3.527. [DOI] [PubMed] [Google Scholar]
- 33.Coffey MJ. Phare SM. Peters–Golden M. Role of leukotrienes in killing of Mycobacterium bovis by neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2004;71:185–190. doi: 10.1016/j.plefa.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Contreras MA. Greiner RS. Chang MC. Myers CS. Salem N., Jr. Rapoport SI. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J Neurochem. 2000;75:2392–2400. doi: 10.1046/j.1471-4159.2000.0752392.x. [DOI] [PubMed] [Google Scholar]
- 35.Cooper AJ. Kristal BS. Multiple roles of glutathione in the central nervous system. Biol Chem. 1997;378:793–802. [PubMed] [Google Scholar]
- 36.Dahlen SE. Bjork J. Hedqvist P. Arfors KE. Hammarstrom S. Lindgren JA. Samuelsson B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: In vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci USA. 1981;78:3887–2891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deininger MH. Kremsner PG. Meyermann R. Schluesener HJ. Focal accumulation of cyclooxygenase-1 (COX-1) and COX-2 expressing cells in cerebral malaria. J Neuroimmunol. 2000;106:198–205. doi: 10.1016/s0165-5728(00)00187-9. [DOI] [PubMed] [Google Scholar]
- 38.Duling BR. Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res. 1970;27:669–678. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- 38a.Echtay KS. Mitochondrial uncoupling proteins–what is their physiological role. Free Radic Biol Med. 2007;43:1351–1371. doi: 10.1016/j.freeradbiomed.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Eichberg J. Phospholipids in Nervous Tissues. Wiley. New York: 1985. pp. 110–118. [Google Scholar]
- 40.Esterbauer H. Schaur RJ. Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 41.Fang J. Holmgren A. Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J Am Chem Soc. 2006;128:1879–1885. doi: 10.1021/ja057358l. [DOI] [PubMed] [Google Scholar]
- 42.Farooqui AA. Horrocks LA. Farooqui T. Modulation of inflammation in brain: A matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 43.Farooqui AA. Ong WY. Horrocks LA. Inhibitors of brain phospholipase A2 activity: Their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 44.Flamand N. Mancuso P. Serezani CH. Brock TG. Leukotrienes: Mediators that have been typecast as villains. Cell Mol Life Sci. 2007;64:2657–2670. doi: 10.1007/s00018-007-7228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaetani P. Marzatico F. Rodriguez y. Baena R. Pacchiarini L. Vigano T. Grignani G. Crivellari MT. Benzi G. Arachidonic acid metabolism and pathophysiologic aspects of subarachnoid hemorrhage in rats. Stroke. 1990;21:328–332. doi: 10.1161/01.str.21.2.328. [DOI] [PubMed] [Google Scholar]
- 46.Ganong WF. Review of Medical Physiology. Norwalk, CT: Appleton & Lange; 1987. p. 505. [Google Scholar]
- 47.Gebremedhin D. Ma YH. Falck JR. Roman RJ. Van Rollins M. Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263:H519–525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 48.Gladstone DJ. Black SE. Hakim AM. Toward wisdom from failure: Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 49.Glickman MH. Klinman JP. Lipoxygenase reaction mechanism: Demonstration that hydrogen abstraction from substrate precedes dioxygen binding during catalytic turnover. Biochemistry. 1996;35:12882–12892. doi: 10.1021/bi960985q. [DOI] [PubMed] [Google Scholar]
- 50.Goppelt–Struebe M. Beiche F. Cyclooxygenase-2 in the spinal cord: Localization and regulation after a peripheral inflammatory stimulus. Adv Exp Med Biol. 1997;433:213–216. doi: 10.1007/978-1-4899-1810-9_45. [DOI] [PubMed] [Google Scholar]
- 51.Grimsrud PA. Xie H. Griffin TJ. Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gursoy–Ozdemir Y. Can A. Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- 53. This reference has been deleted.
