Abstract
A remarkable aspect of adult neurogenesis is that the tight regulation of subventricular zone (SVZ) neuroblast migration is altered after ischemic stroke and newborn neurons emigrate towards the injury. This phenomenon is an essential component of endogenous repair and also serves to illuminate normal mechanisms and rules that govern SVZ migration. Stroke causes inflammation that leads to cytokine and chemokine release, and SVZ neuroblasts that express their receptors are recruited. Metalloproteinases create pathways and new blood vessels provide a scaffold to facilitate neuroblast migration between the SVZ and the infarct. Most experiments have studied the peri-lesion parenchyma and relatively little is known about SVZ remodeling after stroke. Migration in the SVZ is tightly regulated by cellular interactions and molecular signaling; how are these altered after stroke to allow emigration? Do ependymal cells contribute to this process, given their reported neurogenic potential? How does stroke affect ependymal cell regulation of cerebrospinal fluid flow? Given the heterogeneity of SVZ progenitors, do all types of neuroblasts migrate out, or is this confined to specific subtypes of cells? We discuss these and other questions in our review and propose experiments to address them. Antioxid. Redox Signal. 14, 1877–1888.
Introduction
Adult neurogenesis is the generation of new neurons from resident neural stem cell (NSC) populations in the adult brain (65). In many mammalian species, including rodents, higher primates, and humans, heterogeneous neural stem cell populations reside in two distinct neurogenic niches in the adult brain, the subventricular zone (SVZ) surrounding the lateral ventricles (Fig, 1), and the subgranular zone (SGZ) of the hippocampal dentate gyrus (22, 24, 27, 98). In the SVZ, proliferating neural stem cells and progenitor cells reside in a specialized cellular and extracellular matrix niche (79), and give rise to neuroblasts that migrate long distances through the rostral migratory stream (RMS) to the olfactory bulb (OB), where they differentiate into periglomerular and granule GABAergic interneurons (68). In the SGZ, neurogenesis gives rise to granule interneurons and provides excitatory inputs to area CA3 (47). Although their functions are incompletely understood, evidence suggests SVZ neurogenesis is involved in olfactory discrimination, while SGZ neurogenesis may play a role in memory formation (138). Defective or insufficient adult neurogenesis has been associated with a wide spectrum of neurodevelopmental and neurodegenerative diseases ranging from schizophrenia to Alzheimer's disease (106).
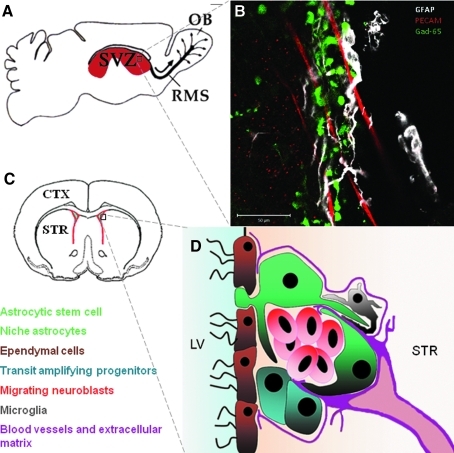
FIG. 1.
Anatomical location and cell composition of the SVZ. (A) Schematic showing location of SVZ (red) in the sagittal plane. Neural stem cells and transit amplifying progenitors give rise to neuroblasts which migrate long distance in the rostral migratory stream (RMS) to the olfactory bulb. The RMS route is shown with a thick black line and the normal direction with a thick arrow. Neuroblasts migrate out of the RMS into the olfactory bulb (OB) in random fashion (thin arrows). (B) Whole-mount immunohistochemistry showing SVZ astrocytes (glial fibrillary acidic protein, GFAP - white), neuroblasts (Gad (65)-GFP, green) and blood vessels (platelet endothelial cell adhesion molecule PECAM, red). Note that neuroblasts are frequently associated with astrocytes and blood vessels. (C) Coronal cross section showing location of SVZ (red). (D) Cell composition of SVZ with cells color coded. Note that SVZ astrocytic stem cells have a basal process protruding amongst the ependymal cells and make ventricular contact. Niche astrocytes are generally located on the striatal (STR) side of the SVZ. Small microglial cells have processes that interdigitate other cells. Mercier has described a complex network (fractones) of specialized extracellular matrix around SVZ cells emanating from blood vessels. LV lateral ventricle. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Remarkably, adult neurogenesis contributes to endogenous repair after different types of brain damage such as traumatic brain injury, multiple sclerosis, intracranial hemorrhage, and stroke (14, 43, 77, 110). After focal ischemic stroke, in addition to increased cell proliferation in the SVZ, newly born neural precursors are able to migrate long distances and localize near injured brain regions. At the site of injury, these neural precursors may mediate neuroprotection and immune modulation (123). Furthermore, a small number of these cells will survive, differentiate and display evidence of synaptic integration (5, 36, 91, 126). Importantly, strategies that increase SVZ neurogenesis and migration towards the ischemic brain regions improve functional and histological outcomes after stroke (107, 108, 111, 112), while elimination of SVZ neurogenesis worsens them (45). There is also evidence that the human SVZ responds to ischemic injury (46, 71, 75). There is thus great impetus to understand the extent of the attempted repair process and the mechanisms which regulate each aspect of this phenomenon. The ultimate goal is to understand why endogenous brain repair is insufficient and to ask whether we will be able to manipulate the system for therapeutic brain repair. Intense research over the last decade has focused on molecular and cellular signals from the ischemic brain regions that induce SVZ proliferation, and recruit newborn neural precursors (reviewed in Refs. 48, 59, 76, 88, 123). In the current review, we will present a novel perspective on the cellular and molecular mechanisms that facilitate SVZ neuroblast migration into the ischemic striatum. We will focus on how dynamic changes within the SVZ neurogenic niche after ischemic stroke permit neuroblasts to exit their tightly regulated migratory pathway.
Tangential Migration During SVZ Adult Neurogenesis
In order to understand the mechanisms that govern the migration of SVZ neuroblasts into the striatum after ischemic stroke, it is important to appreciate constitutive tangential neuroblast migration, from the SVZ, through the RMS to the olfactory bulb (SVZ–RMS–OB). Niche mechanisms operating at cellular and molecular levels tightly regulate this long-distance migration (Fig. 2). For a comprehensive review, see Ref. 16. Neuroblasts form chains and migrate along one another, under the influence of cell-surface molecules, such as polysialylated NCAM and α6β1 integrin, and extracellular matrix proteins (18, 21, 26, 37, 40, 120). In addition to homotypic migration, neuroblast chains are closely associated with blood vessels that form a migratory scaffold and mediate neuroblast migration through BDNF signaling (12, 104, 119). Glial processes ensheath neuroblast chains to form “glial tubes” and regulate cell movements via GABA signaling (11). Niche astrocytes also mediate neuregulin/ErbB4 interactions that maintain astrocytic cytoarchitecture and normal neuroblast migration (29). SVZ microglia are constitutively semi-activated and neuroblasts express several chemokine receptors, including CXCR4 and CCR2, the receptors for SDF-1α and monocyte chemoattractant protein 1 (MCP-1), respectively (31, 116). Although the role of chemokines in normal SVZ neuroblast migration is yet to be determined, chemokines are known to affect normal migration in other contexts, for example, SDF-1α plays important roles in granule neuron migration in cerebellar development (70). The chemorepulsive Slit-Robo signaling pathway appears to play an important role in directing neuroblasts away from the SVZ. Slit is expressed in the SVZ, septum and choroid plexus, and its receptors, Robo-2 and Robo-3, are expressed by neuroblasts (86, 125). CSF flow, which is propelled by the beating cilia of ependymal cells, establishes a chemo-repulsive Slit gradient to direct neuroblast migration rostrally towards the olfactory bulb (99, 125). In addition, there is evidence that the olfactory bulb may be the source of chemoattractants that direct neuroblasts. Prokineticin-2, Netrin-1, GDNF, and BDNF have been suggested to orient neuroblast migration towards the olfactory bulb (17, 83, 85). Furthermore, while removal of the olfactory bulb does not completely stop migration of neuroblasts, nor disturb the directionality of migration, there is evidence that loss of the olfactory bulb results in decreased numbers of SVZ neuroblasts which migrate into the RMS (41, 51, 62).
