Abstract
BACKGROUND
CC chemokine receptor 5 antagonists are a new class of antiretroviral agents.
METHODS
We conducted two double-blind, placebo-controlled, phase 3 studies — Maraviroc versus Optimized Therapy in Viremic Antiretroviral Treatment-Experienced Patients (MOTIVATE) 1 and MOTIVATE 2 — with patients who had R5 human immunodeficiency virus type 1 (HIV-1) only. They had been treated with or had resistance to three antiretroviral-drug classes and had HIV-1 RNA levels of more than 5000 copies per milliliter. The patients were randomly assigned to one of three antiretroviral regimens consisting of maraviroc once daily, maraviroc twice daily, or placebo, each of which included optimized background therapy (OBT) based on treatment history and drug-resistance testing. Safety and efficacy were assessed after 48 weeks.
RESULTS
A total of 1049 patients received the randomly assigned study drug; the mean baseline HIV-1 RNA level was 72,400 copies per milliliter, and the median CD4 cell count was 169 per cubic millimeter. At 48 weeks, in both studies, the mean change in HIV-1 RNA from baseline was greater with maraviroc than with placebo: −1.66 and −1.82 log10 copies per milliliter with the once-daily and twice-daily regimens, respectively, versus −0.80 with placebo in MOTIVATE 1, and −1.72 and −1.87 log10 copies per milliliter, respectively, versus −0.76 with placebo in MOTIVATE 2. More patients receiving maraviroc once or twice daily had HIV-1 RNA levels of less than 50 copies per milliliter (42% and 47%, respectively, vs. 16% in the placebo group in MOTIVATE 1; 45% in both maraviroc groups vs. 18% in MOTIVATE 2; P<0.001 for both comparisons in each study). The change from baseline in CD4 counts was also greater with maraviroc once or twice daily than with placebo (increases of 113 and 122 per cubic millimeter, respectively, vs. 54 in MOTIVATE 1; increases of 122 and 128 per cubic millimeter, respectively, vs. 69 in MOTIVATE 2; P<0.001 for both comparisons in each study). Frequencies of adverse events were similar among the groups.
CONCLUSIONS
Maraviroc, as compared with placebo, resulted in significantly greater suppression of HIV-1 and greater increases in CD4 cell counts at 48 weeks in previously treated patients with R5 HIV-1 who were receiving OBT. (ClinicalTrials.gov numbers, NCT00098306 and NCT00098722.)
For the past decade, treatment of human immunodeficiency virus type 1 (HIV-1) has consisted of a multiple-drug regimen targeting one or more of three HIV-1 proteins: reverse transcriptase, protease, and the glycoprotein envelope subunit gp41.1 Although these antiretroviral combinations are successful in suppressing viral replication and delaying disease progression, drug resistance and toxic effects may occur.2–4 There is therefore a need for better-tolerated, convenient antiretroviral agents with reduced toxicity and activity against multidrug-resistant viruses.
Agents with novel mechanisms of action provide options for patients with drug-resistant virus.4 CC chemokine receptor 5 (CCR5) is an attractive therapeutic target, since people with the delta32 deletion in both copies of the CCR5 gene lack CCR5 on the cell surface and are relatively resistant to HIV infection, whereas people who are heterozygous for the deletion have reduced expression of CCR5 on the cell surface and have delayed declines in the CD4 cell count and slower progression of HIV disease.5–8 These data support the rationale for testing CCR5 antagonists in people infected with R5 HIV-1. R5 HIV-1 predominates in the early stages of infection, whereas the X4 virus may emerge over time.9,10 The results of coreceptor-tropism assays in patients for whom at least two antiretroviral regimens have failed indicated that 50 to 62% were infected with R5 HIV-1 only.11–13 Thus, a sizable proportion of previously treated patients may benefit from treatment with a CCR5 antagonist. Maraviroc binds specifically and selectively to CCR5 on the surface of the CD4 cell and blocks HIV-1 binding.14–16 Maraviroc has potent activity against R5 HIV-1 strains in vitro, including drug-resistant isolates.14 In asymptomatic patients with HIV-1 infected with R5 virus only, as shown by a coreceptor-tropism assay, 10 days of maraviroc monotherapy reduced levels of HIV-1 RNA by nearly 2 log10 copies per milliliter.17 In contrast, maraviroc conferred little or no virologic benefit in patients with evidence of X4 HIV-1 strains.18
The Maraviroc versus Optimized Therapy in Viremic Antiretroviral Treatment-Experienced Patients (MOTIVATE) studies 1 and 2 were designed to evaluate the efficacy and safety of maraviroc once daily or twice daily, as compared with placebo, when added to optimized background therapy (based on treatment history and drug-resistance testing) in patients with R5 HIV-1 who had previously been treated with three classes of anti-retroviral drugs or who had resistant virus.
METHODS
STUDY DESIGN
MOTIVATE 1 and MOTIVATE 2 are parallel, randomized, double-blind, placebo-controlled, multinational phase 3 studies. The trials were designed with a 48-week treatment period, preceded by a 4-to-6-week screening period, and incorporated a single planned interim analysis at 24 weeks and an extension to 96 weeks. MOTIVATE 1 was conducted in Canada and the United States, and MOTIVATE 2 in Australia, Europe, and the United States. The design, conduct, and protocol-specified analyses were identical for the two studies.
At the screening visit, previous antiretroviral treatment was assessed, safety laboratory tests were conducted, and all patients underwent testing for HIV-1 coreceptor tropism with the use of a validated phenotypic assay (Trofile, Monogram Biosciences).19,20 Patients were also tested for plasma levels of HIV-1 RNA (Amplicor HIV-1 Monitor v1.5, Roche Diagnostics) and genotypic and phenotypic resistance to approved antiretroviral drugs (PhenoSense GT assay, Monogram Biosciences), including gp41 sequencing for enfuvirtide resistance (British Columbia Centre for Excellence in HIV/AIDS, Vancouver, Canada).