- 54.Hamberg M. Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci USA. 1974;71:3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hambrecht GS. Adesuyi SA. Holt S. Ellis EF. Brain 12-HETE formation in different species, brain regions, and in brain microvessels. Neurochem Res. 1987;12:1029–1033. doi: 10.1007/BF00970932. [DOI] [PubMed] [Google Scholar]
- 56.Hardebo JE. Wieloch T. Kahrstrom J. Excitatory amino acids and cerebrovascular tone. Acta Physiol Scand. 1989;136:483–485. doi: 10.1111/j.1748-1716.1989.tb08690.x. [DOI] [PubMed] [Google Scholar]
- 57.Hariri RJ. Ghajar JB. Pomerantz KB. Hajjar DP. Giannuzzi RF. Tomich E. Andrews DW. Patterson RH., Jr Human glial cell production of lipoxygenase-generated eicosanoids: A potential role in the pathophysiology of vascular changes following traumatic brain injury. J Trauma. 1989;29:1203–1210. doi: 10.1097/00005373-198909000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Hata R. Maeda K. Hermann D. Mies G. Hossmann KA. Dynamics of regional brain metabolism and gene expression after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:306–315. doi: 10.1097/00004647-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Hata R. Maeda K. Hermann D. Mies G. Hossmann KA. Evolution of brain infarction after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2000;20:937–946. doi: 10.1097/00004647-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Hedlund E. Gustafsson JA. Warner M. Cytochrome P450 in the brain; A review. Curr Drug Metab. 2001;2:245–263. doi: 10.2174/1389200013338513. [DOI] [PubMed] [Google Scholar]
- 61.Hirashima Y. Endo S. Ohmori T. Kato R. Takaku A. Platelet-activating factor (PAF) concentration and PAF acetylhydrolase activity in cerebrospinal fluid of patients with subarachnoid hemorrhage. J Neurosurg. 1994;80:31–36. doi: 10.3171/jns.1994.80.1.0031. [DOI] [PubMed] [Google Scholar]
- 62.Hirashima Y. Nakamura S. Endo S. Kuwayama N. Naruse Y. Takaku A. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem Res. 1997;22:1249–1255. doi: 10.1023/a:1021985030331. [DOI] [PubMed] [Google Scholar]
- 63.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 64.Hua Y. Keep RF. Hoff JT. Xi G. Brain injury after intracerebral hemorrhage: The role of thrombin and iron. Stroke. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 65.Huang FP. Xi G. Keep RF. Hua Y. Nemoianu A. Hoff JT. Brain edema after experimental intracerebral hemorrhage: Role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 66.Iadecola C. Forster C. Nogawa S. Clark HB. Ross ME. Cyclooxygenase-2 immunoreactivity in the human brain following cerebral ischemia. Acta Neuropathol. 1999;98:9–14. doi: 10.1007/s004010051045. [DOI] [PubMed] [Google Scholar]
- 67.Iadecola C. Gorelick PB. The Janus face of cyclooxygenase-2 in ischemic stroke: Shifting toward downstream targets. Stroke. 2005;36:182–185. doi: 10.1161/01.STR.0000153797.33611.d8. [DOI] [PubMed] [Google Scholar]
- 68.Im JY. Kim D. Paik SG. Han PL. Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Radic Biol Med. 2006;41:960–972. doi: 10.1016/j.freeradbiomed.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Ingvar DH. Lassen NA. Treatment of focal cerebral ischemia with hyperbaric oxygen. Report of 4 cases. Acta Neurol Scand. 1965;41:92–95. doi: 10.1111/j.1600-0404.1965.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 70.Jiang C. Haddad GG. A direct mechanism for sensing low oxygen levels by central neurons. Proc Natl Acad Sci USA. 1994;91:7198–7201. doi: 10.1073/pnas.91.15.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin G. Arai K. Murata Y. Wang S. Stins MF. Lo EH. van Leyen K. Protecting against cerebrovascular injury: Contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones CR. Arai T. Rapoport SI. Evidence for the involvement of docosahexaenoic acid in cholinergic stimulated signal transduction at the synapse. Neurochem Res. 1997;22:663–670. doi: 10.1023/a:1027341707837. [DOI] [PubMed] [Google Scholar]