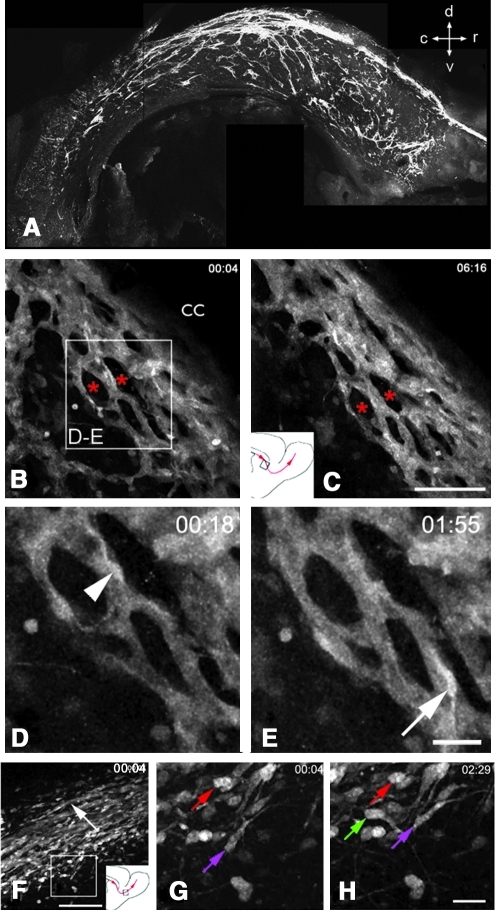
FIG. 2.
SVZ neuroblasts migrate in chains. (A) Whole-mount immmunohistochemistry of doublecortin showing low magnification view of neuroblast chain orientation in the SVZ viewed in a sagittal plane. Note that in the dorsal SVZ, most chains are oriented rostral-caudally, but that in ventral locations, they have a variety of orientations. c, caudal; d, dorsal; r, rostral; v, ventral. (B, C) Neuroblast chains visualized in Dcx-GFP mice under the corpus callosum (cc) were remarkably stable for over 6 hours (time stamps in hours and minutes in upper right corner). Asterisks show examples of areas avoided by these chains and are likely populated by SVZ astrocytes, transit amplifying progenitors, and microglia. Inset in B shows location of D, E). Inset in C shows location of movie. (D, E) shows neuroblasts migrating along chains. (F) low magnification of Dcx-GFP neuroblast chains migrating in RMS into the OB. White box shows location of G, H, and schematic shows location of movie. (G, H) show that cells at the edge of the RMS that are largely stationary (red and purple arrows) and one motile neuroblast (green arrow). Most cells that emigrate from the SVZ and RMS slow down precipitously. [B–H adapted from (84).] (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Post-Stroke SVZ Neuroblast Migration
Following animal models of stroke, large numbers of SVZ neuroblasts are able to overcome the tight regulation described above to exit their normal pathway and migrate towards the injured brain regions (5, 44, 91, 133). Neuroblast emigration commences 3–4 days after stroke, remains robust for several weeks, and has been observed to continue for as long as 4 months (113). Several molecular signals and cellular interactions that direct neuroblasts towards the ischemic brain regions have been described. The role of reactive astrocytes and activated microglia/macrophages at the site of injury is important, as are blood vessels and astrocytic processes that form migratory scaffolds, recapitulating the cellular interactions of constitutive SVZ neuroblast migration (Fig. 3).
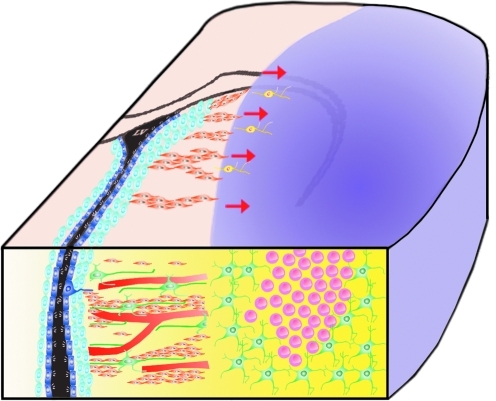
FIG. 3.
Neuroblast emigration towards the ischemic striatum after MCAO stroke. The location of the middle cerebral artery occlusion lesion is shown in purple. Red arrows show direction of neuroblast emigration. Ischemic stroke lead to a robust inflammatory response in the infarct area, with accumulation of large numbers of reactive astrocytes (green cells) and activated microglia/macrophages (pink cells). Inflammatory cells release various chemokines and chemoattractants such as stromal-derived factor 1 alpha (SDF-1α) and monocyte chemoattractant factor 1 (MCP-1). Neuroblasts from the SVZ migrate up chemotactic gradients along blood vessels and astrocytic processes towards the injury. Relatively little is known about the changes in the SVZ neurogenic niche which may facilitate the exit of neuroblasts out of their normal migratory pathway into the striatum. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Role of reactive astrocytes and activated microglia/macrophages
With the onset of ischemic stroke, delivery of oxygenated blood to the brain is drastically decreased. Neurons have high metabolic demands and come under increasing oxidative stress (55). This results in the accumulation of reactive oxygen species and ischemic cell death. Neuronal cell death leads to activation of the complement cascade and the initiation of the inflammatory response (49). In ischemic brain regions, reactive astrocytes and activated microglia/macrophages secrete a variety of substances, including a number of chemokines and chemoattractants that recruit SVZ neuroblasts towards the ischemic striatum. Reactive astrocytes secrete SDF-1α, and neuroblasts express the corresponding CXCR4 receptor (38, 42, 116). Overexpression of SDF-1α results in increased neuroblast recruitment, while functional blocking antibodies or siRNA to the CXCR4 receptor had the opposite effect (65, 95, 112). ERK signaling may mediate the effect of SDF-1α on neuroblast migration (69). Interestingly, a recent report suggests that complement-derived anaphylatoxin C3a potentiates neuroblast migration at low SDF-1α concentration, while inhibiting neuroblast migration at high SDF-1α concentration in an ERK1/2 phosphorylation-dependent process (103). This would imply that neuroblasts can respond dynamically to the concentration of this chemokine. Monocyte chemoattractant protein-1 (MCP-1) also induces post-stroke neuroblast emigration into the striatum. MCP-1 is highly upregulated by reactive astrocytes and activated microglia/macrophages in the ischemic striatum and cortex, while emigrating neuroblasts express its cognate receptor CCR2 (66, 116, 128). Accordingly, MCP-1(-/-) and CCR2(-/-) mice had significantly decreased post-stroke neuroblast emigration (128). However, it is worthwhile to note that in a separate study, CCR2(-/-) mice exhibited decreased post-stroke neutrophil recruitment and reduced expression of pro-inflammatory cytokines such as TNFα, resulting in reduced infarct size after middle cerebral artery occlusion (MCAO) compared to wild-type mice (20).