At the time of randomization, 4 to 7 days before administration of the first dose of the study drug, a second HIV-1 RNA measurement was obtained. An antiretroviral regimen to be used as optimized background therapy was selected by the investigator on the basis of the treatment history, safety considerations, and drug-resistance test results, in consultation with the study’s medical monitor. Eligible patients were randomly assigned in a 2:2:1 ratio to receive optimized background therapy in combination with maraviroc once daily, maraviroc twice daily, or matching placebo. Patients were stratified at the time of randomization according to the use or nonuse of enfuvirtide and the plasma HIV-1 RNA level at the time of screening (<100,000 or ≥100,000 copies per milliliter).
Treatment failure was defined as one or more of four virologic end points (confirmed by a second consecutive measurement within 14 days): an increase in the level of HIV-1 RNA to a value that was at least three times the baseline level at or after week 2; a decrease of less than 0.5 log10 copies per milliliter at or after week 8; a decrease of less than 1.0 log10 copies per milliliter at or after week 8, after a decrease of 2.0 or more log10 copies per milliliter; and an increase to 5000 or more copies per milliliter after levels of 400 copies or less per milliliter had been recorded on two consecutive visits. Coreceptor tropism and drug-resistance testing were performed in all patients who met these criteria, as well as in patients who had HIV-1 RNA levels of 500 copies per milliliter or more at or after week 4 (for tropism) or at weeks 24 and 48 (for drug resistance).
The study protocol was approved by the institutional review board or independent ethics committee at each study center. Written informed consent was obtained from all participants. The studies were performed in accordance with International Conference on Harmonization Good Clinical Practice guidelines and applicable local regulatory requirements and laws. An independent data and safety monitoring board was responsible for oversight of the progress of the studies, the study data, and safety considerations.
The study was designed by the sponsor, Pfizer Global Research and Development, with input from the study investigators. Data were gathered by the study investigators and the sponsor, with data summaries provided by an independent statistician (Covance CAPS, Berkshire, United Kingdom) from the Statistical Data Analysis Center to the data and safety monitoring board for periodic review. All statistical analyses were carried out by the study sponsor according to a predefined plan. Two of the authors wrote the paper, with extensive input from the study team. The study team vouches for the accuracy and completeness of the data and analysis as presented.
STUDY POPULATION
All patients were at least 16 years old, were infected with R5 HIV-1 only (as shown by a coreceptor tropism assay), and had taken one or more agents from three antiretroviral classes (nucleoside analogues, non-nucleoside analogues, protease inhibitors [at least two drugs from this class], or fusion inhibitors) for 6 months or more or had documented genotypic or phenotypic resistance to drugs from at least three of these classes. Patients had been receiving a stable antiretroviral regimen or no antiretroviral therapy for more than 4 weeks and had a plasma HIV-1 RNA level of more than 5000 copies per milliliter at the screening visit. Patients were ineligible if there was evidence of infection with X4 HIV-1, if the tropism assay failed to show a result, or if they had received any investigational HIV-entry inhibitor for more than 14 days.
STUDY MEDICATION
In addition to optimized background therapy, patients received oral maraviroc or placebo (twice daily), without regard to food; the study drugs were identical in appearance. Maraviroc doses equivalent to 300 mg once or twice daily were selected on the basis of prior drug–drug interaction data, with patients whose optimized background therapy included a protease inhibitor (other than tipranavir) or delavirdine receiving maraviroc at a once- or twice-daily dose of 150 mg.17,21,22 With all other regimens, patients received 300 mg of maraviroc once or twice daily. Optimized background therapy, selected by the investigator on the basis of the treatment history and drug-resistance testing, comprised three to six open-label–approved antiretroviral agents (with or without low-dose ritonavir), chosen from nucleoside analogues, nonnucleoside analogues, protease inhibitors, and fusion inhibitors. Use of tipranavir was permitted after drug–drug interaction data became available (February 2006). Investigational agents (at the time including darunavir, etravirine, and raltegravir) were not permitted. If there were toxic effects that could be attributed to a specific component of optimized background therapy, a drug of the same class could be substituted after consultation with the study’s medical monitor.
EFFICACY ANALYSIS
The primary end point was the mean change in log10-transformed levels of HIV-1 RNA from base-line to 48 weeks. This end point was consistent with both treatment guidelines1 and published studies of investigational agents in this patient population.23,24 Secondary end points included HIV-1 RNA levels of less than 50 and less than 400 copies per milliliter, a decrease in HIV-1 RNA from baseline of 1.0 log10 copies per milliliter or more, a change in the CD4 cell count from baseline, and time to treatment failure.
SAFETY ANALYSIS
Safety end points included adverse events, adverse events leading to discontinuation of the study drug, serious adverse events (including death and category C events i.e., acquired immunodeficiency syndrome [AIDS]–defining events),25 and laboratory-test abnormalities. Study procedures included monitoring patients for adverse events and performing laboratory tests for safety at all study visits, measuring vital signs, and obtaining a 12-lead electrocardiogram at selected study visits. Adverse events were graded in accordance with the adverse-event grading scale developed by the Division of AIDS at the National Institutes of Health,26 with causality assessed by the investigator.
STATISTICAL ANALYSIS
Assuming a standard deviation of 0.8, with a two-sided significance level of 0.025 (with Bonferroni’s adjustment for multiple comparisons of the primary end point), we calculated that a sample of 500 patients (randomly assigned in a 2:2:1 ratio to receive maraviroc once daily, maraviroc twice daily, or placebo) would provide more than 95% power to detect a treatment difference in the HIV-1 RNA level of 0.5 log10 copies per milliliter between each maraviroc group and the placebo group in each study. The relative efficacy of the two different maraviroc doses was not formally evaluated.