- 73.Karni A. Meyer G. Jezzard P. Adams MM. Turner R. Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 74.Katsuki H. Okuda S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog Neurobiol. 1995;46:607–636. doi: 10.1016/0301-0082(95)00016-o. [DOI] [PubMed] [Google Scholar]
- 75.Kawano T. Anrather J. Zhou P. Park L. Wang G. Frys KA. Kunz A. Cho S. Orio M. Iadecola C. Prostaglandin E2 EP1 receptors: Downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 76.Kehrer JP. Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 77.Keller JN. Mark RJ. Bruce AJ. Blanc E. Rothstein JD. Uchida K. Waeg G. Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997;80:685–696. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 78.Kim GW. Kondo T. Noshita N. Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: Implications for the production and role of superoxide radicals. Stroke. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- 79.Kinouchi H. Imaizumi S. Yoshimoto T. Yamamoto H. Motomiya M. Changes of polyphosphoinositides, lysophospholipid, and free fatty acids in transient cerebral ischemia of rat brain. Mol Chem Neuropathol. 1990;12:215–228. doi: 10.1007/BF03159946. [DOI] [PubMed] [Google Scholar]
- 80.Kis B. Snipes JA. Busija DW. Acetaminophen and the cyclooxygenase-3 puzzle: Sorting out facts, fictions, and uncertainties. J Pharmacol Exp Ther. 2005;315:1–7. doi: 10.1124/jpet.105.085431. [DOI] [PubMed] [Google Scholar]
- 81.Kis B. Snipes JA. Isse T. Nagy K. Busija DW. Putative cyclooxygenase-3 expression in rat brain cells. J Cereb Blood Flow Metab. 2003;23:1287–1292. doi: 10.1097/01.WCB.0000090681.07515.81. [DOI] [PubMed] [Google Scholar]
- 82.Klabunde RE. Cardiovascular Physiology Concepts. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 85–86. [Google Scholar]
- 83.Koerner IP. Jacks R. DeBarber AE. Koop D. Mao P. Grant DF. Alkayed NJ. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci. 2007;27:4642–4649. doi: 10.1523/JNEUROSCI.0056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kramer BC. Yabut JA. Cheong J. Jnobaptiste R. Robakis T. Olanow CW. Mytilineou C. Toxicity of glutathione depletion in mesencephalic cultures: A role for arachidonic acid and its lipoxygenase metabolites. Eur J Neurosci. 2004;19:280–286. doi: 10.1111/j.1460-9568.2004.03111.x. [DOI] [PubMed] [Google Scholar]
- 85.Kroetz DL. Zeldin DC. Cytochrome P450 pathways of arachidonic acid metabolism. Curr Opin Lipidol. 2002;13:273–283. doi: 10.1097/00041433-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Kukreja RC. Kontos HA. Hess ML. Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res. 1986;59:612–619. doi: 10.1161/01.res.59.6.612. [DOI] [PubMed] [Google Scholar]
- 87.Kurumbail RG. Stevens AM. Gierse JK. McDonald JJ. Stegeman RA. Pak JY. Gildehaus D. Miyashiro JM. Penning TD. Seibert K. Isakson PC. Stallings WC. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 88.Lahaie I. Hardy P. Hou X. Hassessian H. Asselin P. Lachapelle P. Almazan G. Varma DR. Morrow JD. Roberts LJ., 2nd Chemtob S. A novel mechanism for vasoconstrictor action of 8-isoprostaglandin F2 alpha on retinal vessels. Am J Physiol. 1998;274:R1406–1416. doi: 10.1152/ajpregu.1998.274.5.R1406. [DOI] [PubMed] [Google Scholar]
- 89.Lancelot E. Callebert J. Revaud ML. Boulu RG. Plotkine M. Detection of hydroxyl radicals in rat striatum during transient focal cerebral ischemia: Possible implication in tissue damage. Neurosci Lett. 1995;197:85–88. doi: 10.1016/0304-3940(95)11901-8. [DOI] [PubMed] [Google Scholar]
- 90.Lapchak PA. Hemorrhagic transformation following ischemic stroke: Significance, causes, and relationship to therapy and treatment. Curr Neurol Neurosci Rep. 2002;2:38–43. doi: 10.1007/s11910-002-0051-0. [DOI] [PubMed] [Google Scholar]
- 91.Lauritzen I. Heurteaux C. Lazdunski M. Expression of group II phospholipase A2 in rat brain after severe forebrain ischemia and in endotoxic shock. Brain Res. 1994;651:353–356. doi: 10.1016/0006-8993(94)90719-6. [DOI] [PubMed] [Google Scholar]