In addition to classic chemokines, activated microglia/macrophages secrete vascular endothelial growth factor (VEGF) which has chemoattractant, angiogenic, and neurogenic properties (93). In vitro, VEGF is a chemoattractant and promotes neuroblast migration through VEGFR-2 (6, 132). Transgenic overexpression of VEGF increased SVZ cell proliferation and the number of neuroblasts in the ischemic brain regions after ischemic stroke (118). However, the authors of the study point out that in vivo it is difficult to distinguish whether the increased number of neuroblasts observed is due to a direct effect on migration, or merely increased proliferation. Activated microglia/macrophages in the ischemic brain also express high levels of osteopontin, an acidic glycoprotein, which has chemoattractive properties, as well as cell adhesion and extracellular matrix signaling functions via activation of integrin receptors (23, 78). After ischemic stroke, osteopontin mediates neuroblast emigration in a β1-integrin-dependent manner (127). Osmotic pump infusion of a functional blocking antibody to osteopontin decreased neuroblast emigration into the striatum without affecting SVZ proliferation, compared to infusion of IgG controls. In vitro, blockade of β1-integrin which is expressed by neurosphere-derived migrating neural precursor cells, decreased cell migration on a laminin substrate (40), further supporting the notion that integrins are integral to neuroblast migration. Reactive astrocytes and activated microglia/macrophages are also involved in tissue remodelling of the ischemic striatum, and express matrix metalloproteinases (MMPs) which cleave and degrade extracellular-matrix components to facilitate neuroblast migration through the striatum (56, 97). Thus, a variety of different molecules are secreted by glial cells in response to stroke. These molecules diffuse from the injury to the SVZ and recruit migratory neuroblasts (Table 1).
Table 1.
Molecules Regulating SVZ Neuroblast Migration
| Families of molecules | Constitutive SVZ-RMS-OB migration | Emigration to Model of Stroke |
|---|---|---|
| Chemoattractant (ligand/receptor) | BDNF/TrkB | ? |
| GDNF/GFRα1 | ? | |
| VEGF/VEGFR-1,2 | VEGF/VEGFR-2 | |
| ProK2/ProKR2 | ? | |
| Netrin-1 | ? | |
| Anosmin-1 | ? | |
| HGF/Met | ? | |
| SHH/Patched | ? | |
| ? | SDF-1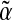 CXCR4 CXCR4 |
|
| ? | MCP-1/CCR2 | |
| ? | Osteopontin | |
| ? | Ang-1/Tie-2 | |
| Chemorepellant (ligand/receptor) | Slit1,2/Robo2,3 | ? |
| Signaling (ligand/receptor) | Ephrin-B2/EphB2 | ? |
| Reelin/disabled | ? | |
| Neuregulin/ErbB4 | ? | |
| EGF/ErbB1 | ? | |
| FGF2/FGFR1 | ? | |
| NPY/Y1 | ? | |
| Adhesion (ECM or homotypic) | NCAM/NCAM | ? |
| PSA-NCAM | ? | |
| α6β1 Integrin/Laminin | ? | |
| Tenascin | ? | |
| Connexins 43 & 45 | ? | |
| Matrix metaloproteinases | ? | MMP(s) |
| Neurotransmitters | GABA | ? |
| Glutamate | ? | |
| Cytoskeletal Proteins | Dcx | ? |
?, unknown.
Role of angiogenic vasculature as substrate for migration
Following ischemic stroke, there is increased endothelial proliferation and formation of new blood vessels (angiogenesis) in the peri-infarct region (7, 54, 63, 74, 137). Endothelial proliferation in the peri-infarct region is seen as early as 12–24 hours post-stroke, resulting in significantly increased blood vessel density by 3 days (8). Peri-infarct angiogenesis is increased for more than 3 weeks following cerebral ischemia (35). Angiogenesis functions to increase blood supply to vulnerable brain regions, and to provide trophic support that plays an important role to reduce further neuronal death in the days and weeks following stroke (57, 92, 101). Endothelial cells secrete growth factors such as BDNF and angiopoietin (Ang-1) to promote neuroblast recruitment towards the ischemic brain tissue. BDNF is known to promote proliferation, migration, survival, and differentiation of SVZ neural precursor cells (9, 57). After focal stroke, endothelial cells express Ang-1, which acts on Tie-2 receptors on neuroblasts to promote emigration (89). A recent study found that Ang-1 also promotes SVZ proliferation constitutively (96). In addition, extracellular matrix proteins associated with blood vessels such as laminin are important for the maintenance of neural precursor cells (79, 102). Thus, the vasculature changes after stroke and makes important contributions to the molecular milieu into which SVZ neuroblasts are enticed to migrate.
Migrating cells frequently exhibit haptotaxis and durotaxis, migration up adhesion or mechanical stiffness gradients, respectively. Thus the question arises; do emigrating SVZ neuroblasts have preferred physical substrates for emigration? As discussed above, SVZ neuroblast migration to the olfactory bulb is partly mediated by the vascular scaffold that is present in the SVZ–RMS–OB migratory pathway. Several studies using different models of cerebral ischemia have shown histologically that neuroblasts migrating into the striatum after stroke are frequently in close proximity to blood vessels (89, 113, 126). Time-lapse microscopy of acute slices after focal ischemia suggests that some of the Dcx+ neuroblasts made contacts with blood vessels during active migration, both in the early phase as the neuroblasts exit the SVZ, as well as in the striatum (53, 134). These experiments suggest that blood vessels not only form dynamic molecular interactions with neuroblasts to provide directionality and trophic support in a hostile environment, but in a recapitulation of the normal migratory pathway also provide a physical substrate for migration.
Role of SVZ Remodelling in Neuroblast Emigration to Stroke
The experiments described above point to a model whereby release of soluble factors recruit neuroblasts towards ischemic brain regions with guidance from astrocytic processes and blood vessels. Overwhelmingly, the emphasis has focused on factors outside the SVZ that act to divert neuroblasts. We propose that de novo cellular or molecular processes localized in the SVZ facilitate early events in post-stroke neuroblast emigration (i.e., exit from the SVZ-RMS-OB migratory pathway). What are the changes within the SVZ which facilitate neuroblast emigration? Are SVZ components such as scaffolds neuroblast chains, astrocytic “glial tubes”, vascular scaffolds, and extracellular matrix proteins, which regulate normal SVZ migration, altered after ischemic stroke?
Molecular and migratory properties of SVZ neuroblasts are altered after stroke
Indeed, SVZ neuroblasts undergo intrinsic changes after focal stroke. Neuroblasts express matrix metalloproteinases (MMPs) which can cleave and degrade extracellular matrix components. MMP-3 and MMP-9 are upregulated by SVZ neuroblasts in response to chemokine stimulation and focal ischemia (6, 56). Neuroblast emigration into the ischemic striatum is significantly reduced by administration of the broad spectrum MMP inhibitor, GM6001, while MMP-3 and MMP-9 blockade with specific siRNAs decreased neuroblast migration in vitro (6, 56). However, it is unclear whether MMPs are required to merely “digest” a pathway through the extracellular matrix to facilitate neuroblast emigration, or whether MMPs directly influence extracellular matrix protein interactions such as integrin and laminin, and thus affect intracellular signaling. Nevertheless, these studies suggest a model wherein the striatum presents a molecular/mechanical barrier through which neuroblasts have to bore to reach the lesion. It may be that this feature is what restricts neuroblasts from migrating errantly into the striatum under normal conditions. It would be interesting to test this hypothesis by decreasing extracellular matrix stiffness with chondroitinase, as has recently been carried out to augment spinal cord axon outgrowth (28).
Attempts have been made to characterize changes in gene expression in the SVZ following ischemic stroke. Using combination of gene microarray and real time RT-PCR, Liu and colleagues showed the upregulation of several genes, including Hif-1α, Notch4, and Ephrin B2, in both dissected SVZ tissue and cultured SVZ neurospheres after MCAO (64). Their functional implication in the post-stroke SVZ niche and their effects on neuroblast emigration remains to be elucidated. Another important approach will be to isolate neuroblasts which are migrating through the ischemic striatum and compare their gene/protein expression at a single cell level in comparison to non-emigrating neuroblasts.