Genotypic, phenotypic, and overall susceptibility scores at screening were calculated to assess the number of potentially active agents in the optimized background therapy. Scores were assigned with the use of a binary system in which each drug was coded as 1 for full susceptibility and 0 for reduced susceptibility, based on genotypic or phenotypic data. The overall susceptibility score was based on the net assessment from the resistance tests, which was derived from both genotypic and phenotypic susceptibility data with the use of a proprietary algorithm, except that for enfuvirtide, the overall susceptibility score was based solely on the genotype. A drug was classified as active if the resistance test was unavailable at the time of screening.
Efficacy data were analyzed for all patients who had undergone randomization and received at least one dose of the assigned study drug. An analysis-of-covariance model was used, with the viral load at screening, use or nonuse of enfuvirtide, and study group as the main covariables. For the primary efficacy analysis, if the study drug was discontinued for any reason at or before week 48, the change from baseline was recorded as zero. The change from baseline was also recorded as zero if baseline data were missing or there was no assessment of HIV-1 RNA level at a specific time point. An HIV-1 RNA level of less than 50 copies per milliliter was designated as 49 copies per milliliter for the analyses. Missing values were classified as nonresponses. A change in the CD4 cell count from baseline was analyzed with an analysis-of-covariance model, with the CD4 cell count at screening, use or nonuse of enfuvirtide, and study group as the covariables. For the analysis of the change in the CD4 cell count from baseline, the last-observation-carried-forward method was used.
Safety data were analyzed for all patients who underwent randomization and received at least one dose of the assigned study drug. Adverse events were assessed until 7 days after discontinuation of the study drug or until the time from the end of therapy in the blinded study to the start of therapy with open-label antiretroviral drugs, whichever was shorter. Serious adverse events were assessed until 28 days after discontinuation of the study drug. Serious, treatment-related adverse events were reported regardless of whether the study drug was discontinued. All reported P values are two-sided and have not been adjusted for multiple comparisons.
RESULTS
STUDY POPULATION
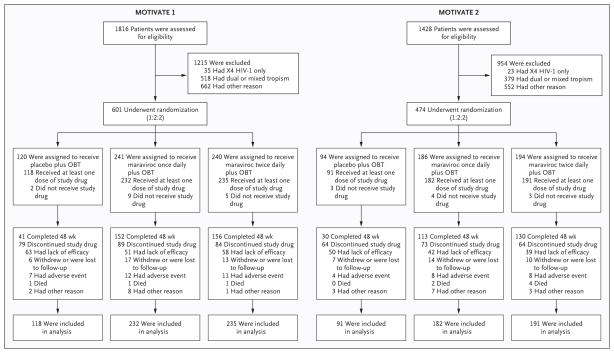
Of the 3244 patients screened for the two studies between November 2004 and March 2006, 61% had R5 HIV-1 only, as shown by a coreceptor-tropism assay. Of these patients, 1075 underwent randomization at 239 study sites, with more patients enrolled in MOTIVATE 1 (601) than in MOTIVATE 2 (474); 1049 patients received at least one dose of the study drug (Fig. 1).
Figure 1. Screening, Randomization, and Completion of Treatment.
Exclusion from the studies for other reason included failure to meet all inclusion criteria or meeting one or more exclusion criteria — for example, patients with a screening HIV-1 RNA level of less than 5000 copies per milliliter, no evidence of triple-class experience or triple-class–resistant virus, or an excluded laboratory-test value. Lack of efficacy was assessed by the study investigator on the basis of protocol-defined criteria for treatment failure, as discussed in the Methods section. The analysis was limited to study patients who both underwent randomization and received at least one dose of the study drug. OBT denotes optimized background therapy.
The demographic and baseline characteristics of the patients were similar between the two studies and were balanced among the study groups, with mean HIV-1 RNA levels of 4.85 and 4.86 log10 copies per milliliter and median CD4 counts of 159 and 176 cells per cubic millimeter in MOTIVATE 1 and MOTIVATE 2, respectively (Table 1). Of the 1049 patients who received at least one dose of the study drug, 766 (73%), 651 (62%), and 708 (67%) had genotypic, phenotypic, and overall susceptibility scores of 2 or less, respectively. Approximately 57% of patients had CD4 counts of less then 200 cells per cubic millimeter. A total of 82% of patients were taking antiretroviral drugs at study entry; 18% had not taken antiretroviral drugs within 7 days before study entry.
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | MOTIVATE 1 (N = 585) | MOTIVATE 2 (N = 464) | MOTIVATE 1 and 2 Pooled Data (N = 1049) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 118) | Maraviroc Once Daily (N = 232) | Maraviroc Twice Daily (N = 235) | Placebo (N = 91) | Maraviroc Once Daily (N = 182) | Maraviroc Twice Daily (N = 191) | Placebo (N = 209) | Maraviroc Once Daily (N = 414) | Maraviroc Twice Daily (N = 426) | |
| Age — yr | |||||||||
| Mean | 46 | 46 | 46 | 45 | 45 | 47 | 46 | 46 | 46 |
| Range | 31–71 | 19–75 | 25–69 | 29–72 | 17–75 | 21–73 | 29–72 | 17–75 | 21–73 |
| Male sex — no. (%) | 106 (90) | 210 (91) | 212 (90) | 79 (87) | 153 (84) | 170 (89) | 185 (89) | 363 (88) | 382 (90) |
| Race or ethnic group — no. (%)† | |||||||||
| White | 99 (84) | 187 (81) | 197 (84) | 79 (87) | 149 (82) | 166 (87) | 178 (85) | 336 (81) | 363 (85) |
| Black | 15 (13) | 39 (17) | 33 (14) | 11 (12) | 31 (17) | 18 (9) | 26 (12) | 70 (17) | 51 (12) |
| Asian, other, or unspecified | 4 (3) | 6 (3) | 5 (2) | 1 (1) | 12 (1) | 7 (4) | 5 (2) | 8 (2) | 12 (3) |
| Mean HIV-1 RNA — log10 copies/ml‡ | 4.84 | 4.85 | 4.86 | 4.89 | 4.87 | 4.84 | 4.86 | 4.86 | 4.85 |
| Median CD4 count — cells/mm3‡ | 160 | 168 | 150 | 174 | 173 | 182 | 171 | 171 | 167 |
All regimens in MOTIVATE 1 and MOTIVATE 2 include optimized background therapy. All patients who received at least one dose of study treatment are included in the table. There was no evidence of imbalance in baseline characteristics among patients in either the MOTIVATE 1 or the MOTIVATE 2 study.