- 92.Liang X. Wu L. Wang Q. Hand T. Bilak M. McCullough L. Andreasson K. Function of COX-2 and prostaglandins in neurological disease. J Mol Neurosci. 2007;33:94–99. doi: 10.1007/s12031-007-0058-8. [DOI] [PubMed] [Google Scholar]
- 93.Lin LL. Wartmann M. Lin AY. Knopf JL. Seth A. Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 94.Lin TN. Wang Q. Simonyi A. Chen JJ. Cheung WM. He YY. Xu J. Sun AY. Hsu CY. Sun GY. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J Neurochem. 2004;90:637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- 95.Liu M. Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab. 2005;25:939–948. doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- 96.Liu S. Shi H. Liu W. Furuichi T. Timmins GS. Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- 97.Lizasoain I. Moro MA. Knowles RG. Darley–Usmar V. Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem J. 1996;314:877–880. doi: 10.1042/bj3140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lloyd–Jones D. Adams RJ. Brown TM. Carnethon M. Dai S. De Simone G. Ferguson TB. Ford E. Furie K. Gillespie C. Go A. Greenlund K. Haase N. Hailpern S. Ho PM. Howard V. Kissela B. Kittner S. Lackland D. Lisabeth L. Marelli A. McDermott MM. Meigs J. Mozaffarian D. Mussolino M. Nichol G. Roger V. Rosamond W. Sacco R. Sorlie P. Stafford R. Thom T. Wasserthiel–Smoller S. Wong ND. Wylie–Rosett J. Heart Disease and Stroke Statistics—2010 Update. A Report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 99.Lou M. Eschenfelder CC. Herdegen T. Brecht S. Deuschl G. Therapeutic window for use of hyperbaric oxygenation in focal transient ischemia in rats. Stroke. 2004;35:578–583. doi: 10.1161/01.STR.0000111599.77426.A0. [DOI] [PubMed] [Google Scholar]
- 100.Mancini JA. Abramovitz M. Cox ME. Wong E. Charleson S. Perrier H. Wang Z. Prasit P. Vickers PJ. 5-lipoxygenase-activating protein is an arachidonate binding protein. FEBS Lett. 1993;318:277–281. doi: 10.1016/0014-5793(93)80528-3. [DOI] [PubMed] [Google Scholar]
- 101.Mark RJ. Pang Z. Geddes JW. Uchida K. Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: Involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McCullough L. Wu L. Haughey N. Liang X. Hand T. Wang Q. Breyer RM. Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGeer PL. McGeer EG. Yasojima K. Expression of COX-1 and COX-2 mRNAs in atherosclerotic plaques. Exp Gerontol. 2002;37:925–929. doi: 10.1016/s0531-5565(02)00028-1. [DOI] [PubMed] [Google Scholar]
- 104.Michaelis UR. Falck JR. Schmidt R. Busse R. Fleming I. Cytochrome P4502C9-derived epoxyeicosatrienoic acids induce the expression of cyclooxygenase-2 in endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:321–326. doi: 10.1161/01.ATV.0000151648.58516.eb. [DOI] [PubMed] [Google Scholar]
- 105.Michelassi F. Landa L. Hill RD. Lowenstein E. Watkins WD. Petkau AJ. Zapol WM. Leukotriene D4: A potent coronary artery vasoconstrictor associated with impaired ventricular contraction. Science. 1982;217:841–843. doi: 10.1126/science.6808665. [DOI] [PubMed] [Google Scholar]
- 106.Miettinen S. Fusco FR. Yrjanheikki J. Keinanen R. Hirvonen T. Roivainen R. Narhi M. Hokfelt T. Koistinaho J. Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-D-aspartic acid-receptors and phospholipase A2. Proc Natl Acad Sci USA. 1997;94:6500–6505. doi: 10.1073/pnas.94.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miyake H. Kadoya A. Ohyashiki T. Increase in molecular rigidity of the protein conformation of brain Na+-K+-ATPase by modification with 4-hydroxy-2-nonenal. Biol Pharm Bull. 2003;26:1652–1656. doi: 10.1248/bpb.26.1652. [DOI] [PubMed] [Google Scholar]