Time-lapse microscopy demonstrated that neuroblasts actively exiting the SVZ migrate significantly faster than neuroblasts inside the SVZ (134). This was surprising since 2-photon time-lapse data from our lab show that SVZ neuroblasts significantly slow down when emigrating from the (84) (Fig. 2) and it suggests that mechanisms such as GABAergic signaling that regulate speed, as mentioned above, may be significantly altered after stroke. In vitro, SVZ cells derived from post-stroke animals spent less time in cytokinesis, and migrated further when plated on Matrigel substrate (135). Using 2-photon time-lapse imaging, we have shown that neuroblast chains remain very stable during normal migration (84) (Fig. 2). After ischemic stroke, there is evidence in static sections that neuroblast chains may be dispersed as neuroblasts exit their normal migratory pathway (44). It will be important to detail the extent to which neuroblast chains are disturbed and the molecular signals within the SVZ which facilitate neuroblast emigration. For example, reelin has been shown to be a chain detachment signal for migrating neuroblasts as they commence radial migration in the olfactory bulb (33). Does stroke alter reelin signaling to facilitate neuroblast exit from the SVZ? Interestingly, after stroke, reelin deficient mice have larger stroke volume, and showed decreased number of neuroblasts in the ischemic border, despite preserved SVZ cell proliferation (124). This suggests that reduced neuroblast emigration may exacerbate stroke injury.
Stroke may induce migration in SVZ cells that are normally stationary
The first thorough, and classic, descriptions of the SVZ and RMS were by Altman in the 1960s. They clearly delineated a proliferative population of cells that were concentrated in the SVZ and a migratory population of cells that predominated in the RMS (2, 3). This general notion has been challenged to a certain extent by the finding that migratory neuroblasts occasionally divide in the SVZ and RMS. In addition, transit amplifying progenitor cells have been proposed to be constitutively migratory (1). Dcx labels the entire neuroblast population, yet we found Dcx-negative cells that were motile, suggesting that we were observing migratory stem or progenitor cells (84). We therefore directly examined the hypothesis that SVZ astrocytes or transit amplifying progenitor cells may be motile. Despite analyzing close to 900 cells in multiple transgenic lines with 2-photon time-lapse microscopy, we did not find any evidence of astrocyte (stem cells and niche astrocytes) or transit amplifying progenitor cells that were motile (50). The question then arises whether the SVZ stem or progenitor cells can become motile after stroke? This would be extremely interesting since these precursor cells are less committed and may better effectuate repair than neuroblasts. Indeed there is evidence that multiple types of SVZ cells migrate into the striatum after injury. SVZ cells with all the characteristics of transit amplifying progenitor cells emigrate towards the striatum following the 6-hydroxydopamine model of Parkinson's disease (19). Furthermore, using tamoxifen-inducible Cre-recombinase reporter mice driven by the nestin promoter (nestin-CreERT2:R26R-YFP), which permit labeling and fate-mapping of nestin+ SVZ cells, Cunningham and colleagues report significant waves of astrocyte and oligodendrocyte-precursor cell emigration after ischemic stroke (58). In their studies, astrocyte emigration appears to precede neuroblast emigration. It remains to be determined whether the same mechanisms facilitate emigration of other SVZ cell types and to what extent they interact with emigrating neuroblasts.
Several sublineages of SVZ neuroblasts have recently been discovered (53, 80, 117, 121, 123, 131). The lineages arise from distinct dorsoventral embryonic regions, their stem cells reside in spatially nested domains of the adult SVZ, and they give rise to different interneurons in the olfactory bulbs. These comprise periglomerular calretinin-, calbindin-, and tyrosine hydroxylase-positive neurons, as well as GABA-ergic granule neurons. These new findings have given rise to myriad questions including whether the migratory properties of the lineages are equivalent. SVZ-derived interneuron migration ends either in the periglomerular or granule cell layers during the last stage of migration in the olfactory bulb, suggesting the lineages differ in their response to stop signals in the OB. Thus far, the greatest migratory difference seems to be between periglomerular neurons and granule neurons, simply because the two groups arrest migration in distinct layers of the olfactory bulb. Do certain lineage(s) of neuroblasts emigrate preferentially after stroke? Interestingly, a couple of studies suggest that calretinin+ neurons preferentially emigrate; they were frequently found in neonatal hypoxia/ischaemia injuries (130) and stroke (61). To directly answer the question of differences in SVZ lineage emigration, radial glia viral infection at P0 or transgenic reporter lines could be used to label specific SVZ subpopulations.
Stroke may induce fate switches in SVZ cells
There has been much controversy and inconsistency as to whether emigrating neuroblasts are able to generate projection neurons, the cells mostly responsible for functional loss after stroke. Early studies in rats (5, 91) reported the generation of striatal medium spiny neurons, while work in mice suggested that only parvalbumin+ striatal interneurons (111), or calretinin+ interneurons were generated (61). The conflicting results could be explained by inter-species differences, or the heterogeneity of SVZ cell types described above. However, it is important to point out that normally the SVZ makes small interneurons with short or no axons. Nevertheless, SVZ cells have been shown to emigrate into the striatum, survive, and differentiate into neurons with evidence of synaptic formation and electrophysiological activity (36). It is not known whether these neurons form long projections to target structures such as the globus pallidus after stroke. Focal apoptosis-induced neurogenesis in periventricular regions gave rise to a few projection neurons which were retrograde labeled from their projection targets (72). Also, SVZ progenitors can be re-specified into a glutamatergic phenotype and exhibit cortical recruitment after injury (10, 13). Thus, a key goal is to coax SVZ neuroblasts to differentiate into projection neurons. Partial reprogramming with induced pluripotential stem cell approaches may revert SVZ progenitors to the fate competence their forebears exhibited in the embryonic lateral ganglionic eminence, thus allowing them to generate striatal projection neurons.
Stroke may also re-specify the fate of SVZ neuroblasts and induce glial phenotypes. SVZ neuroblast plasticity was demonstrated when they were isolated via PSA-NCAM expression based magnetic cell sorting, transplanted into the striatum, and differentiated into astrocytic and oligodendrocytic cells (100). In a dramatic recent example, SVZ neuroblasts could express glial proteins, mediated by the BMP antagonist chordin after a demyelinating lesion (39). Thus, the SVZ may also contribute glial precursors to restore or replace oligodendrocytes or astrocytes lost to the injury.
SVZ astrocyte and ependymal cell alterations after stroke
Adult SVZ astrocytes envelop migrating neuroblasts in a glial tube-like configuration (21, 22). Although SVZ neuroblasts are capable of migrating in chains in the absence of SVZ astrocyte tubes (120), it is clear that astrocytes contribute to neuroblast migration in vivo. SVZ astrocytes regulate GABA that decreases neuroblast speed (11), and they also dampen BDNF's effect on migration by reducing its bioavailability via TrkB receptors (104). It may also simply be that the morphology of SVZ astrocytes helps guide them to the RMS and OB; they bear long processes that elongate in the anterior/posterior direction (81). Mice deficient in ErbB4 signaling exhibit disruption of SVZ astrocyte cytoarchitecture and concurrent alterations in SVZ migration (4). The extent to which SVZ astrocyte cytoarchitecture changes after stroke and whether such morphological changes contribute to emigration is unclear but deserves to be examined.