Race or ethnic group was reported by the study participants — 13% of the patients were of Hispanic or Latino ethnicity; these patients could be included in any of the three specified groups.
The baseline value for each patient was calculated as the mean of up to three assessments made before receipt of the study drug (at screening, randomization, and the baseline visit).
DISCONTINUATION OF TREATMENT
In the two studies combined, a total of 143 (68%) of the patients taking placebo discontinued treatment by week 48, as compared with 162 (39%) of those taking maraviroc once daily and 148 (35%) of those taking maraviroc twice daily (Fig. 1). The most common reason for discontinuation was lack of efficacy in the opinion of the site investigator, based on protocol-defined treatment failure. Discontinuation was related to adverse events in 11 patients (5%) taking placebo as compared with 20 (5%) taking maraviroc once daily and 19 (4%) taking maraviroc twice daily. The median duration of treatment was 144 days in the placebo group, as compared with 335 and 336 days in the maraviroc once-daily and twice-daily groups, respectively.
EFFICACY
The primary end-point results were similar between the two studies (Table 2). In MOTIVATE 1, the mean change in plasma levels of HIV-1 RNA from baseline at 48 weeks was −0.80 log10 copies per milliliter in the placebo group versus −1.66 log10 copies per milliliter in the group that received maraviroc once daily (difference, −0.85; 97.5% confidence interval [CI], −1.22 to −0.49) and −1.82 log10 copies per milliliter in the group that received maraviroc twice daily (difference, −1.02; 97.5% CI, −1.39 to −0.66). The corresponding figures for MOTIVATE 2 were −0.76 log10 copies per milliliter in the placebo group versus −1.72 log10 copies per milliliter in the group that received maraviroc once daily (difference, −0.96; 97.5% CI, −1.38 to −0.54) and −1.87 log10 copies per milliliter in the group that received maraviroc twice daily (difference, −1.11; 97.5% CI, −1.52 to −0.70).
Table 2.
Efficacy at 48 Weeks.*
| End Point | MOTIVATE 1 (N = 585) | MOTIVATE 2 (N = 464) | MOTIVATE 1 and 2 Pooled Data (N = 1049) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 118) | Maraviroc Once Daily (N = 232) | Maraviroc Twice Daily (N = 235) | Placebo (N = 91) | Maraviroc Once Daily (N = 182) | Maraviroc Twice Daily (N = 191) | Placebo (N = 209) | Maraviroc Once Daily (N = 414) | Maraviroc Twice Daily (N = 426) | |
| HIV-1 RNA — log10 copies/ml† | |||||||||
| Mean change from baseline | −0.80 | −1.66 | −1.82 | −0.76 | −1.72 | −1.87 | −0.79 | −1.68 | −1.84 |
| Difference from placebo group (97.5% CI) | −0.85 (−1.22 to −0.49) | −1.02 (−1.39 to −0.66) | −0.96 (−1.38 to −0.54) | −1.11 (−1.52 to 0.70) | −0.90 (−1.17 to −0.62) | −1.05 (−1.33 to −0.78) | |||
| HIV-1 RNA — no. (%) | |||||||||
| <50 copies/ml | 19 (16) | 97 (42) | 109 (46) | 16 (18) | 82 (45) | 85 (45) | 35 (17) | 179 (43) | 194 (46) |
| <400 copies/ml | 26 (22) | 118 (51) | 135 (57) | 21 (23) | 96 (53) | 105 (55) | 47 (22) | 214 (52) | 239 (56) |
| Decrease from baseline of ≥1.0 log10 or to <400 copies/ml | 37 (31) | 134 (58) | 148 (63) | 25 (27) | 107 (59) | 121 (63) | 61 (29) | 241 (58) | 270 (63) |
| CD4 count — cells/mm3 | |||||||||
| Mean change from baseline‡ | 54 | 113 | 122 | 69 | 122 | 128 | 61 | 116 | 124 |
| Difference from placebo group (95% CI) | 59 (34 to 84) | 69 (44 to 93) | 52 (23 to 81) | 59 (30 to 87) | 55 (36 to 74) | 63 (44 to 82) | |||
All regimens in MOTIVATE 1 and MOTIVATE 2 included optimized background therapy. Efficacy was assessed for all patients who received at least one dose of the study drug. P<0.001 for each comparison of a maraviroc group with the placebo group in the individual and combined studies.
Missing values for patients who discontinued the study for any reason at or before week 48 were defined as equal to the baseline values.
Data were available for 116 patients in the placebo group, 227 in the group receiving maraviroc once daily, and 233 in the group receiving maraviroc twice daily in MOTIVATE 1 and for 90, 180, and 185 patients, respectively, in MOTIVATE 2. This was a last-observation-carried-forward analysis.
In the pooled analysis, the mean change from baseline to week 48 in plasma HIV-1 RNA levels was −0.79 log10 copies per milliliter in the placebo group as compared with −1.68 log10 copies per milliliter in the group that received maraviroc once daily (difference, −0.90; 97.5% CI, −1.17 to −0.62) and −1.84 log10 copies per milliliter in the group that received maraviroc twice daily (difference, −1.05; 97.5% CI, −1.33 to −0.78) (Table 2).