- 108.Munzenmaier DH. Harder DR. Cerebral microvascular endothelial cell tube formation: Role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- 109.Musiek ES. Breeding RS. Milne GL. Zanoni G. Morrow JD. McLaughlin B. Cyclopentenone isoprostanes are novel bioactive products of lipid oxidation which enhance neurodegeneration. J Neurochem. 2006;97:1301–1313. doi: 10.1111/j.1471-4159.2006.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakayama M. Uchimura K. Zhu RL. Nagayama T. Rose ME. Stetler RA. Isakson PC. Chen J. Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Naraba H. Murakami M. Matsumoto H. Shimbara S. Ueno A. Kudo I. Oh-ishi S. Segregated coupling of phospholipases A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages. J Immunol. 1998;160:2974–2982. [PubMed] [Google Scholar]
- 112.Nazarewicz RR. Zenebe WJ. Parihar A. Parihar MS. Vaccaro M. Rink C. Sen CK. Ghafourifar P. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch Biochem Biophys. 2007;468:114–120. doi: 10.1016/j.abb.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Negishi H. Ikeda K. Nara Y. Yamori Y. Increased hydroxyl radicals in the hippocampus of stroke-prone spontaneously hypertensive rats during transient ischemia and recirculation. Neurosci Lett. 2001;306:206–208. doi: 10.1016/s0304-3940(01)01893-6. [DOI] [PubMed] [Google Scholar]
- 114.Neubauer JA. Sunderram J. Oxygen-sensing neurons in the central nervous system. J Appl Physiol. 2004;96:367–374. doi: 10.1152/japplphysiol.00831.2003. [DOI] [PubMed] [Google Scholar]
- 115. This reference has been deleted.
- 116.Neuringer M. Connor WE. Lin DS. Barstad L. Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci USA. 1986;83:4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nighoghossian N. Trouillas P. Adeleine P. Salord F. Hyperbaric oxygen in the treatment of acute ischemic stroke. A double-blind pilot study. Stroke. 1995;26:1369–1372. doi: 10.1161/01.str.26.8.1369. [DOI] [PubMed] [Google Scholar]
- 118.Nito C. Kamada H. Endo H. Niizuma K. Myer DJ. Chan PH. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 2008;28:1686–1396. doi: 10.1038/jcbfm.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Node K. Huo Y. Ruan X. Yang B. Spiecker M. Ley K. Zeldin DC. Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nogawa S. Zhang F. Ross ME. Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Osuka K. Watanabe Y. Yamauchi K. Nakazawa A. Usuda N. Tokuda M. Yoshida J. Activation of the JAK-STAT signaling pathway in the rat basilar artery after subarachnoid hemorrhage. Brain Res. 2006;1072:1–7. doi: 10.1016/j.brainres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 122.Ousman SS. David S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia. 2000;30:92–104. [PubMed] [Google Scholar]
- 123.Owada Y. Tominaga T. Yoshimoto T. Kondo H. Molecular cloning of rat cDNA for cytosolic phospholipase A2 and the increased gene expression in the dentate gyrus following transient forebrain ischemia. Brain Res Mol Brain Res. 1994;25:364–368. doi: 10.1016/0169-328x(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 124.Pan J. Konstas AA. Bateman B. Ortolano GA. Pile–Spellman J. Reperfusion injury following cerebral ischemia: Pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007;49:93–102. doi: 10.1007/s00234-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pantano P. Baron JC. Lebrun-Grandie P. Duquesnoy N. Bousser MG. Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke. 1984;15:635–641. doi: 10.1161/01.str.15.4.635. [DOI] [PubMed] [Google Scholar]
- 126.Parola M. Bellomo G. Robino G. Barrera G. Dianzani MU. 4-Hydroxynonenal as a biological signal: Molecular basis and pathophysiological implications. Antioxid Redox Signal. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- 127.Pearigen P. Gwinn R. Simon RP. The effects in vivo of hypoxia on brain injury. Brain Res. 1996;725:184–191. doi: 10.1016/0006-8993(96)00215-6. [DOI] [PubMed] [Google Scholar]