Both SVZ astrocytes and ependymal cells are derived from radial glia, the stem cells of embryonic neural development (105, 115). The role played by GFAP+ SVZ astrocytes in neuroblast emigration is not thoroughly understood. Early reports suggested that new astrocytic processes emanate from the SVZ into the striatum after injury (110) and that neuroblast emigration into the striatum occurs along vimentin+ astrocytic processes in a similar manner to the SVZ–RMS–OB pathway (91, 111). Radial glia-like cells have been found in the uninjured murine brain, emanating from the ventral SVZ into the nucleus accumbens (109). These specialized astrocyte-like cells express BLBP and GLAST, markers of radial glia and are juxtaposed to Dcx+ neuroblasts that appear to migrate along them (109, 129). Although a cerebral cortex model of TBI does not seem to alter rates of ventral emigration along these fibers, it would be interesting to test if ischemic injuries of the ventral forebrain do so. Until now, it has been difficult to ascertain the exact role of normal and post-injury adult radial glia-like cells, but it is intriguing to speculate that injuries may partially reprogram SVZ astrocytes or ependymal cells to express a radial glial phenotype. Were this true, they could not only provide a convenient substrate for emigration but in theory could increase the fate potential of their progeny.
The role of ependymal cells after stroke remains poorly understood, although clues are emerging suggesting that they may contribute to the neurogenic response. Although ependymal cells are largely post-mitotic in the normal brain (105), they can proliferate and give rise to neuroblasts and astrocytes after cerebral ischemia (15). In another study, S100β+ ependymal cells incorporated BrdU and took on a radial glia-like morphology, extending processes into the SVZ (136). Interestingly, some neuroblasts are closely associated with these radial glial-like processes, reminiscent of migration during cortical development (87) and the radial glia transformation of SVZ astrocytes described above. An obvious and unanswered question from this observation is whether ependymal cells have a role in facilitating neuroblast emigration out of the SVZ. Ependymal cells regulate the SVZ niche through expression of Noggin, which is a BMP antagonist and promotes neurogenesis (60). As discussed earlier, the beating cilia of ependymal cells generates CSF flow and creates the Slit gradient shown to direct neuroblasts towards the OB. Stroke is one of the most common causes of noncommunicating hydrocephalus in humans and it will be interesting to examine if loss of ependymal cells after stroke lead to disruption of normal CSF flow. Thus, it is likely that ependymal cells contribute to the post-stroke SVZ response, by regulating the niche and even becoming neurogenic themselves.
A role for SVZ microglia in post-stroke SVZ response?
Several data suggest that SVZ microglia are specialized and could contribute to SVZ neuroblast emigration. As noted above, SVZ microglia in the SVZ are normally semi-activated (31). Interestingly, the same study showed that compared to adjacent striatal and cortical microglia, they have a dampened activation response to cerebral cortex lesions. Another set of data suggesting SVZ microglia are a breed apart showed their rates of proliferaton in vitro is far greater than non-neurogenic niche microglial (73). We found that after cortical lesions, fluorescent bead-labeled SVZ microglia emigrated rapidly toward the lesion, along paths very similar to those subsequently taken by neuroblasts (31). In other studies, a viral model of MS in which SVZ neuroblasts emigrate to periventricular regions was characterized by very early infiltration into these regions of hematopoietic cells (30). We hypothesize that SVZ microglia could influence SVZ neuroblast emigration by clearing paths or creating molecular gradients for neuroblast emigration.
The most commonly used MCAO models do not result in lesions which encompass the SVZ. Thus, it is unlikely that significant SVZ cell death occurs after stroke, predicting that SVZ microglial function should not be significantly altered. Thored and colleagues have shown that similar to cortical lesions, after stroke, SVZ microglia activation is not as pronounced as in the adjacent ischemic striatum (114). However, they also showed that SVZ microglia upregulated insulin-like growth factor-1 (IGF-1) after stroke which boosted proliferation and neurogenesis. This is interesting since activated microglia dampen hippocampal neurogenesis (25, 82). It is clear that the role of microglia in the post-stroke SVZ deserves further investigation since they may significantly contribute to the regulation of neurogenesis.
The SVZ vasculature is altered after ischemic stroke
Seven days after MCAO, endothelial proliferation in the SVZ is increased (113). Similarly, 7 days following thermocoagulatory cortical lesions, SVZ blood vessels increase vascular permeability, with concomitant increases in endothelial cell proliferation and VEGF expression (32). VEGF and MMPs which are upregulated in the SVZ after focal ischemia are known to increase blood vessel permeability (32). Furthermore, given the presence of the vascular scaffold and the molecular regulation of neuroblast migration via BDNF signaling (104), it will be interesting to see how these are altered to facilitate neuroblast emigration after focal ischaemia. The orientation of blood vessels in the SVZ may be diverted after stroke to orient cell migration from rostral to lateral.
The role of hypoxia in the SVZ after ischemic stroke
The triggers for the changes in the SVZ niche described above are not clear. Cellular and molecular signals from the inflammatory response in the ischemic tissue clearly play important roles. However, after the MCAO model of ischemic stroke, the SVZ is transiently rendered hypoxic to <10 mm Hg (113). Hypoxia induces upregulation of hypoxia-inducible factor 1 alpha (Hif-1α) in neural precursor cells (34). Hif-1α, a transcription factor with downstream targets such as VEGF and erythropoietin, is involved in many critical functions, including angiogenesis, cell proliferation, and glucose metabolism (94). Therefore, even transient hypoxia, which does not lead to profound cell death, can have considerable effects on the neurogenic niche.
Conclusion and Future Directions
The migration of SVZ neuroblasts towards areas of ischemic injury remains one of the most remarkable aspects of adult neurogenesis. A key question of various forms of cellular replacement therapy for neurological diseases is one of delivery to the site of injury. Therefore, in addition to exploring their therapeutic potential, a better understanding of mechanisms which regulate neuroblast emigration after stroke will help address this question. Much progress has been made to understand the cellular and molecular interactions which facilitate neuroblast emigration, and we are beginning to grasp that significant and complex changes occur at a system/niche level, both in the ischemic/peri-ischemic tissue, and in the SVZ neurogenic niche.
Abbreviations Used
- Ang-1
angiopoietin
- BDNF
brain-derived neurotrophic factor
- BMP
bone morphogenic protein
- BrdU
5-bromo-2-deoxyuridine
- CCR2
chemokine (C-C motif) receptor
- CSF
cerebral spinal fluid
- CXCR4
C-X-C chemokine receptor type 4
- Dcx
doublecortin
- GDNF
glial cell-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- Hif-1α
hypoxia-inducible factor 1 alpha
- IGF-1
insulin growth factor-1
- MCAO
middle cerebral artery occlusion
- MCP-1
monocyte chemoattractant protein-1
- MMP
matrix metalloproteinase
- OB
olfactory bulb
- PSA-NCAM
polysialylated neuronal cell adhesion molecule
- RMS
rostral migratory stream
- RT-PCR
reverse transcription polymerase chain reaction
- SDF-1α
stromal derived factor 1 alpha
- SGZ
subgranular zone
- SVZ
subventricular zone
- TBI
traumatic brain injury
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
Acknowledgments
CCY was supported by a Rhodes scholarship. FGS was supported by NIH Grant RO1 NS-42253. We thank Ms. Judith van der Harg for technical assistance with the wholemount immunohistochemistry, and Dr. Isabelle Comte for original artwork. KJB and AMB supported by MRC, BRC, and Foundation Leduc.