For each of the secondary virologic end points, including the rates of virologic suppression to less than 400 and less than 50 copies per milliliter over the 48-week period (Table 2 and Fig. 2A), the response with maraviroc once daily and twice daily was also significantly better than with placebo: for suppression to less than 400 copies per milliliter, the response rate was 51% (maraviroc once daily) and 57% (maraviroc twice daily) versus 22% (placebo) in MOTIVATE 1 and 53% and 55% versus 23%, respectively, in MOTIVATE 2; for suppression to less than 50 copies per milliliter, the respective response rates were 42% and 47% versus 16% in MOTIVATE 1 and 45% and 45% versus 18% in MOTIVATE 2 (P<0.001 for pairwise comparisons). Detailed subgroup analyses are presented by Fätkenheuer et al. in this issue of the Journal.27
Figure 2. Treatment Responses at 48 Weeks.
HIV-1 RNA suppression (Panel A), the time to protocol-defined treatment failure (Panel B), and the change in CD4 cell count (Panel C) are shown as pooled data for MOTIVATE 1 and MOTIVATE 2. To review the same data for each individual study, see the Supplementary Appendix, available with the full text of this article at www.nejm.org. All patients received at least one dose of study treatment. The HIV-1 RNA value was defined as the baseline level if missing or if the patient discontinued treatment before the end of the 48-week study period. For the CD4 cell-count analysis, the last observation was carried forward. MVC denotes maraviroc, and OBT optimized background therapy.
The time to treatment failure in the two studies combined was similar in the two maraviroc groups and was significantly longer than in the placebo group (P<0.001 for pairwise comparisons) (Fig. 2B). In the two studies combined, the increase in the CD4 cell count from baseline to week 48 was similar in the two maraviroc groups and significantly greater than the increase in the placebo group (P<0.001 for pairwise comparisons) (Table 2 and Fig. 2C). In MOTIVATE 1, the mean increases in the CD4 count in the group that received maraviroc once daily and in the group that received maraviroc twice daily were 113 and 122 cells per cubic millimeter, respectively, as compared with 54 cells per cubic millimeter in the placebo group; in MOTIVATE 2, the mean increases were 122 and 128 cells per cubic millimeter in the groups receiving maraviroc once daily and twice daily, respectively, as compared with 69 cells per cubic millimeter in the placebo group.
SAFETY
There were no clinically relevant differences in safety events between the two studies; therefore, analyses of pooled data are presented. Safety data are unadjusted for the duration of therapy. The higher rate of study-drug discontinuation in the placebo group means that these patients spent less time receiving the study drug than did patients in the maraviroc groups: 111 patient-years for placebo versus 300 patient-years for maraviroc once daily and 309 patient-years for maraviroc twice daily.
The rate of adverse events from any cause was lower with placebo (85%) than with maraviroc once daily (91%) or twice daily (92%) (P = 0.01); there were no significant differences in the rates of treatment-related adverse events (Table 3). Post hoc analyses, unadjusted for treatment duration or multiple testing, showed that for grade 2, 3, or 4 adverse events occurring in 5% or more of patients, the only event rates that differed significantly among the study groups were those for fever (4% in the placebo group, 2% in the group that received maraviroc once daily, and 6% in the group that received maraviroc twice daily; P = 0.04) and headache (6%, 5%, and 2%, respectively; P = 0.03). Discontinuation because of adverse events related to the study treatment was uncommon, and the discontinuation rate was the same in the three groups (placebo, 6 of 209 patients [3%]; maraviroc once daily, 12 of 414 [3%]; and maraviroc twice daily, 13 of 426 [3%]).
Table 3.
Adverse Events (MOTIVATE 1 and MOTIVATE 2 Study Populations Combined).*
| Placebo (N = 209) | Maraviroc Once Daily (N = 414) | Maraviroc Twice Daily (N = 426) | |
|---|---|---|---|
| Duration of treatment — patient-yr | 111 | 300 | 309 |
| Patients with ≥1 adverse event (of any grade) — no. of patients (%) | |||
| All causes† | 177 (85) | 375 (91) | 393 (92) |
| Related to treatment | 94 (45) | 205 (50) | 219 (51) |
| Grade 2–4 adverse events (all causes) occurring in at least 5% of patients — no. of patients (%) | |||
| Diarrhea | 20 (10) | 43 (10) | 32 (8) |
| Fatigue | 13 (6) | 13 (3) | 21 (4) |
| Fever‡ | 9 (4) | 9 (2) | 24 (6) |
| Headache§ | 12 (6) | 22 (5) | 9 (2) |
| Nausea | 15 (7) | 25 (6) | 25 (6) |
| Upper respiratory tract infection | 3 (1) | 16 (4) | 20 (5) |
| Death¶ | 2 (1) | 6 (1) | 9 (2) |
| Category C (AIDS-defining) adverse events — no. | |||
| AIDS encephalopathy | 0 | 1 | 0 |
| Cryptosporidium gastroenteritis | 1 | 1 | 0 |
| Cytomegalovirus infection | 0 | 1 | 2 |
| Esophageal candidiasis‡ | 2 | 12 | 3 |
| Herpesvirus infection | 4 | 11 | 7 |
| Kaposi’s sarcoma | 3 | 1 | 2 |
| Lymphoma|| | 2 | 2 | 2 |
| Mycobacterial infection | 0 | 0 | 1 |
| Mycobacterium avium infection | 2 | 0 | 2 |
| Pneumocystis jiroveci pneumonia | 0 | 0 | 2 |
| Pneumonia** | 4 | 3 | 1 |
| Progressive multifocal leukoencephalopathy | 1 | 0 | 1 |
| Total no. of category C events | 19 | 32 | 23 |
| No. of patients (%) | 16 (8) | 29 (7) | 23 (5) |
| Aspartate aminotransferase elevation (maximum, all causes, without regard to baseline) — no. of patients/total no. of patients (%)†† | |||
| Grade 3 (>5 to 10 × upper limit of normal) | 6/207 (3) | 12/408 (3) | 14/421 (3) |
| Grade 4 (>10 × upper limit of normal) | 0/207 | 4/408 (1) | 6/421 (1) |
| Alanine aminotransferase elevation (maximum, all causes, without regard to baseline) — no. of patients/total no. of patients (%)†† | |||
| Grade 3 (>5 to 10 × upper limit of normal) | 6/207 (3) | 16/408 (4) | 7/421 (2) |
| Grade 4 (>10 × upper limit of normal) | 1/207 (<1) | 2/408 (<1) | 4/421 (1) |
All regimens included optimized background therapy. There was no evidence of significant differences in adverse events among the study groups except as noted. Comparisons were conducted post hoc and were not adjusted for duration of treatment or multiple testing.