- 128.Peng X. Zhang C. Alkayed NJ. Harder DR. Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 129.Phillis JW. Horrocks LA. Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: Their role and involvement in neurological disorders. Brain Res Rev. 2006;52:201–243. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 130.Rao AM. Hatcher JF. Kindy MS. Dempsey RJ. Arachidonic acid and leukotriene C4: Role in transient cerebral ischemia of gerbils. Neurochem Res. 1999;24:1225–1232. doi: 10.1023/a:1020916905312. [DOI] [PubMed] [Google Scholar]
- 131.Rapoport SI. Arachidonic acid and the brain. J Nutr. 2008;138:2515–2520. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rapoport SI. Chang MC. Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- 133.Rink C. Roy S. Khan M. Ananth P. Kuppusamy P. Sen CK. Khanna S. Oxygen-sensitive outcomes and gene expression in acute ischemic stroke. J Cereb Blood Flow Metab. 2010;30:1275–1287. doi: 10.1038/jcbfm.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rordorf G. Uemura Y. Bonventre JV. Characterization of phospholipase A2 (PLA2) activity in gerbil brain: Enhanced activities of cytosolic, mitochondrial, and microsomal forms after ischemia and reperfusion. J Neurosci. 1991;11:1829–1836. doi: 10.1523/JNEUROSCI.11-06-01829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosell A. Cuadrado E. Ortega–Aznar A. Hernandez–Guillamon M. Lo EH. Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 136.Rusyniak DE. Kirk MA. May JD. Kao LW. Brizendine EJ. Welch JL. Cordell WH. Alonso RJ. Hyperbaric oxygen therapy in acute ischemic stroke: Results of the Hyperbaric Oxygen in Acute Ischemic Stroke Trial Pilot Study. Stroke. 2003;34:571–574. doi: 10.1161/01.str.0000050644.48393.d0. [DOI] [PubMed] [Google Scholar]
- 137.Sadrzadeh SM. Anderson DK. Panter SS. Hallaway PE. Eaton JW. Hemoglobin potentiates central nervous system damage. J Clin Invest. 1987;79:662–664. doi: 10.1172/JCI112865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sadrzadeh SM. Eaton JW. Hemoglobin-mediated oxidant damage to the central nervous system requires endogenous ascorbate. J Clin Invest. 1988;82:1510–1515. doi: 10.1172/JCI113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saluja I. Song D. O'Regan MH. Phillis JW. Role of phospholipase A2 in the release of free fatty acids during ischemia-reperfusion in the rat cerebral cortex. Neurosci Lett. 1997;233:97–100. doi: 10.1016/s0304-3940(97)00646-0. [DOI] [PubMed] [Google Scholar]
- 140.Sapirstein A. Bonventre JV. Phospholipases A2 in ischemic and toxic brain injury. Neurochem Res. 2000;25:745–753. doi: 10.1023/a:1007583708713. [DOI] [PubMed] [Google Scholar]
- 141.Saver JL. Time is brain–quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 142.Schellenberg RR. Foster A. Differential activity of leukotrienes upon human pulmonary vein and artery. Prostaglandins. 1984;27:475–482. doi: 10.1016/0090-6980(84)90205-3. [DOI] [PubMed] [Google Scholar]
- 143.Schilling T. Lehmann F. Ruckert B. Eder C. Physiological mechanisms of lysophosphatidylcholine-induced de-ramification of murine microglia. J Physiol. 2004;557:105–120. doi: 10.1113/jphysiol.2004.060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scott KF. Bryant KJ. Bidgood MJ. Functional coupling and differential regulation of the phospholipase A2-cyclooxygenase pathways in inflammation. J Leukoc Biol. 1999;66:535–541. doi: 10.1002/jlb.66.4.535. [DOI] [PubMed] [Google Scholar]
- 145.Semenza GL. Hydroxylation of HIF-1: Oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 146.Serezani CH. Perrela JH. Russo M. Peters–Golden M. Jancar S. Leukotrienes are essential for the control of Leishmania amazonensis infection and contribute to strain variation in susceptibility. J Immunol. 2006;177:3201–3208. doi: 10.4049/jimmunol.177.5.3201. [DOI] [PubMed] [Google Scholar]
- 147.Shimizu T. Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 148.Shohami E. Rosenthal J. Lavy S. The effect of incomplete cerebral ischemia on prostaglandin levels in rat brain. Stroke. 1982;13:494–499. doi: 10.1161/01.str.13.4.494. [DOI] [PubMed] [Google Scholar]
- 149. This reference has been deleted.