References
- 1.Aguirre A. Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 3.Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 4.Anton ES. Ghashghaei HT. Weber JL. McCann C. Fischer TM. Cheung ID. Gassmann M. Messing A. Klein R. Schwab MH. Lloyd KCK. Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 5.Arvidsson A. Collin T. Kirik D. Kokaia Z. Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 6.Barkho BZ. Munoz AE. Li X. Li L. Cunningham LA. Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–3149. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck H. Acker T. Wiessner C. Allegrini PR. Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157:1473–1483. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck H. Plate K. Angiogenesis after cerebral ischemia. Acta Neuropathologica. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 9.Benraiss A. Chmielnicki E. Lerner K. Roh D. Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berninger B. Guillemot F. Götz M. Directing neurotransmitter identity of neurones derived from expanded adult neural stem cells. Eur J Neurosci. 2007;25:2581–2590. doi: 10.1111/j.1460-9568.2007.05509.x. [DOI] [PubMed] [Google Scholar]
- 11.Bolteus AJ. Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bovetti S. Hsieh Y. Bovolin P. Perroteau I. Kazunori T. Puche AC. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brill MS. Ninkovic J. Winpenny E. Hodge RD. Ozen I. Yang R. Lepier A. Gascón S. Erdelyi F. Szabo G. Parras C. Guillemot F. Frotscher M. Berninger B. Hevner RF. Raineteau O. Götz M. Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci. 2009;12:1524–1533. doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calzà L. Giardino L. Pozza M. Bettelli C. Micera A. Aloe L. Proliferation and phenotype regulation in the subventricular zone during experimental allergic encephalomyelitis: In vivo evidence of a role for nerve growth factor. Proc Natl Acad Sci USA. 1998;95:3209–3214. doi: 10.1073/pnas.95.6.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlen M. Meletis K. Goritz C. Darsalia V. Evergren E. Tanigaki K. Amendola M. Barnabe–Heider F. Yeung MSY. Naldini L. Honjo T. Kokaia Z. Shupliakov O. Cassidy RM. Lindvall O. Frisen J. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 16.Cayre M. Canoll P. Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiaramello S. Dalmasso G. Bezin L. Marcel D. Jourdan F. Peretto P. Fasolo A. Marchis SD. BDNF/ TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signaling pathways. Eur J Neurosci. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- 18.Cremer H. Lange R. Christoph A. Plomann M. Vopper G. Roes J. Brown R. Baldwin S. Kraemer P. Scheff S. Barthels D. Rajewsky K. Wille W. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 19.De Chevigny A. Cooper O. Vinuela A. Reske–Nielsen C. Lagace DC. Eisch AJ. Isacson O. Fate mapping and lineage analyses demonstrate the production of a large number of striatal neuroblasts after transforming growth factor alpha and noggin striatal infusions into the dopamine-depleted striatum. Stem Cells. 2008;26:2349–2360. doi: 10.1634/stemcells.2008-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimitrijevic OB. Stamatovic SM. Keep RF. Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 21.Doetsch F. Alvarez–Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doetsch F. Garcia–Verdugo JM. Alvarez–Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle KP. Yang T. Lessov NS. Ciesielski TM. Stevens SL. Simon RP. King JS. Stenzel-Poore MP. Nasal administration of osteopontin peptide mimetics confers neuroprotection in stroke. J Cereb Blood Flow Metab. 2008;28:1235–1248. doi: 10.1038/jcbfm.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckenhoff M. Rakic P. Nature and fate of proliferative cells in the hippocampal dentate gyrus during the life span of the rhesus monkey. J Neurosci. 1988;8:2729–2747. doi: 10.1523/JNEUROSCI.08-08-02729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekdahl CT. Claasen J. Bonde S. Kokaia Z. Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emsley JG. Hagg T. alpha6beta1 integrin directs migration of neuronal precursors in adult mouse forebrain. Exp Neurol. 2003;183:273–285. doi: 10.1016/s0014-4886(03)00209-7. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson PS. Perfilieva E. Bjork–Eriksson T. Alborn A. Nordborg C. Peterson DA. Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 28.García–Alías G. Barkhuysen S. Buckle M. Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- 29.Ghashghaei HT. Weber J. Pevny L. Schmid R. Schwab MH. Lloyd KCK. Eisenstat DD. Lai C. Anton ES. The role of neuregulin–ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci USA. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goings GE. Greisman A. James RE. Abram LK. Begolka WS. Miller SD. Szele FG. Hematopoietic cell activation in the subventricular zone after Theiler's virus infection. J Neuroinflamm. 2008;5:44. doi: 10.1186/1742-2094-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goings GE. Kozlowski DA. Szele FG. Differential activation of microglia in neurogenic versus non-neurogenic regions of the forebrain. Glia. 2006;54:329–342. doi: 10.1002/glia.20381. [DOI] [PubMed] [Google Scholar]
- 32.Gotts JE. Chesselet M. Vascular changes in the subventricular zone after distal cortical lesions. Exp Neurol. 2005;194:139–150. doi: 10.1016/j.expneurol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Hack I. Bancila M. Loulier K. Carroll P. Cremer H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci. 2002;5:939–945. doi: 10.1038/nn923. [DOI] [PubMed] [Google Scholar]
- 34.Harms KM. Li L. Cunningham LA. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1α–regulated VEGF signaling. PLoS ONE. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi T. Noshita N. Sugawara T. Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 36.Hou S. Wang Y. Xu M. Shen D. Wang J. Huang F. Yu Z. Sun F. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008;39:2837–2844. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- 37.Hu H. Tomasiewicz H. Magnuson T. Rutishauser U. The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron. 1996;16:735–743. doi: 10.1016/s0896-6273(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 38.Imitola J. Raddassi K. Park KI. Mueller F. Nieto M. Teng YD. Frenkel D. Li J. Sidman RL. Walsh CA. Snyder EY. Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jablonska B. Aguirre A. Raymond M. Szabo G. Kitabatake Y. Sailor KA. Ming G. Song H. Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacques T. Relvas J. Nishimura S. Pytela R. Edwards G. Streuli C. ffrench-Constant C. Neural precursor cell chain migration and division are regulated through different beta1 integrins. Development. 1998;125:3167–3177. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- 41.Jankovski A. Garcia C. Soriano E. Sotelo C. Proliferation, migration and differentiation of neuronal progenitor cells in the adult mouse subventricular zone surgically separated from its olfactory bulb. Eur J Neurosci. 1998;10:3853–3868. doi: 10.1046/j.1460-9568.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 42.Ji JF. He BP. Dheen ST. Tay SSW. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci Lett. 2004;355:236–240. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 43.Jin K. Minami M. Lan JQ. Mao XO. Batteur S. Simon RP. Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin K. Sun Y. Xie L. Peel A. Mao XO. Batteur S. Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 45.Jin K. Wang X. Xie L. Mao XO. Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci USA. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin K. Wang X. Xie L. Mao XO. Zhu W. Wang Y. Shen J. Mao Y. Banwait S. Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kernie SG. Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim GH. Mocco J. Hahn DK. Kellner CP. Komotar RJ. Ducruet AF. Mack WJ. Connolly ES. Protective effect of C5a receptor inhibition after murine reperfused stroke. Neurosurgery. 2008;63:122–125. doi: 10.1227/01.NEU.0000335079.70222.8D. discussion 125–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Y. Comte I. Szabo G. Hockberger P. Szele FG. Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration. PLoS One. 2009;4:e8122. doi: 10.1371/journal.pone.0008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirschenbaum B. Doetsch F. Lois C. Alvarez–Buylla A. Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci. 1999;19:2171–2180. doi: 10.1523/JNEUROSCI.19-06-02171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohwi M. Petryniak MA. Long JE. Ekker M. Obata K. Yanagawa Y. Rubenstein JLR. Alvarez–Buylla A. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kojima T. Hirota Y. Ema M. Takahashi S. Miyoshi I. Okano H. Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- 54.Kovacs Z. Ikezaki K. Samoto K. Inamura T. Fukui M. Kawamata T. Finklestein SP. VEGF and flt: Expression time kinetics in rat brain infarct. Stroke. 1996;27:1865–1873. doi: 10.1161/01.str.27.10.1865. [DOI] [PubMed] [Google Scholar]