P = 0.01 for these values (three-way comparison).
P = 0.04 for these values (three-way comparison).
P = 0.03 for these values (three-way comparison).
Causes of death were as follows: in the placebo group, pneumonia and sudden death; in the group that received maraviroc once daily, anorexia, bacterial pneumonia, cerebrovascular accident, myocardial infarction/acute heart failure/coronary-artery atheroma, respiratory failure, and septic shock; and in the group that received maraviroc twice daily, cardiorespiratory arrest, cerebrovascular accident, chronic obstructive pulmonary disease, progression of HIV infection, hypertensive heart disease, multiple organ failure, suicide, T-cell lymphoma, unknown.
This category includes B-cell and T-cell lymphomas.
This category includes viral and bacterial pneumonias.
This category includes all patients for whom there was at least one observation of the given test while on study treatment or within 7 days of the last dose of study treatment.
Rates of serious adverse events were similar among the treatment groups (placebo, 18%; maraviroc once daily, 18%; and maraviroc twice daily, 21%). A total of 17 patients died during treatment, with no significant differences among the three groups (placebo, 2 patients [1%], 1 of whom had received open-label maraviroc; maraviroc once daily, 6 [1%]; and maraviroc twice daily, 9 [2%]); none of the deaths were considered to be treatment-related. There were 74 category C events in 68 patients during treatment, with no significant differences among groups, except for esophageal candidiasis (in 2 patients in the placebo group, 12 in the group that received maraviroc once daily, and 3 in the group that received maraviroc twice daily, P = 0.04) (Table 3). Five of the 12 cancers identified as category C events (including 2 of 6 lymphomas) occurred in patients receiving placebo.
The incidence of laboratory-test abnormalities was similar among the study groups, with no significant differences in the incidence of grade 3 or grade 4 abnormalities (see the Supplementary Appendix, available with the full text of this article at www.nejm.org). In the combined placebo groups, approximately 3% of patients had grade 3 or grade 4 increases in levels of aspartate aminotransferase or alanine aminotransferase, as compared with approximately 3 to 4% in the maraviroc groups, without adjustment for the duration of therapy (Table 3). There were no significant differences in the incidence of hepatic abnormalities except for a grade 1 elevation in the aspartate aminotransferase level (37% in the placebo group, 31% in the group that received maraviroc once daily, and 26% in the group that received maraviroc twice daily) and a grade 2 elevation in the alanine aminotransferase level (7%, 5%, and 10%, respectively; P=0.02 for both comparisons for all three groups) (see the Supplementary Appendix).
DISCUSSION
These primary analyses of two ongoing phase 3 studies show that in previously treated patients infected with R5 HIV-1 only (as shown by a co-receptor-tropism assay) who are receiving optimized background therapy, the addition of maraviroc, as compared with placebo, is associated with a significantly greater reduction in the level of HIV-1 RNA and a greater increase in the CD4 cell count. The efficacy of maraviroc over placebo was further supported by the finding that 42 to 47% of patients taking maraviroc had HIV-1 RNA levels of less than 50 copies per milliliter at 48 weeks, as compared with 16% and 18% of those taking placebo.
Although the short-term efficacy of maraviroc was first shown in two phase 2 monotherapy studies in patients infected with R5 HIV-1,17 the MOTIVATE studies show that longer-term efficacy can be achieved by blocking a host protein, rather than a viral protein, with a small-molecule CCR5 coreceptor antagonist taken orally. These results are similar to those reported in studies evaluating other new drugs that target HIV directly28–33 and show that antiretroviral agents that target host and viral proteins can be used together to achieve virologic suppression in previously treated patients with HIV-1 infection. These results also support current antiretroviral treatment guidelines that recommend a new regimen with two (or preferably three) fully active agents from multiple drug classes for previously treated patients.4
The virologic response rates in the placebo groups in the current studies were notably higher than those seen at 48 weeks in the control groups of similarly designed studies — for example, the T-20 versus Optimized Background Regimen Only studies (TORO) involving patients with extensive previous treatment (HIV-1 RNA, <50 copies per milliliter overall in 17% vs. 8% of patients, respectively).31 This result is probably due to improvements in the drugs available to construct an optimized background regimen, including the use of enfuvirtide itself as part of the regimen in the MOTIVATE studies. In the recently completed studies of raltegravir — Blocking Integrase in Treatment Experienced Patients with a Novel Compound against HIV, Merck (BENCHMRK) — that allowed use of darunavir, even higher response rates (31 to 35%) were achieved in the placebo groups.32,33 The DUET studies of etravirine that mandated use of darunavir resulted in a response rate of 40% in the placebo groups.29,30 In the MOTIVATE studies, investigational agents (e.g., darunavir, etravirine, and raltegravir) were not permitted because no information on drug–drug interactions was available at the time, and only 15% of patients received tipranavir. Given the current availability of these newer antiretroviral drugs, combination regimens with maraviroc could be even more effective.
Treatment-related adverse events, study-drug discontinuation related to adverse events, AIDS-defining events, and deaths did not differ significantly across the three study groups, with the exception of esophageal candidiasis, which was more common in the group that received maraviroc once daily than in the placebo group or the group that received maraviroc twice daily. However, this difference must be interpreted cautiously, since the analysis was post hoc and was not adjusted for the duration of treatment exposure or for multiple testing. There were no significant differences between the maraviroc groups and the placebo group with respect to severe hepatotoxic effects, lymphoma, or other cancers, as reported in prior studies of other CCR5 antagonists.34,35 Given the relatively limited clinical experience with maraviroc to date, careful, longer-term safety monitoring would be prudent.