- 150.Simmons DL. Botting RM. Hla T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 151.Singhal AB. Dijkhuizen RM. Rosen BR. Lo EH. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology. 2002;58:945–952. doi: 10.1212/wnl.58.6.945. [DOI] [PubMed] [Google Scholar]
- 152.Smith WL. DeWitt DL. Garavito RM. Cyclooxygenases: Structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 153.Smith WL. Garavito RM. DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 154.Sorrenti V. Di Giacomo C. Campisi A. Perez–Polo JR. Vanella A. Nitric oxide synthetase activity in cerebral post-ischemic reperfusion and effects of L-N(G)-nitroarginine and 7-nitroindazole on the survival. Neurochem Res. 1999;24:861–866. doi: 10.1023/a:1020906030328. [DOI] [PubMed] [Google Scholar]
- 155.Sorrenti V. Salerno L. Di Giacomo C. Acquaviva R. Siracusa MA. Vanella A. Imidazole derivatives as antioxidants and selective inhibitors of nNOS. Nitric Oxide. 2006;14:45–50. doi: 10.1016/j.niox.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 156.Spector AA. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J Mol Neurosci. 2001;16:159–165. doi: 10.1385/JMN:16:2-3:159. discussion 215–221. [DOI] [PubMed] [Google Scholar]
- 157.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50:S52–56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stankiewicz JM. Brass SD. Role of iron in neurotoxicity: A cause for concern in the elderly? Curr Opin Clin Nutr Metab Care. 2009;12:22–29. doi: 10.1097/MCO.0b013e32831ba07c. [DOI] [PubMed] [Google Scholar]
- 159.Stephenson D. Rash K. Smalstig B. Roberts E. Johnstone E. Sharp J. Panetta J. Little S. Kramer R. Clemens J. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27:110–128. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 160.Stephenson DT. Manetta JV. White DL. Chiou XG. Cox L. Gitter B. May PC. Sharp JD. Kramer RM. Clemens JA. Calcium-sensitive cytosolic phospholipase A2 (cPLA2) is expressed in human brain astrocytes. Brain Res. 1994;637:97–105. doi: 10.1016/0006-8993(94)91221-1. [DOI] [PubMed] [Google Scholar]
- 161.Stevens MK. Yaksh TL. Time course of release in vivo of PGE2, PGF2 alpha, 6-keto-PGF1 alpha, and TxB2 into the brain extracellular space after 15 min of complete global ischemia in the presence and absence of cyclooxygenase inhibition. J Cereb Blood Flow Metab. 1988;8:790–798. doi: 10.1038/jcbfm.1988.134. [DOI] [PubMed] [Google Scholar]
- 162.Stock C. Schilling T. Schwab A. Eder C. Lysophosphatidylcholine stimulates IL-1beta release from microglia via a P2X7 receptor-independent mechanism. J Immunol. 2006;177:8560–8568. doi: 10.4049/jimmunol.177.12.8560. [DOI] [PubMed] [Google Scholar]
- 163.Strobel HW. Thompson CM. Antonovic L. Cytochromes P450 in brain: Function and significance. Curr Drug Metab. 2001;2:199–214. doi: 10.2174/1389200013338577. [DOI] [PubMed] [Google Scholar]
- 164.Sun GY. Foudin LL. On the status of lysolecithin in rat cerebral cortex during ischemia. J Neurochem. 1984;43:1081–1086. doi: 10.1111/j.1471-4159.1984.tb12847.x. [DOI] [PubMed] [Google Scholar]
- 165.Symon L. Branston NM. Strong AJ. Hope TD. The concepts of thresholds of ischaemia in relation to brain structure and function. J Clin Pathol Suppl (R Coll Pathol) 1977;11:149–154. doi: 10.1136/jcp.s3-11.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tabuchi S. Uozumi N. Ishii S. Shimizu Y. Watanabe T. Shimizu T. Mice deficient in cytosolic phospholipase A2 are less susceptible to cerebral ischemia/reperfusion injury. Acta Neurochir Suppl. 2003;86:169–172. doi: 10.1007/978-3-7091-0651-8_36. [DOI] [PubMed] [Google Scholar]