- 55.Lakhan S. Kirchgessner A. Hofer M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J Translat Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S. Kim H. Rogowska J. Zhao B. Bhide P. Parent JM. Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leventhal C. Rafii S. Rafii D. Shahar A. Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 58.Li L. Harms KM. Ventura PB. Lagace DC. Eisch AJ. Cunningham LA. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia. 2010;58:1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lichtenwalner RJ. Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2005;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- 60.Lim DA. Tramontin AD. Trevejo JM. Herrera DG. García–Verdugo JM. Alvarez–Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 61.Liu F. You Y. Li X. Ma T. Nie Y. Wei B. Li T. Lin H. Yang Z. Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts. J Neurosci. 2009;29:5075–5087. doi: 10.1523/JNEUROSCI.0201-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu G. Rao Y. Neuronal migration from the forebrain to the olfactory bulb requires a new attractant persistent in the olfactory bulb. J Neurosci. 2003;23:6651–6659. doi: 10.1523/JNEUROSCI.23-16-06651.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu HM. Neovasculature and blood–brain barrier in ischemic brain infarct. Acta Neuropathol. 1988;75:422–426. doi: 10.1007/BF00687796. [DOI] [PubMed] [Google Scholar]
- 64.Liu X. Zhang Z. Zhang R. Gregg S. Meng H. Chopp M. Comparison of in vivo and in vitro gene expression profiles in subventricular zone neural progenitor cells from the adult mouse after middle cerebral artery occlusion. Neuroscience. 2007;146:1053–1061. doi: 10.1016/j.neuroscience.2007.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu XS. Chopp M. Santra M. Hozeska–Solgot A. Zhang RL. Wang L. Teng H. Lu M. Zhang ZG. Functional response to SDF1[alpha] through over-expression of CXCR4 on adult subventricular zone progenitor cells. Brain Res. 2008;1226:18–26. doi: 10.1016/j.brainres.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu XS. Zhang ZG. Zhang RL. Gregg SR. Wang L. Yier T. Chopp M. Chemokine ligand 2 (CCL2) induces migration and differentiation of subventricular zone cells after stroke. J Neurosci Res. 2007;85:2120–2125. doi: 10.1002/jnr.21359. [DOI] [PubMed] [Google Scholar]
- 67.Lois C. Alvarez–Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lois C. Alvarez–Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 69.Luo Y. Cai J. Xue H. Miura T. Rao MS. Functional SDF1α/CXCR4 signaling in the developing spinal cord. J Neurochem. 2005;93:452–462. doi: 10.1111/j.1471-4159.2005.03049.x. [DOI] [PubMed] [Google Scholar]
- 70.Ma Q. Jones D. Borghesani PR. Segal RA. Nagasawa T. Kishimoto T. Bronson RT. Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macas J. Nern C. Plate KH. Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magavi SS. Leavitt BR. Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 73.Marshall GP. Demir M. Steindler DA. Laywell ED. Subventricular zone microglia possess a unique capacity for massive in vitro expansion. Glia. 2008;56:1799–1808. doi: 10.1002/glia.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marti HJH. Bernaudin M. Bellail A. Schoch H. Euler M. Petit E. Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marti–Fabregas J. Romaguera–Ros M. Gomez–Pinedo U. Martinez–Ramirez S. Jimenez–Xarrie E. Marin R. Marti–Vilalta J. Garcia–Verdugo J. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357–365. doi: 10.1212/WNL.0b013e3181cbccec. [DOI] [PubMed] [Google Scholar]
- 76.Massouh M. Saghatelyan A. De-routing neuronal precursors in the adult brain to sites of injury: Role of the vasculature. Neuropharmacology. 2010;58:877–883. doi: 10.1016/j.neuropharm.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 77.Masuda T. Isobe Y. Aihara N. Furuyama F. Misumi S. Kim T. Nishino H. Hida H. Increase in neurogenesis and neuroblast migration after a small intracerebral hemorrhage in rats. Neurosci Lett. 2007;425:114–119. doi: 10.1016/j.neulet.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 78.Meller R. Stevens SL. Minami M. Cameron JA. King S. Rosenzweig H. Doyle K. Lessov NS. Simon RP. Stenzel–Poore MP. Neuroprotection by osteopontin in stroke. J Cereb Blood Flow Metab. 2005;25:217–225. doi: 10.1038/sj.jcbfm.9600022. [DOI] [PubMed] [Google Scholar]
- 79.Mercier F. Kitasako JT. Hatton GI. Anatomy of the brain neurogenic zones revisited: Fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- 80.Merkle FT. Mirzadeh Z. Alvarez–Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 81.Mirzadeh Z. Merkle FT. Soriano–Navarro M. Garcia–Verdugo JM. Alvarez–Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Monje ML. Toda H. Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 83.Murase S. Horwitz AF. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J Neurosci. 2002;22:3568–3579. doi: 10.1523/JNEUROSCI.22-09-03568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nam SC. Kim Y. Dryanovski D. Walker A. Goings G. Woolfrey K. Kang SS. Chu C. Chenn A. Erdelyi F. Szabo G. Hockberger P. Szele FG. Dynamic features of postnatal subventricular zone cell motility: A two-photon time-lapse study. J Comp Neurol. 2007;505:190–208. doi: 10.1002/cne.21473. [DOI] [PubMed] [Google Scholar]
- 85.Ng KL. Li J. Cheng MY. Leslie FM. Lee AG. Zhou Q. Dependence of olfactory bulb neurogenesis on Prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen Ba–Charvet KTN. Brose K. Marillat V. Kidd T. Goodman CS. Tessier–Lavigne M. Sotelo C. Chédotal A. Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron. 1999;22:463–473. doi: 10.1016/s0896-6273(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 87.Noctor SC. Flint AC. Weissman TA. Dammerman RS. Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 88.Ohab JJ. Carmichael ST. Poststroke neurogenesis: Emerging principles of migration and localization of immature neurons. Neuroscientist. 2008;14:369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- 89.Ohab JJ. Fleming S. Blesch A. Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paratcha G. Ibáñez CF. Ledda F. GDNF is a chemoattractant factor for neuronal precursor cells in the rostral migratory stream. Mol Cell Neurosci. 2006;31:505–514. doi: 10.1016/j.mcn.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 91.Parent JM. Vexler ZS. Gong C. Derugin N. Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 92.Plane JM. Andjelkovic AV. Keep RF. Parent JM. Intact and injured endothelial cells differentially modulate postnatal murine forebrain neural stem cells. Neurobiol Dis. 2010;37:218–227. doi: 10.1016/j.nbd.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plate KH. Beck H. Danner S. Allegrini PR. Wiessner C. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol. 1999;58:654–666. doi: 10.1097/00005072-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Pugh CW. Ratcliffe PJ. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 95.Robin AM. Zhang ZG. Wang L. Zhang RL. Katakowski M. Zhang L. Wang Y. Zhang C. Chopp M. Stromal cell-derived factor 1[alpha] mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 96.Rosa AI. Goncalves J. Cortes L. Bernardino L. Malva JO. Agasse F. The angiogenic factor Angiopoietin-1 is a proneurogenic peptide on subventricular zone stem/progenitor cells. J Neurosci. 