In general, studies of people with the CCR5 delta32 deletion show some immunologic effects. For example, the CCR5 delta32 mutation is associated with fewer signs and symptoms of rheumatoid arthritis36 but is also associated with more severe infection and increased mortality from West Nile virus in certain groups of patients.37 There are also data indicating that this host genetic deletion is associated with fewer cancers in patients with HIV infection.38 A congenital absence of CCR5 receptors, however, may not be equivalent to blocking of the CCR5 receptor with a small-molecule inhibitor; this again underscores the need for careful, longer-term safety monitoring is warranted.
These studies have several limitations. Although they were conducted on three continents, only about 10% of enrollees were women and less than 20% were nonwhite, which means that the generalizability of the results to other populations may be limited. The coreceptor-tropism assay used may not detect minority populations of X4 virus at baseline; the virus may emerge either before or during CCR5-antagonist therapy and may ultimately lead to reduced virologic responses. An enhanced tropism assay that better detects minority viral populations is now available.39 Use of newer antiretroviral drugs in the study regimens was limited (tipranavir) or prohibited (darunavir, etravirine, and raltegravir) because of the lack of data on drug–drug interactions with maraviroc or lack of availability of these investigational agents at the time. Safety comparisons were conducted post hoc and were not adjusted for treatment duration and multiple comparisons. Finally, although maraviroc, as compared with placebo, had superior virologic activity and resulted in higher CD4 cell counts with a similar safety profile in these 48-week studies, further follow-up is required to assess longer-term virologic and immunologic responses and drug-related side effects.
In summary, the results of these studies show that in previously treated patients with R5 HIV-1 infection only (as shown by a coreceptortropism assay), maraviroc, together with an antiretroviral regimen optimized on the basis of treatment history and drug-resistance testing, is effective and generally tolerated for at least 48 weeks.
Supplementary Material
Acknowledgments
MOTIVATE 1 and MOTIVATE 2 were sponsored by Pfizer Global Research and Development and were supported by Pfizer and by a grant from the National Institutes of Health (AI-51966, to Dr. Gulick). Assistance with manuscript preparation, funded by Pfizer, was provided by Health Interactions (London).
Dr. Gulick reports receiving consulting fees from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Glaxo-SmithKline, Merck, Monogram, Pfizer, Schering-Plough, Tibotec, Virco, and ViroStatics and grant support from Boehringer Ingelheim, Gilead, Merck, Pfizer, Schering-Plough, and Tibotec. Dr. Lalezari reports receiving consulting and lecture fees from Pfizer. Dr. Goodrich and Ms. Ridgway report being employees of Pfizer, the study sponsor. Dr. Clumeck reports receiving consulting fees from Abbott, Gilead, Merck, and Pfizer. Dr. DeJesus reports receiving consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, and GlaxoSmithKline, lecture fees from Gilead, GlaxoSmithKline, and Merck, and grant support from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Pfizer, Roche, Schering-Plough, and Tibotec. Dr. Horban reports receiving consulting fees from Janssen. Dr. Nadler reports holding equity ownership and stock options in Gilead. Dr. Clotet reports receiving consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Janssen, Merck, Pfizer, Roche, and Siemens, lecture fees from Bristol-Myers Squibb, Gilead, Janssen, Merck, and Roche, and grant support from Merck and Pfizer. Dr. Wohlfeiler reports receiving consulting and lecture fees from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Monogram, Pfizer, and Tibotec and lecture fees from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Monogram, and Tibotec. Dr. McHale reports being previously employed by Pfizer and holding equity ownership and stock options in Pfizer. Mr. Sullivan and Drs. Felstead, Dunne, van der Ryst, and Mayer report being employees of Pfizer and holding equity ownership and stock options in Pfizer. No other potential conflict of interest relevant to this article was reported.
We thank the study participants as well as the study investigators and site staff at the 239 participating study sites (for a complete list of the investigators, site staff, and study sites, see the Supplementary Appendix).
References
- 1.AIDSinfo. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Rockville, MD: Department of Health and Human Services; Oct 10, 2006. [Accessed September 9, 2008]. http://aidsinfo.nih.gov/Guidelines/ArchivedGuidelines.aspx. [Google Scholar]
- 2.Phillips AN, Dunn D, Sabin C, et al. Long term probability of detection of HIV-1 drug resistance after starting anti-retroviral therapy in routine clinical practice. AIDS. 2005;19:487–94. doi: 10.1097/01.aids.0000162337.58557.3d. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Devereux H, Kinloch-de-Loes S, et al. Immunological, virological and clinical response to highly active anti-retroviral therapy treatment regimens in a complete clinic population. AIDS. 2000;14:1545–52. doi: 10.1097/00002030-200007280-00010. [DOI] [PubMed] [Google Scholar]
- 4.AIDSinfo. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Rockville, MD: Department of Health and Human Services; Jan 29, 2008. [Accessed September 9, 2008]. http://aidsinfo.nih.gov/guidelines. [Google Scholar]
- 5.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 6.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–62. doi: 10.1126/science.273.5283.1856. [Erratum, Science 1996; 274:1069.] [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–3. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 8.de Roda Husman AM, Koot M, Cornelissen M, et al. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann Intern Med. 1997;127:882–90. doi: 10.7326/0003-4819-127-10-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1–infected individuals. J Exp Med. 1997;185:621–8. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philpott SM. HIV-1 coreceptor usage, transmission, and disease progression. Curr HIV Res. 2003;1:217–27. doi: 10.2174/1570162033485357. [DOI] [PubMed] [Google Scholar]