- 167.Takano T. Panesar M. Papillon J. Cybulsky AV. Cyclooxygenases-1 and 2 couple to cytosolic but not group IIA phospholipase A2 in COS-1 cells. Prostaglandins Other Lipid Mediat. 2000;60:15–26. doi: 10.1016/s0090-6980(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 168.Takayasu M. Dacey RG., Jr. Effects of inhibitory and excitatory amino acid neurotransmitters on isolated cerebral parenchymal arterioles. Brain Res. 1989;482:393–396. doi: 10.1016/0006-8993(89)91207-9. [DOI] [PubMed] [Google Scholar]
- 169.Tsai AG. Cabrales P. Intaglietta M. The physics of oxygen delivery: Facts and controversies. Antioxid Redox Signal. 2009;12:683–691. doi: 10.1089/ars.2009.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 171.Uauy RD. Birch DG. Birch EE. Tyson JE. Hoffman DR. Effect of dietary omega-3 fatty acids on retinal function of very-low-birth-weight neonates. Pediatr Res. 1990;28:485–492. doi: 10.1203/00006450-199011000-00014. [DOI] [PubMed] [Google Scholar]
- 172.Umemura A. Mabe H. Nagai H. Sugino F. Action of phospholipases A2 and C on free fatty acid release during complete ischemia in rat neocortex. Effect of phospholipase C inhibitor and N-methyl-D-aspartate antagonist. J Neurosurg. 1992;76:648–651. doi: 10.3171/jns.1992.76.4.0648. [DOI] [PubMed] [Google Scholar]
- 173.Unterberg A. Schmidt W. Wahl M. Baethmann A. Role of leukotrienes as mediator compounds in brain edema. Adv Neurol. 1990;52:211–214. [PubMed] [Google Scholar]
- 174.Virag L. Szabo E. Gergely P. Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140–141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- 175.Xu J. Weng YI. Simonyi A. Krugh BW. Liao Z. Weisman GA. Sun GY. Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachadonic acid release in primary murine astrocytes. J Neurochem. 2002;83:259–270. doi: 10.1046/j.1471-4159.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- 175a.Yao H. Acute stroke therapy: highlighting the ischemic penumbra. In: Haddad GG, editor; Yu SP, editor. Brain Hypoxia and Ischemia. New York: Humana Press; 2009. pp. 307–322. [Google Scholar]
- 176.Yoshihara Y. Watanabe Y. Translocation of phospholipase A2 from cytosol to membranes in rat brain induced by calcium ions. Biochem Biophys Res Commun. 1990;170:484–490. doi: 10.1016/0006-291x(90)92117-i. [DOI] [PubMed] [Google Scholar]
- 177.Young C. Gean PW. Wu SP. Lin CH. Shen YZ. Cancellation of low-frequency stimulation-induced long-term depression by docosahexaenoic acid in the rat hippocampus. Neurosci Lett. 1998;247:198–200. doi: 10.1016/s0304-3940(98)00272-9. [DOI] [PubMed] [Google Scholar]
- 178.Zauner A. Daugherty WP. Bullock MR. Warner DS. Brain oxygenation and energy metabolism: Part I—Biological function and pathophysiology. Neurosurgery. 2002;51:289–301. discussion 302. [PubMed] [Google Scholar]
- 179.Zeiger SL. Musiek ES. Zanoni G. Vidari G. Morrow JD. Milne GJ. McLaughlin B. Neurotoxic lipid peroxidation species formed by ischemic stroke increase injury. Free Radic Biol Med. 2009;47:1422–1431. doi: 10.1016/j.freeradbiomed.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 181.Zeldin DC. Liao JK. Reply: Cytochrome P450-derived eicosanoids and the vascular wall. Trends Pharmacol Sci. 2000;21:127–128. doi: 10.1016/s0165-6147(00)01454-1. [DOI] [PubMed] [Google Scholar]
- 182.Zhang J. Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Invest. 1992;90:1193–1199. doi: 10.1172/JCI115980. [DOI] [PMC free article] [PubMed] [Google Scholar]