2010;30:4573–4584. doi: 10.1523/JNEUROSCI.5597-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosenberg GA. Cunningham LA. Wallace J. Alexander S. Estrada EY. Grossetete M. Razhagi A. Miller K. Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: Activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 98.Sanai N. Tramontin AD. Quinones–Hinojosa A. Barbaro NM. Gupta N. Kunwar S. Lawton MT. McDermott MW. Parsa AT. Manuel–Garcia Verdugo J. Berger MS. Alvarez–Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 99.Sawamoto K. Wichterle H. Gonzalez–Perez O. Cholfin JA. Yamada M. Spassky N. Murcia NS. Garcia–Verdugo JM. Marin O. Rubenstein JLR. Tessier–Lavigne M. Okano H. Alvarez–Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 100.Seidenfaden R. Desoeuvre A. Bosio A. Virard I. Cremer H. Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol Cell Neurosci. 2006;32:187–198. doi: 10.1016/j.mcn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Shen Q. Goderie SK. Jin L. Karanth N. Sun Y. Abramova N. Vincent P. Pumiglia K. Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 102.Shen Q. Wang Y. Kokovay E. Lin G. Chuang S. Goderie SK. Roysam B. Temple S. Adult svz stem cells lie in a vascular niche: A quantitative analysis of niche cell–cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shinjyo N. Ståhlberg A. Dragunow M. Pekny M. Pekna M. Complement-derived Anaphylatoxin C3a regulates in vitro differentiation and migration of neural progenitor cells. Stem Cells. 2009;27:2824–2832. doi: 10.1002/stem.225. [DOI] [PubMed] [Google Scholar]
- 104.Snapyan M. Lemasson M. Brill MS. Blais M. Massouh M. Ninkovic J. Gravel C. Berthod F. Gotz M. Barker PA. Parent A. Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spassky N. Merkle FT. Flames N. Tramontin AD. García–Verdugo JM. Alvarez–Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steiner B. Wolf S. Kempermann G. Adult neurogenesis and neurodegenerative disease. Regen Med. 2006;1:15–28. doi: 10.2217/17460751.1.1.15. [DOI] [PubMed] [Google Scholar]
- 107.Sugiura S. Kitagawa K. Tanaka S. Todo K. Omura–Matsuoka E. Sasaki T. Mabuchi T. Matsushita K. Yagita Y. Hori M. Adenovirus-mediated gene transfer of heparin-binding epidermal growth factor-like growth factor enhances neurogenesis and angiogenesis after focal cerebral ischemia in rats. Stroke. 2005;36:859–864. doi: 10.1161/01.STR.0000158905.22871.95. [DOI] [PubMed] [Google Scholar]
- 108.Sun Y. Jin K. Xie L. Childs J. Mao XO. Logvinova A. Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sundholm–Peters NL. Yang HKC. Goings GE. Walker AS. Szele FG. Radial glia-like cells at the base of the lateral ventricles in adult mice. J Neurocytol. 2004;33:153–164. doi: 10.1023/B:NEUR.0000029654.70632.3a. [DOI] [PubMed] [Google Scholar]
- 110.Szele FG. Chesselet MF. Cortical lesions induce an increase in cell number and PSA-NCAM expression in the subventricular zone of adult rats. J Comp Neurol. 1996;368:439–454. doi: 10.1002/(SICI)1096-9861(19960506)368:3<439::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 111.Teramoto T. Qiu J. Plumier J. Moskowitz MA. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest. 2003;111:1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thored P. Arvidsson A. Cacci E. Ahlenius H. Kallur T. Darsalia V. Ekdahl CT. Kokaia Z. Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 113.Thored P. Wood J. Arvidsson A. Cammenga J. Kokaia Z. Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 114.Thored P. Heldmann U. Gomes–Leal W. Gisler R. Darsalia V. Taneera J. Nygren JM. Jacobsen S-EW. Ekdahl CT. Kokaia Z. Lindvall O. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 115.Tramontin AD. García–Verdugo JM. Lim DA. Alvarez–Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): A continuum of the neural stem cell compartment. Cereb Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- 116.Tran PB. Banisadr G. Ren D. Chenn A. Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1034. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ventura RE. Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27:4297–4302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y. Jin K. Mao XO. Xie L. Banwait S. Marti HH. Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- 119.Whitman MC. Fan W. Rela L. Rodriguez–Gil DJ. Greer CA. Blood vessels form a migratory scaffold in the rostral migratory stream. J Comp Neurol. 2009;516:94–104. doi: 10.1002/cne.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wichterle H. García–Verdugo JM. Alvarez–Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron. 1997;18:779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- 121.Willaime–Morawek S. van der Kooy D. Cortex- and striatum- derived neural stem cells produce distinct progeny in the olfactory bulb and striatum. Eur J Neurosci. 2008;27:2354–2362. doi: 10.1111/j.1460-9568.2008.06206.x. [DOI] [PubMed] [Google Scholar]
- 122.Willaime–Morawek S. Seaberg RM. Batista C. Labbé E. Attisano L. Gorski JA. Jones KR. Kam A. Morshead CM. van der Kooy D. Embryonic cortical neural stem cells migrate ventrally and persist as postnatal striatal stem cells. J Cell Biol. 2006;175:159–168. doi: 10.1083/jcb.200604123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiltrout C. Lang B. Yan Y. Dempsey RJ. Vemuganti R. Repairing brain after stroke: A review on post-ischemic neurogenesis. Neurochem Internat. 2007;50:1028–1041. doi: 10.1016/j.neuint.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 124.Won SJ. Kim SH. Xie L. Wang Y. Mao XO. Jin K. Greenberg DA. Reelin-deficient mice show impaired neurogenesis and increased stroke size. Exper Neurol. 2006;198:250–259. doi: 10.1016/j.expneurol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Wu W. Wong K. Chen J. Jiang Z. Dupuis S. Wu JY. Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamashita T. Ninomiya M. Hernández Acosta P. García–Verdugo JM. Sunabori T. Sakaguchi M. Adachi K. Kojima T. Hirota Y. Kawase T. Araki N. Abe K. Okano H. Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yan Y. Lang BT. Vemuganti R. Dempsey RJ. Osteopontin is a mediator of the lateral migration of neuroblasts from the subventricular zone after focal cerebral ischemia. Neurochem Internat. 2009;55:826–832. doi: 10.1016/j.neuint.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 128.Yan Y. Sailor KA. Lang BT. Park S. Vemuganti R. Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- 129.Yang HK. Sundholm–Peters NL. Goings GE. Walker AS. Hyland K. Szele FG. Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain. J Neurosci Res. 2004;76:282–295. doi: 10.1002/jnr.20071. [DOI] [PubMed] [Google Scholar]
- 130.Yang Z. You Y. Levison SW. Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J Comp Neurol. 2008;511:19–33. doi: 10.1002/cne.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Young KM. Fogarty M. Kessaris N. Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang H. Vutskits L. Pepper MS. Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163:1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang RL. Zhang ZG. Zhang L. Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 134.Zhang RL. Chopp M. Gregg SR. Toh Y. Roberts C. LeTourneau Y. Buller B. Jia L. Pourabdollah S. Zhang ZG. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J Cereb Blood Flow Metab. 2009;29:1240–1250. doi: 10.1038/jcbfm.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang RL. LeTourneau Y. Gregg SR. Wang Y. Toh Y. Robin AM. Gang Zhang Z. Chopp M. Neuroblast Division during migration toward the ischemic striatum: A study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang RL. Zhang ZG. Wang Y. LeTourneau Y. Liu XS. Zhang X. Gregg SR. Wang L. Chopp M. Stroke induces ependymal cell transformation into radial glia in the subventricular zone of the adult rodent brain. J Cereb Blood Flow Metab. 2007;27:1201–1212. doi: 10.1038/sj.jcbfm.9600430. [DOI] [PubMed] [Google Scholar]
- 137.Zhang ZG. Zhang L. Jiang Q. Zhang R. Davies K. Powers C. Bruggen NV. Chopp M. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao C. Deng W. Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]