- 11.Whitcomb JM, Huang W, Fransen S, et al. Analysis of baseline enfuvirtide (T20) susceptibility and co-receptor tropism in two phase III study populations. Presented at the 10th Conference on Retroviruses and Opportunistic Infections; Boston. February 10–14, 2003; abstract. [Google Scholar]
- 12.Melby T, Despirito M, Demasi R, Heilek-Snyder G, Greenberg ML, Graham N. HIV-1 coreceptor use in triple-class treatment-experienced patients: baseline prevalence, correlates, and relationship to enfuvirtide response. J Infect Dis. 2006;194:238–46. doi: 10.1086/504693. [DOI] [PubMed] [Google Scholar]
- 13.Wilkin TJ, Su Z, Kuritzkes DR, et al. HIV type 1 chemokine coreceptor use among antiretroviral-experienced patients screened for a clinical trial of a CCR5 inhibitor: AIDS Clinical Trial Group A5211. Clin Infect Dis. 2007;44:591–5. doi: 10.1086/511035. [Erratum, Clin Infect Dis 2007;44:1399.] [DOI] [PubMed] [Google Scholar]
- 14.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427,857): a potent, orally bioavailable, and selective small-molecule inhibitor of the chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Anti-microb Agents Chemother. 2005;49:4721–32. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westby M, van der Ryst E. CCR5 antagonists: host-targeted antivirals for the treatment of HIV infection. Antivir Chem Chemother. 2005;16:339–54. doi: 10.1177/095632020501600601. [DOI] [PubMed] [Google Scholar]
- 16.Selzentry prescribing information. New York: Pfizer; 2007. (package insert) [Google Scholar]
- 17.Fätkenheuer G, Pozniak AL, Johnson MA, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11:1170–2. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 18.Goodrich J, Saag M, van der Ryst E, et al. 48-Week safety and efficacy of maraviroc in combination with optimized background therapy (OBT) for the treatment of antiretroviral-experienced patients infected with dual/mixed-tropic HIV-1. Presented at the 45th Annual Meeting of the Infectious Diseases Society of America; San Diego, CA. October 4–7, 2007; abstract. [Google Scholar]
- 19.Limoli K, Whitcomb J, Kiss L, et al. Technical validation defines the performance of Trofile–Monogram’s HIV co-receptor tropism assay. Presented at the XVI International AIDS Conference; Toronto. August 13–18, 2006; abstract. [Google Scholar]
- 20.Whitcomb JM, Huang W, Fransen S, et al. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob Agents Chemother. 2007;51:566–75. doi: 10.1128/AAC.00853-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel S, Jenkins TM, Whitlock LA, Ridgway CE, Muirhead GJ. Effects of CYP3A4 inducers with and without CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br J Clin Pharmacol. 2008;65(Suppl 1):38–46. doi: 10.1111/j.1365-2125.2008.03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abel S, Russell D, Taylor-Worth RJ, Ridgway CE, Muirhead GJ. Effects of CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br J Clin Pharmacol. 2008;65(Suppl 1):27–37. doi: 10.1111/j.1365-2125.2008.03133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalezari JP, Henry K, O’Hearn M, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175–85. doi: 10.1056/NEJMoa035026. [Erratum, N Engl J Med 2003; 349:1100.] [DOI] [PubMed] [Google Scholar]
- 24.Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med. 2003;348:2186–95. doi: 10.1056/NEJMoa035211. [DOI] [PubMed] [Google Scholar]
- 25.1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 26.Regulatory Compliance Center. [Accessed September 9, 2008];Division of AIDS: table for grading severity of adult adverse experiences. 1992 http://rcc.tech-res.com/tox_tables.htm.
- 27.Fätkenheuer G, Nelson M, Lazzarin A, et al. Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med. 2008;359:1442–55. doi: 10.1056/NEJMoa0803154. [DOI] [PubMed] [Google Scholar]
- 28.Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–78. doi: 10.1016/S0140-6736(07)60497-8. [Erratum, Lancet 2008;371:116.] [DOI] [PubMed] [Google Scholar]
- 29.Haubrich R, Cahn P, Grinsztejn B, et al. DUET-1: week-48 results of a phase III randomized double-blind trial to evaluate the efficacy and safety of TMC125 vs placebo in 612 treatment-experienced HIV-1-infected patients. Presented at the 15th Conference on Retroviruses and Opportunistic Infections; Boston. February 3–6, 2008; abstract. [Google Scholar]
- 30.Johnson M, Campbell T, Clotet B, et al. DUET-2: week-48 results of a phase III randomized double-blind trial to evaluate the efficacy and safety of TMC125 vs placebo in 591 treatment-experienced HIV-1-infected patients. Presented at the 15th Conference on Retroviruses and Opportunistic Infections; Boston. February 3–6, 2008; abstract. [Google Scholar]
- 31.Nelson M, Arastéh K, Clotet B, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 Versus Optimized Background Regimen Only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005;40:404–12. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 32.Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–54. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 33.Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infeciton. N Engl J Med. 2008;359:355–65. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- 34.Nichols WG, Steel HM, Bonny T, et al. Hepatotoxicity observed in clinical trials of aplaviroc ( GW873140) Antimicrob Agents Chemother. 2008;52:858–65. doi: 10.1128/AAC.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS Clinical Trials Group 5211. J Infect Dis. 2007;196:304–12. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 36.Garred P, Madsen HO, Petersen J, et al. CC chemokine receptor 5 polymorphism in rheumatoid arthritis. J Rheumatol. 1998;25:1462–5. [PubMed] [Google Scholar]
- 37.Glass WG, McDermott DH, Lim JK, et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dean M, Jacobson LP, McFarlane G, et al. Reduced risk of AIDS lymphoma in individuals heterozygous for the CCR5-delta32 mutation. Cancer Res. 1999;59:3561–4. [PubMed] [Google Scholar]
- 39.Trinh L, Han D, Huang W, et al. Technical validation of an enhanced sensitivity Trofile HIV co-receptor tropism assay for selecting patients for therapy with entry inhibitors targeting CCR5. Presented at the XVII International HIV Drug Resistance Workshop; Sitges, Spain. June 10–14, 2008; abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.